Self-Reported Practices and Emotions in Prescribing Opioids for Chronic Noncancer Pain: A Cross-Sectional Study of German Physicians
Abstract
:1. Introduction
2. Methods
2.1. Study Population and Inclusion Criteria
2.2. Survey Questionnaire
2.2.1. Baseline Characteristics
2.2.2. Opioid-Prescribing Behavior: Type and Formulation of Opioids, Indications
2.2.3. Physicians’ Emotional Response to Patients’ Demands for Dose Escalation
2.2.4. Risk Literacy
2.2.5. Piloting
2.3. Statistical Analysis
3. Results
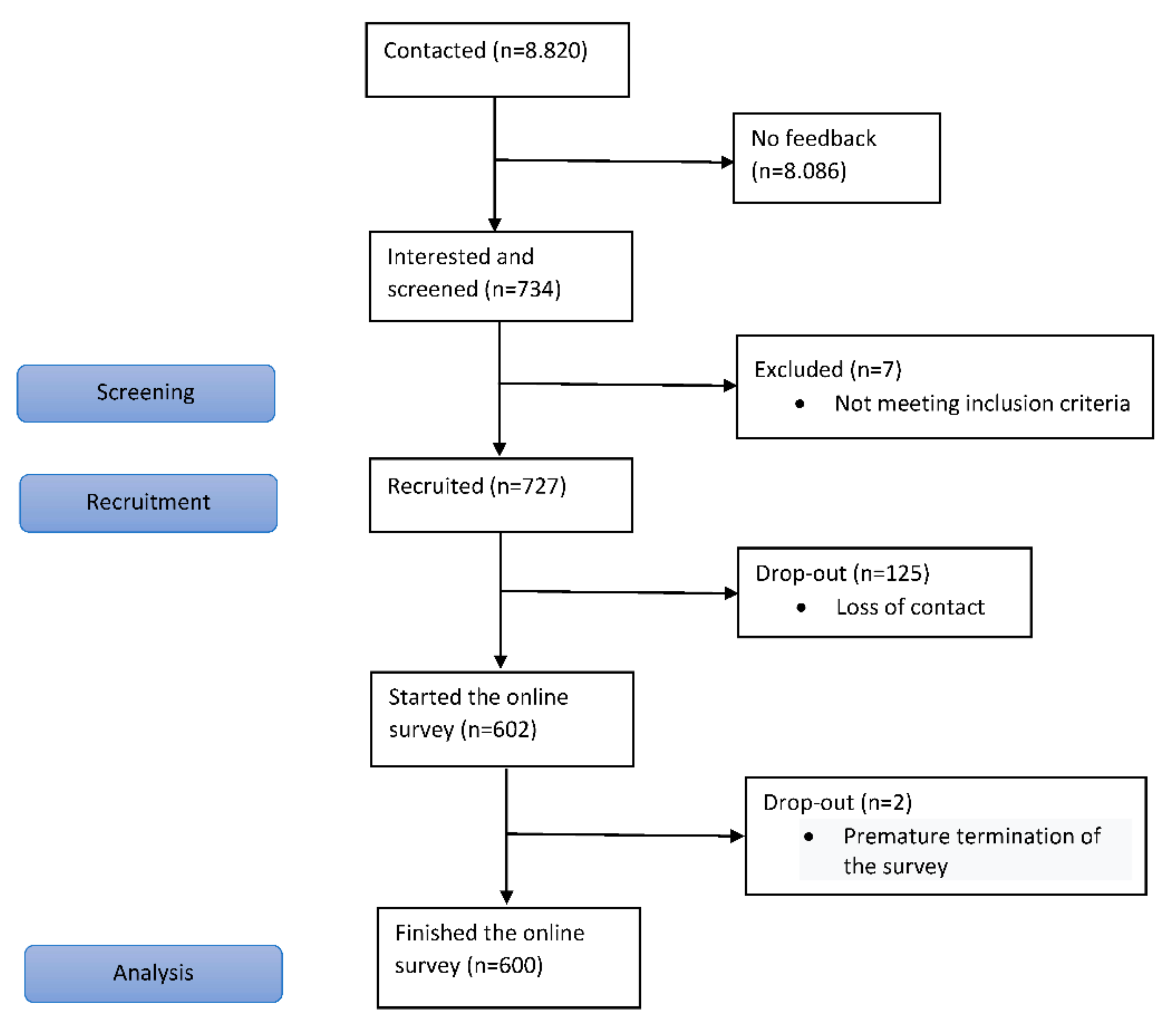
3.1. Recruitment of Participating Physicians
3.2. Demographic and Professional Characteristics
3.3. Self-Reports of the Opioid Ingredients and Galenics Prescribed
3.4. The Indications Detailed in the Self-Reported Opioid Prescribing Behavior
3.5. Physicians’ Self-Reported Emotional Reactions to Patient Requests to Increase Opioid Dosages
3.6. Covariate Analysis of Non-Guideline-Compliant Opioid Prescribing Behavior
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Portenoy, R.K.; Foley, K.M. Chronic use of opioid analgesics in non-malignant pain: Report of 38 cases. Pain 1986, 25, 171–186. [Google Scholar] [CrossRef]
- Bialas, P.; Maier, C.; Klose, P.; Häuser, W. Efficacy and harms of long-term opioid therapy in chronic non-cancer pain: Systematic review and metaanalysis of open-label extensions trials. Eur. J. Pain 2020, 24, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro, L.E.; Furlan, A.D.; Deshpande, A.; Mailis-Gagnon, A.; Atlas, S.; Turk, D.C. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst. Rev. 2013, 8, CD004959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, R.; Deyo, R.; Friedly, J.; Skelly, A.; Weimer, M.; Fu, R.; Dana, T.; Kraegel, P.; Griffin, J.; Grusing, S. Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 2017, 166, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.D.; Sandoval, J.A.; Mailis-Gagnon, A.; Tunks, E. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. CMAJ 2006, 174, 1589–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.A.; McQuay, H.J. Prevalence of opioid adverse events in chronic non-malignant pain: Systematic review of randomised trials of oral opioids. Arthritis Res. Ther. 2005, 7, R1046–R1051. [Google Scholar] [CrossRef] [Green Version]
- Nury, E.; Schmucker, C.; Nagavci, B.L.; Motschall, E.; Nitschke, K.; Schulte, E.; Wegwarth, O.; Meerpohl, J.J. The effectiveness and risk of long-term opioid therapy versus placebo and non-opioid therapy in patients with chronic non-cancer pain: A systematic review and meta-analysis. Pain 2022, 163, 610–636. [Google Scholar] [CrossRef]
- Garland, E.L.; Froeliger, B.; Zeidan, F.; Partin, K.; Howard, M.O. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neurosci. Biobehav. Rev. 2013, 37, 2597–2607. [Google Scholar] [CrossRef] [Green Version]
- McDermott, K.A.; Griffin, M.L.; McHugh, R.K.; Fitzmaurice, G.M.; Jamison, R.N.; Provost, S.E.; Weiss, R.D. Long-term naturalistic follow-up of chronic pain in adults with prescription opioid use disorder. Drug Alcohol Depend. 2019, 205, 107675. [Google Scholar] [CrossRef]
- Vowles, K.E.; McEntee, M.L.; Julnes, P.S.; Frohe, T.; Ney, J.P.; van der Goes, D.N. Rates of opioid misuses, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 2015, 156, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Busse, J.W.; Craigie, S.; Juurlink, D.N.; Buckley, D.N.; Wang, L.; Couban, R.J.; Agoritsas, T.; Akl, E.A.; Carrasco-Labra, A.; Cooper, L.; et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017, 189, E659–E666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häuser, W.; Bock, F.; Hüppe, M.; Nothacker, M.; Norda, H.; Radbruch, L.; Schiltenwolf, M.; Schuler, M.; Tölle, T.; Viniol, A.; et al. Koautoren für die Konsensusgruppe der 2. Aktualisierung der S3-Leitlinie LONTS. Empfehlungen der zweiten Aktualisierung der Leitlinie LONTS. Langzeitanwendung von Opioiden bei chronischen nicht-tumorbedingten Schmerzen. Der Schmerz 2020, 34, 204–244. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Asamoah-Boaheng, M.; Badejo, O.A.; Bell, L.V.; Buckley, N.; Busse, J.W.; Campbell, T.S.; Corace, K.; Cooper, L.K.; Flusk, D.; et al. Prescriber adherence to guidelines for chronic noncancer pain management with opioids: Systematic review and meta-analysis. Health Psychol. 2020, 39, 430–451. [Google Scholar] [CrossRef]
- Alenezi, A.; Yahyouche, A.; Paudyal, V. Current status of opioid epidemic in the United Kingdom and strategies for treatment optimisation in chronic pain. Int. J. Clin. Pharm. 2021, 43, 318–322. [Google Scholar] [CrossRef]
- Pierce, M.; van Amsterdam, J.; Kalkman, G.A.; Schellekens, A.; van den Brink, W. Is Europe facing an opioid crisis like the United States? An analysis of opioid use and related adverse effects in 19 European countries between 2010 and 2018. Eur. Psychiatry 2021, 64, e47. [Google Scholar] [CrossRef]
- Häuser, W.; Schug, S.; Furlan, A.D. The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: A perspective from different continents. Pain Rep. 2017, 2, e599. [Google Scholar] [CrossRef]
- Alford, D.P.; Lazure, P.; Murray, S.; Hardesty, I.; Krause, J.R.; White, J.L. National Trends in Prescription Opioid Risk Mitigation Practices: Implications for Prescriber Education. Pain Med. 2019, 20, 907–915. [Google Scholar] [CrossRef]
- Turk, D.C.; Okifuji, A. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? Clin. J. Pain 1997, 13, 330–336. [Google Scholar] [CrossRef]
- Spitz, A.; Moore, A.A.; Papaleontiou, M.; Granieri, E.; Turner, B.J.; Reid, M.C. Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: A qualitative study. BMC Geriatr. 2011, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Bauer, S.R.; Hitchner, L.; Harrison, H.; Gerstenberger, J.; Steiger, S. Predictors of higher-risk chronic opioid prescriptions in an academic primary care setting. Subst. Abus. 2016, 37, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Harle, C.A.; Bauer, S.E.; Hoang, H.Q.; Cook, R.L.; Hurley, R.W.; Fillingim, R.B. Decision support for chronic pain care: How do primary care physicians decide when to prescribe opioids? a qualitative study. BMC Fam. Pract. 2015, 16, 48. [Google Scholar] [CrossRef] [Green Version]
- Muench, J.; Fankhauser, K.; Voss, R.W.; Huguet, N.; Hartung, D.M.; O’Malley, J.; Bailey, S.R.; Cowburn, S.; Wright, D.; Barker, G.; et al. Assessment of Opioid Prescribing Patterns in a Large Network of US Community Health Centers, 2009 to 2018. JAMA Netw. Open 2020, 3, e2013431. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Maestre, C.; Reyes-Pérez, Á.; Esteve, R.; López-Martínez, A.E.; Bernardes, S.; Jensen, M.P. Opioid Pain Medication Prescription for Chronic Pain in Primary Care Centers: The Roles of Pain Acceptance, Pain Intensity, Depressive Symptoms, Pain Catastrophizing, Sex, and Age. Int. J. Environ. Res. Public Health 2020, 17, 6428. [Google Scholar] [CrossRef] [PubMed]
- Wegwarth, O.; Spies, C.; Schulte, E.; Meerpohl, J.J.; Schmucker, C.; Nury, E.; Brockmann, D.; Donner-Banzhoff, N.; Wind, S.; Goebel, E.; et al. Experiencing the risk of overutilising opioids among patients with chronic non-cancer pain in ambulatory care (ERONA): The protocol of an exploratory, randomised controlled trial. BMJ Open 2020, 10, e037642. [Google Scholar] [CrossRef] [PubMed]
- Caverly, T.J.; Prochazka, A.V.; Combs, B.P.; Lucas, B.P.; Mueller, S.R.; Kutner, J.S.; Binswanger, I.; Fagerlin, A.; McCormick, J.; Pfister, S.; et al. Doctors and numbers: An assessment of the critical risk interpretation test. Med. Decis. Mak. 2015, 35, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Petzke, F.; Welsch, P.; Klose, P.; Sommer, C.; Häuser, W. Opioids for chronic low back pain. An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least four weeks double-blind duration. Eur. J. Pain 2020, 24, 497–517. [Google Scholar] [CrossRef] [Green Version]
- Welsch, P.; Klose, P.; Petzke, F.; Häuser, W. Opioids for chronic osteoarthritis pain. An updated systematic review and metaanalysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least four weeks double-blind duration. Eur. J. Pain 2020, 24, 685–703. [Google Scholar] [CrossRef]
- Sommer, C.; Klose, P.; Welsch, P.; Petzke, F.; Häuser, W. Opioids for chronic non-cancer neuropathic pain. An updated systematic review and metaanalysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least four weeks duration. Eur. J. Pain 2020, 24, 3–18. [Google Scholar] [CrossRef]
- Bundesärztekammer (BÄK); Kassenärztliche Bundesvereinigung (KBV); Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Nicht-Spezifischer Kreuzschmerz—Langfassung, 2nd ed.; Version 1; BÄK: Berlin, Germany; KBV: Berlin, Germany; AWMF: Frankfurt am Main, Germany, 2017; Available online: www.kreuzschmerz.versorgungsleitlinien.de (accessed on 24 June 2021). [CrossRef]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.C.; Chenot, J.F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.N.M.; Laetsch, D.C.; Chen, L.J.; Holleczek, B.; Meid, A.D.; Brenner, H.; Schöttker, B. Comparison of Five Lists to Identify Potentially Inappropriate Use of Non-Steroidal Anti-Inflammatory Drugs in Older Adults. Pain Med. 2021, 22, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, A.; von Heideken Wågert, P. Adherence to and the Maintenance of Self-Management Behaviour in Older People with Musculoskeletal Pain-A Scoping Review and Theoretical Models. J. Clin. Med. 2021, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hughes, E. The Opioid That Made a Fortune for Its Maker—And for Its Prescribers. New York Times, 2 May 2018. Available online: https://nyti.ms/2Fz37rr (accessed on 27 March 2021).
- Thomas, K. Doubts Raised about Off-Label Use of Subsys, a Strong Painkiller. New York Times, 13 May 2014. Available online: https://nyti.ms/1piOoWq (accessed on 27 March 2021).
- Miceli, L.; Bednarova, R.; Di Cesare, M.; Santori, E.; Spizzichino, M.; DIMinco, L.; Botti, R.; Casciello, M.; Della Rocca, G. Outpatient therapeutic chronic opioid consumption in Italy: A one-year survey. Minerva Anestesiol. 2017, 83, 33–40. [Google Scholar] [CrossRef]
- Fleischman, W.; Auth, D.; Shah, N.D.; Agrawal, S.; Ross, J.S. Association of a Risk Evaluation and Mitigation Strategy Program with Transmucosal Fentanyl Prescribing. JAMA Netw. Open 2019, 2, e191340. [Google Scholar] [CrossRef] [Green Version]
- Passik, S.D.; Kirsh, K.L. The interface between pain and drug abuse and the evolution of strategies to optimize pain management while minimizing drug abuse. Exp. Clin. Psychopharmacol. 2008, 16, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Bundesärztekammer (BÄK). 2020. Available online: https://www.bundesaerztekammer.de/fileadmin/user_upload/downloads/pdf-Ordner/Statistik_2020/2020-Statistik.pdf (accessed on 17 April 2022).

| Total | |
| N = 600 | |
| N (%) | |
| Gender | |
| female | 221 (36.8) |
| Age (years) | |
| 20–39 | 51 (8.5) |
| 40–59 | 413 (68.8) |
| 60–79 | 136 (22.7) |
| Place of work in Germany | |
| North | 133 (22.2) |
| South | 133 (22.2) |
| East | 160 (26.7) |
| West | 174 (29.0) |
| Work experience (years) | |
| <10 | 46 (7.7) |
| 10–19 | 199 (33.2) |
| 20–29 | 247 (41.2) |
| >30 | 108 (18.0) |
| Type of workplace | |
| Doctor’s office | 386 (64.3) |
| Medical care center | 149 (24.8) |
| Hospital | 58 (9.7) |
| Rehabilitation clinic/nursing home | 7 (1.2) |
| Areas of expertise a | |
| General medicine | 360 (60.0) |
| Internal medicine | 149 (24.8) |
| Anesthesiology | 68 (11.3) |
| Orthopedic surgery | 40 (6.7) |
| Psychiatry/psychotherapy/psychosomatic | 12 (2.0) |
| Neurology | 11 (1.8) |
| General surgery | 11 (1.8) |
| Physical medicine | 3 (0.5) |
| Gynecology | 1 (0.2) |
| Urology | 1 (0.2) |
| “Which of the following Strong Opioids are you Currently Prescribing for the Treatment of Chronic Noncancer Pain and in which Dosage Form?” | Total |
| N = 600 | |
| N (%) | |
| Morphine | |
| Oral extended release | 587 (97.8) |
| Oral immediate release | 517 (86.2) |
| No use | 9 (1.5) |
| Buprenorphine | |
| Transdermal | 482 (80.3) |
| Sublingual | 245 (40.8) |
| No use | 102 (17.0) |
| Fentanyl | |
| Transdermal | 594 (99.0) |
| Oral/nasal immediate release | 294 (49.0) |
| No use | 2 (0.3) |
| Oxycodone | |
| Oral extended release | 545 (90.8) |
| Oral immediate release | 468 (78.0) |
| No use | 5 (0.8) |
| Hydromorphone | |
| Oral extended release | 473 (78.8) |
| Oral immediate release | 207 (34.5) |
| No use | 117 (19.5) |
| Tapentadol | |
| Oral extended release | 515 (85.8) |
| Oral immediate release | 354 (58.3) |
| no use | 51 (8.5) |
| “For which noncancer-related diseases have you prescribed strong opioids as the primary prescriber in the past 12 months?” | Total | Evidence Level According to LONTS b [13] |
| N = 600 | ||
| N (%) | ||
| Chronic nonspecific low-back pain | ||
| Yes | 225 (37.5) | 4–12 weeks: Ia, recommendation for |
| No | 335 (55.8) | 13–26 weeks: Ia, recommendation for |
| Does not apply a | 40 (6.7) | >26 weeks: IIb, open recommendation |
| Osteoarthritis | ||
| Yes | 335 (55.8) | 4–12 weeks: Ia, recommendation for |
| No | 238 (39.7) | 13–26 weeks: Ia, recommendation for |
| Does not apply a | 27 (4.5) | >26 weeks: IIb, open recommendation |
| Diabetic polyneuropathy | ||
| Yes | 248 (41.3) | 4–12 weeks: Ia, strong recommendation for |
| No | 210 (35.0) | 13–26 weeks: no data, open recommendation |
| Does not apply a | 142 (23.7) | >26 weeks: IIb, open recommendation |
| Postherpetic neuralgia | ||
| Yes | 229 (38.2) | 4–12 weeks: Ia, recommendation for |
| No | 273 (45.5) | 13–26 weeks: no data, open recommendation |
| Does not apply | 98 (16.3) | >26 weeks: no data, open recommendation |
| Phantom limb pain | ||
| yes | 289 (48.2) | 4–12 weeks: Ib, open recommendation for |
| no | 186 (31.0) | 13–26 weeks: no data, open recommendation |
| does not apply a | 125 (20.8) | >26 weeks: no data, open recommendation |
| Disc prolapsec | ||
| yes | 370 (61.7) | 4–12 weeks: Ib, open recommendation for |
| no | 200 (33.3) | 13–26 weeks: no data, open recommendation |
| does not apply a | 30 (5.0) | >26 weeks: no data, open recommendation |
| Spinal stenosis | ||
| yes | 251 (41.8) | 4–12 weeks: Ib, open recommendation for c |
| no | 287 (47.8) | 13–26 weeks: no data, open recommendation c |
| does not apply a | 62 (10.3) | >26 weeks: no data, open recommendation c |
| Rheumatoid arthritis | ||
| yes | 263 (43.8) | 4–12 weeks: Ib, open recommendation for |
| no | 298 (49.7) | 13–26 weeks: no data, open recommendation |
| does not apply a | 39 (6.5) | >26 weeks: no data, open recommendation |
| Fibromyalgia syndrome | ||
| yes | 152 (25.3) | 4–12 weeks: Ib, open recommendation for |
| no | 252 (42.0) | 13–26 weeks: no data, open recommendation |
| does not apply a | 196 (32.7) | >26 weeks: no data, open recommendation |
| Secondary headaches | ||
| yes | 136 (22.7) | 4–12 weeks: no data, open recommendation |
| no | 380 (63.3) | 13–26 weeks: no data, open recommendation |
| does not apply a | 84 (14.0) | >26 weeks: no data, open recommendation |
| Vertebral body fractures in osteoporosis | ||
| yes | 231 (38.5) | 4–12 weeks: no data, open recommendation |
| no | 268 (44.7) | 13–26 weeks: no data, open recommendation |
| does not apply a | 101 (16.8) | >26 weeks: no data, open recommendation |
| Chronic postsurgical pain | ||
| yes | 336 (56.0) | 4–12 weeks: no data, open recommendation |
| no | 143 (23.8) | 13–26 weeks: no data, open recommendation |
| does not apply a | 121 (20.2) | >26 weeks: no data, open recommendation |
| Peripheral arterial disease of the lower extremities | ||
| yes | 171 (28.5) | 4–12 weeks: no data, open recommendation |
| no | 337 (56.2) | 13–26 weeks: no data, open recommendation |
| does not apply a | 92 (15.3) | >26 weeks: no data, open recommendation |
| Grade 3 and 4 pressure ulcers | ||
| yes | 362 (60.3) | 4–12 weeks: no data, open recommendation |
| no | 158 (26.3) | 13–26 weeks: no data, open recommendation |
| does not apply a | 80 (13.3) | >26 weeks: no data, open recommendation |
| Chronic pain associated with fixed contractures | ||
| yes | 231 (38.5) | 4–12 weeks: no data, open recommendation |
| no | 254 (42.3) | 13–26 weeks: no data, open recommendation |
| does not apply a | 115 (19.2) | >26 weeks: no data, open recommendation |
| Central neuropathic pain | ||
| yes | 112 (18.7) | 4–12 weeks: no data, open recommendation |
| no | 327 (54.5) | 13–26 weeks: no data, open recommendation |
| does not apply a | 161 (26.8) | >26 weeks: no data, open recommendation |
| Chronic regional pain syndrome I and II | ||
| yes | 274 (45.7) | 4–12 weeks: no data, open recommendation |
| no | 172 (28.7) | 13–26 weeks: no data, open recommendation |
| does not apply a | 154 (25.7) | >26 weeks: no data, open recommendation |
| Chronic pelvic pain | ||
| yes | 73 (12.2) | 4–12 weeks: no data, open recommendation |
| no | 217 (36.2) | 13–26 weeks: no data, open recommendation |
| does not apply a | 310 (51.7) | >26 weeks: no data, open recommendation |
| Chronic inflammatory bowel disease | >26 weeks: IIIb, recommendation against | |
| yes | 252 (42.0) | |
| no | 248 (41.3) | |
| does not apply a | 100 (16.7) | |
| Primary headaches | >26 weeks: IIIb, strong recommendation against | |
| yes | 119 (19.8) | |
| no | 406 (67.7) | |
| does not apply a | 75 (12.5) | |
| Functional disorders | no data; independent of time: strong recommendation against | |
| yes | 158 (26.3) | |
| no | 366 (61.0) | |
| does not apply a | 76 (12.7) | |
| Chronic pancreatitis | >26 weeks: IIIb, strong recommendation against | |
| yes | 180 (30.0) | |
| no | 316 (52.7) | |
| does not apply a | 104 (17.3) | |
| Craniomandibular dysfunction | no recommendation | |
| yes | 86 (14.3) | |
| no | 259 (43.2) | |
| does not apply a | 255 (42.5 | |
| Persistent idiopathic facial pain | no recommendation | |
| yes | 197 (32.8) | |
| no | 264 (44.0) | |
| does not apply a | 139 (23.2) | |
| Neuralgia (e.g., trigeminus) | no recommendation | |
| yes | 241 (40.2) | |
| no | 295 (49.2) | |
| does not apply a | 64 (10.7) | |
| Multiple sclerosis | No statement on this indication in LONTS b | |
| yes | 136 (22.7) | |
| no | 295 (49.2) | |
| does not apply a | 169 (28.2) |
| Case Vignette: “Please Imagine the Following Situation: A Patient with Chronic Noncancer Related Low-Back Pain who has Already been Prescribed an Opioid for a Long Time Comes to your Consultation with the Request to Increase the Opioid Dose. There are no Indications of a Finding that Requires Intervention, such as a New Neurological Disorder or Other red Flags. Which of the Emotions Described below have you Already Observed in Yourself?” | Total |
| N = 600 | |
| N a (%) | |
| “I can handle the situation quite well.” | 354 (59) |
| “I feel pressured to increase the dose.” | 148 (25) |
| “I feel helpless because I don’t have an easy solution.” | 149 (25) |
| “I experience negative emotions such as anger.” | 135 (23) |
| “I have a bad feeling about increasing the dose.” | 258 (43) |
| Non-Guideline-Compliant Opioid Prescribing Using the Example of Ultra-Fast-Acting Fentanyl for CNCP | |||
|---|---|---|---|
| Independent Variables | Odds Ratio | 95% CI | p |
| Buprenorphine, sublingual Prescribing for CNCP (reference class: no prescribing for CNCP) | 15.4 | 10.1–23.3 | <0.001 |
| Negative emotions a present (reference class: not-present) | 1.7 | 1.2–2.6 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte, E.; Petzke, F.; Spies, C.; Denke, C.; Schäfer, M.; Donner-Banzhoff, N.; Hertwig, R.; Wegwarth, O. Self-Reported Practices and Emotions in Prescribing Opioids for Chronic Noncancer Pain: A Cross-Sectional Study of German Physicians. J. Clin. Med. 2022, 11, 2506. https://doi.org/10.3390/jcm11092506
Schulte E, Petzke F, Spies C, Denke C, Schäfer M, Donner-Banzhoff N, Hertwig R, Wegwarth O. Self-Reported Practices and Emotions in Prescribing Opioids for Chronic Noncancer Pain: A Cross-Sectional Study of German Physicians. Journal of Clinical Medicine. 2022; 11(9):2506. https://doi.org/10.3390/jcm11092506
Chicago/Turabian StyleSchulte, Erika, Frank Petzke, Claudia Spies, Claudia Denke, Michael Schäfer, Norbert Donner-Banzhoff, Ralph Hertwig, and Odette Wegwarth. 2022. "Self-Reported Practices and Emotions in Prescribing Opioids for Chronic Noncancer Pain: A Cross-Sectional Study of German Physicians" Journal of Clinical Medicine 11, no. 9: 2506. https://doi.org/10.3390/jcm11092506
APA StyleSchulte, E., Petzke, F., Spies, C., Denke, C., Schäfer, M., Donner-Banzhoff, N., Hertwig, R., & Wegwarth, O. (2022). Self-Reported Practices and Emotions in Prescribing Opioids for Chronic Noncancer Pain: A Cross-Sectional Study of German Physicians. Journal of Clinical Medicine, 11(9), 2506. https://doi.org/10.3390/jcm11092506






