Texture Analysis in Diagnosing Skin Pigmented Lesions in Normal and Polarized Light—A Preliminary Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Lesions
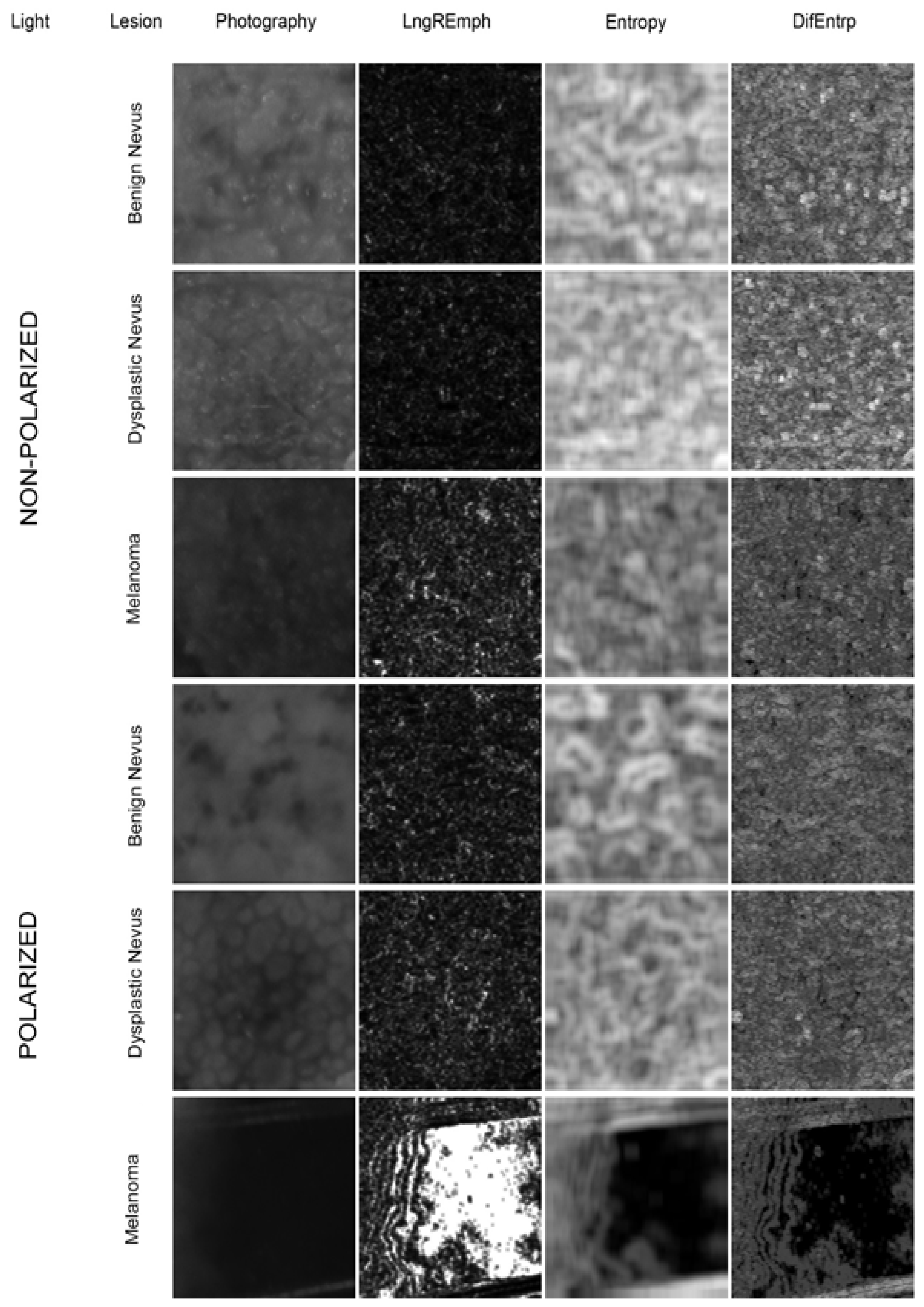
2.2. Image Preparation
2.3. Texture Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- A texture analysis shows differences between benign, dysplastic nevi, and melanomas.
- A texture analysis demonstrates differences between in situ and invasive melanomas.
- Entropy, difference entropy from the co-occurrence matrix and the long-run emphasis moment from the run-length matrix are texture features that can be used for Derm-CAD analysis, and the bone index and texture index can be derived from these three features.
- Polarized light is superior to non-polarized light in visualizing the details of melanocytic lesions, which leads to a more accurate diagnosis and analysis of the lesion.
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, I. New and Evolving Concepts of Melanocytic Nevi and Melanocytomas. Mod. Pathol. 2020, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sardana, K.; Chakravarty, P.; Goel, K. Optimal Management of Common Acquired Melanocytic Nevi (Moles): Current Perspectives. Clin. Cosmet. Investig. Dermatol. 2014, 7, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, D.E. Dysplastic Naevi: An Update. Histopathology 2010, 56, 112–120. [Google Scholar] [CrossRef]
- Mesbah Ardakani, N. Dysplastic/Clark Naevus in the Era of Molecular Pathology. Australas. J. Dermatol. 2019, 60, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Braghiroli, N.; Grant-Kels, J.M.; Oliviero, M.; Rabinovitz, H.; Ferenczi, K.; Scope, A. The Role of Reflectance Confocal Microscopy in Differentiating Melanoma in Situ from Dysplastic Nevi with Severe Atypia: A Cross-Sectional Study. J. Am. Acad. Dermatol. 2020, 83, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Scatena, C.; Murtas, D.; Tomei, S. Cutaneous Melanoma Classification: The Importance of High-Throughput Genomic Technologies. Front. Oncol. 2021, 11, 635488. [Google Scholar] [CrossRef]
- Lattanzi, M.; Lee, Y.; Simpson, D.; Moran, U.; Darvishian, F.; Kim, R.H.; Hernando, E.; Polsky, D.; Hanniford, D.; Shapiro, R.; et al. Primary Melanoma Histologic Subtype: Impact on Survival and Response to Therapy. J. Natl. Cancer Inst. 2019, 111, 180–188. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; Mccubrey, J.A.; Candido, S.; Libra, M. Cutaneous Melanoma: From Pathogenesis to Therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie-Wood, A.R.; Milton, G.W.; de Launey, J.W. Melanoma: Accuracy of Clinical Diagnosis. Australas. J. Dermatol. 1998, 39, 31–33. [Google Scholar] [CrossRef]
- Argenziano, G.; Soyer, H.P. Dermoscopy of Pigmented Skin Lesions--a Valuable Tool for Early Diagnosis of Melanoma. Lancet. Oncol. 2001, 2, 443–449. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Chuchu, N.; Ferrante di Ruffano, L.; Matin, R.N.; Thomson, D.R.; Wong, K.Y.; Aldridge, R.B.; Abbott, R.; Fawzy, M.; et al. Dermoscopy, with and without Visual Inspection, for Diagnosing Melanoma in Adults. Cochrane Database Syst. Rev. 2018, 12, CD011902. [Google Scholar] [CrossRef]
- Ferrante di Ruffano, L.; Takwoingi, Y.; Dinnes, J.; Chuchu, N.; Bayliss, S.E.; Davenport, C.; Matin, R.N.; Godfrey, K.; O’Sullivan, C.; Gulati, A.; et al. Computer-Assisted Diagnosis Techniques (Dermoscopy and Spectroscopy-Based) for Diagnosing Skin Cancer in Adults. Cochrane Database Syst. Rev. 2018, 12, CD013186. [Google Scholar] [CrossRef]
- Garnavi, R.; Aldeen, M.; Bailey, J. Computer-Aided Diagnosis of Melanoma Using Border and Wavelet-Based Texture Analysis. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 1239–1252. [Google Scholar] [CrossRef]
- Murali, A.; Stoecker, W.v.; Moss, R.H. Detection of Solid Pigment in Dermatoscopy Images Using Texture Analysis. Skin Res. Technol. 2000, 6, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Trafalski, M.; Kozakiewicz, M.; Jurczyszyn, K. Application of Fractal Dimension and Texture Analysis to Evaluate the Effectiveness of Treatment of a Venous Lake in the Oral Mucosa Using a 980 Nm Diode Laser-A Preliminary Study. Materials 2021, 14, 4140. [Google Scholar] [CrossRef]
- Jurczyszyn, K.; Trzeciakowski, W.; Kozakiewicz, M.; Kida, D.; Malec, K.; Karolewicz, B.; Konopka, T.; Zborowski, J. Fractal Dimension and Texture Analysis of Lesion Autofluorescence in the Evaluation of Oral Lichen Planus Treatment Effectiveness. Materials 2021, 14, 5448. [Google Scholar] [CrossRef]
- Kassner, A.; Thornhill, R.E. Texture Analysis: A Review of Neurologic MR Imaging Applications. Am. J. Neuroradiol. 2010, 31, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szczypiński, P.M.; Strzelecki, M.; Materka, A.; Klepaczko, A. MaZda—A Software Package for Image Texture Analysis. Comput. Methods Programs Biomed. 2009, 94, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kołaciński, M.; Kozakiewicz, M.; Materka, A. Textural Entropy as a Potential Feature for Quantitative Assessment of Jaw Bone Healing Process. Arch. Med. Sci. 2015, 11, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Haralick, R.M. Statistical and Structural Approaches to Texture. Proc. IEEE 1979, 67, 786–804. [Google Scholar] [CrossRef]
- Materka, A.; Strzelecki, M. Texture Analysis Methods—A Review; COST B11 Report (Presented and Distributed at MC Meeting and Workshop in Brussels, June 1998); Technical University of Lodz: Lodz, Poland, 1998. [Google Scholar]
- Kozakiewicz, M.; Wach, T. New Oral Surgery Materials for Bone Reconstruction—A Comparison of Five Bone Substitute Materials for Dentoalveolar Augmentation. Materials 2020, 13, 2935. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Szymor, P.; Wach, T. Influence of General Mineral Condition on Collagen-Guided Alveolar Crest Augmentation. Materials 2020, 13, 3649. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, Risk Factors, Pathogenesis, Diagnosis and Classification. In Vivo 2014, 28, 1005–1011. [Google Scholar]
- Panda, S.; Dash, S.; Besra, K.; Samantaray, S.; Pathy, P.C.; Rout, N. Clinicopathological Study of Malignant Melanoma in a Regional Cancer Center. Indian J. Cancer 2018, 55, 292–296. [Google Scholar] [CrossRef]
- Ribero, S.; Stucci, L.S.; Marra, E.; Marconcini, R.; Spagnolo, F.; Orgiano, L.; Picasso, V.; Queirolo, P.; Palmieri, G.; Quaglino, P.; et al. Effect of Age on Melanoma Risk, Prognosis and Treatment Response. Acta Derm. Venereol. 2018, 98, 624–629. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Su, J.; Zheng, X.; Chen, M.; Chen, X.; Li, J.; Peng, C.; Kuang, Y.; Zhu, W. A Clinicopathological Analysis of Melanocytic Nevi: A Retrospective Series. Front. Med. 2021, 8, 681668. [Google Scholar] [CrossRef]
- Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; de Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819. [Google Scholar] [CrossRef]
- Lakhani, N.A.; Saraiya, M.; Thompson, T.D.; King, S.C.; Guy, G.P. Total Body Skin Examination for Skin Cancer Screening among U.S. Adults from 2000 to 2010. Prev. Med. 2014, 61, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Holman, D.M.; Berkowitz, Z.; Guy, G.P.; Hawkins, N.A.; Saraiya, M.; Watson, M. Patterns of Sunscreen Use on the Face and Other Exposed Skin among US Adults. J. Am. Acad. Dermatol. 2015, 73, 83–92.e1. [Google Scholar] [CrossRef] [Green Version]
- Barzegari, M.; Ghaninezhad, H.; Mansoori, P.; Taheri, A.; Naraghi, Z.S.; Asgari, M. Computer-Aided Dermoscopy for Diagnosis of Melanoma. BMC Dermatol. 2005, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Marsden, H.; Jaffe, W.; Matin, R.N.; Wali, G.N.; Greenhalgh, J.; McGrath, E.; James, R.; Ladoyanni, E.; Bewley, A.; et al. Assessment of Accuracy of an Artificial Intelligence Algorithm to Detect Melanoma in Images of Skin Lesions. JAMA Netw. Open 2019, 2, e1913436. [Google Scholar] [CrossRef] [Green Version]
- Oukil, S.; Kasmi, R.; Mokrani, K.; García-Zapirain, B. Automatic Segmentation and Melanoma Detection Based on Color and Texture Features in Dermoscopic Images. Skin Res. Technol. 2021, 28, 203–211. [Google Scholar] [CrossRef]
- Jaworek-Korjakowska, J. Computer-Aided Diagnosis of Micro-Malignant Melanoma Lesions Applying Support Vector Machines. BioMed Res. Int. 2016, 2016, 4381972. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Akram, T.; Sharif, M.; Shahzad, A.; Aurangzeb, K.; Alhussein, M.; Haider, S.I.; Altamrah, A. An Implementation of Normal Distribution Based Segmentation and Entropy Controlled Features Selection for Skin Lesion Detection and Classification. BMC Cancer 2018, 18, 638. [Google Scholar] [CrossRef]
- Erol, R.; Bayraktar, M.; Kockara, S.; Kaya, S.; Halic, T. Texture Based Skin Lesion Abruptness Quantification to Detect Malignancy. BMC Bioinform. 2017, 18, 484. [Google Scholar] [CrossRef]
- Li, F.-W.; Luo, S.-K. Identification and Construction of a Predictive Immune-Related LncRNA Signature Model for Melanoma. Int. J. Gen. Med. 2021, 14, 9227–9235. [Google Scholar] [CrossRef]
- Anderson, R.R. Polarized Light Examination and Photography of the Skin. Arch. Dermatol. 1991, 127, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, K.L. Cross-Polarised and Parallel-Polarised Light: Viewing and Photography for Examination and Documentation of Biological Materials in Medicine and Forensics. J. Vis. Commun. Med. 2018, 41, 3–8. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Cui, K.; Chen, Y.; Sun, F.; Sun, X.; Xing, L. Prognostic Role of Computed Tomography Textural Features In Early-Stage Non-Small Cell Lung Cancer Patients Receiving Stereotactic Body Radiotherapy. Cancer Manag. Res. 2019, 11, 9921–9930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnin, A.; Durot, C.; Barat, M.; Djelouah, M.; Grange, F.; Mulé, S.; Soyer, P.; Hoeffel, C. CT Texture Analysis as a Predictor of Favorable Response to Anti-PD1 Monoclonal Antibodies in Metastatic Skin Melanoma. Diagn. Interv. Imaging 2022, 103, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hadzik, J.; Kubasiewicz-Ross, P.; Simka, W.; Gębarowski, T.; Barg, E.; Cieśla-Niechwiadowicz, A.; Trzcionka Szajna, A.; Szajna, E.; Gedrange, T.; Kozakiewicz, M.; et al. Fractal Dimension and Texture Analysis in the Assessment of Experimental Laser-Induced Periodic Surface Structures (LIPSS) Dental Implant Surface—In Vitro Study Preliminary Report. Materials 2022, 15, 2713. [Google Scholar] [CrossRef] [PubMed]
| Imaging | Lesion | LngREmph | Entropy | DifEntrp | TI | BI |
|---|---|---|---|---|---|---|
| Non-polarized Light | BN | 2.64 ± 0.80 | 2.74 ± 0.14 | 0.98 ± 0.13 | 1.12 ± 0.29 | 0.40 ± 0.13 |
| DN | 2.74 ± 0.71 | 2.77 ± 0.12 | 0.99 ± 0.12 | 1.09 ± 0.31 | 0.39 ± 0.14 | |
| MM | 3.16 ± 2.74 | 2.72 ± 0.15 | 0.96 ± 0.13 | 0.97 ± 0.28 | 0.35 ± 0.12 | |
| Note | p = 0.000006 | p = 0.057 | p = 0.214 | p = 0.00004 | p = 0.0006 | |
| MM > DN | MM < DN | MM < DN | ||||
| MM > BN | MM < BN | MM < BN | ||||
| Polarized Light | BN | 5.28 ± 4.32 | 2.44 ± 0.23 | 0.76 ± 0.13 | 0.67 ± 0.33 | 0.21 ± 0.12 |
| DN | 6.20 ± 3.29 | 2.38 ± 0.20 | 0.71 ± 0.10 | 0.49 ± 0.25 | 0.15 ± 0.09 | |
| MM | 7.84 ± 4.69 | 2.21 ± 0.29 | 0.68 ± 0.10 | 0.39 ± 0.23 | 0.12 ± 0.07 | |
| Note | p = 0.0000001 | p = 0.0000001 | p = 0.0000003 | p = 0.0000001 | p = 0.0000001 | |
| MM > DN > BN | MM < DN < BN | MM < DN < BN | MM < DN < BN | MM < DN < BN |
| Feature | Non-Polarized Light | Statistical Significance | Polarized Light | Statistical Significance | ||
|---|---|---|---|---|---|---|
| In Situ | Invasive | In Situ | Invasive | |||
| LngREmph | 2.66 ± 0.48 | 3.65 ± 2.24 | p = 0.005 | 6.01 ± 0.10 | 8.64 ± 4.54 | p = 0.0000003 |
| Entrory | 2.79 ± 0.09 | 2.67 ± 0.22 | p = 0.002 | 2.37 ± 0.27 | 2.15 ± 0.28 | p = 0.00000002 |
| DifEntrp | 1.00 ± 0.10 | 0.93 ± 0.16 | p = 0.001 | 0.73 ± 0.09 | 0.67 ± 0.10 | p = 0.000001 |
| TI | 1.09 ± 0.21 | 0.91 ± 0.17 | p = 0.004 | 0.55 ± 0.28 | 0.32 ± 0.17 | p = 0.00000008 |
| BI | 0.39 ± 0.10 | 0.32 ± 0.14 | p = 0.007 | 0.17 ± 0.09 | 0.10 ± 0.06 | p = 0.0000006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popecki, P.; Jurczyszyn, K.; Ziętek, M.; Kozakiewicz, M. Texture Analysis in Diagnosing Skin Pigmented Lesions in Normal and Polarized Light—A Preliminary Report. J. Clin. Med. 2022, 11, 2505. https://doi.org/10.3390/jcm11092505
Popecki P, Jurczyszyn K, Ziętek M, Kozakiewicz M. Texture Analysis in Diagnosing Skin Pigmented Lesions in Normal and Polarized Light—A Preliminary Report. Journal of Clinical Medicine. 2022; 11(9):2505. https://doi.org/10.3390/jcm11092505
Chicago/Turabian StylePopecki, Paweł, Kamil Jurczyszyn, Marcin Ziętek, and Marcin Kozakiewicz. 2022. "Texture Analysis in Diagnosing Skin Pigmented Lesions in Normal and Polarized Light—A Preliminary Report" Journal of Clinical Medicine 11, no. 9: 2505. https://doi.org/10.3390/jcm11092505
APA StylePopecki, P., Jurczyszyn, K., Ziętek, M., & Kozakiewicz, M. (2022). Texture Analysis in Diagnosing Skin Pigmented Lesions in Normal and Polarized Light—A Preliminary Report. Journal of Clinical Medicine, 11(9), 2505. https://doi.org/10.3390/jcm11092505








