Abstract
Osteonecrosis (ON) is a serious complication of acute lymphocytic leukaemia (ALL) or lymphoblastic lymphoma (LBL) treatment, and there is little information regarding ON in Korean paediatric and young adult patients. This retrospective cohort study assessed the cumulative incidence of and risk factors for ON using national health insurance claims data from 2008 to 2019 in 4861 ALL/LBL patients. The Kaplan–Meier method was used to estimate the cumulative incidence of ON according to age groups; the Cox proportional hazard regression model was used to identify risk factors related to ON development after diagnosing ALL/LBL. A cause-specific hazard model with time-varying covariates was used to assess the effects of risk factors. Overall, 158 (3.25%) patients were diagnosed with ON, among whom 23 underwent orthopaedic surgeries. Older age, radiotherapy (HR = 2.62, 95% confidence interval (CI) 1.87–3.66), HSCT (HR = 2.40, 95% CI 1.74–3.31), steroid use and anthracycline use (HR = 2.76, CI 1.85–4.14) were related to ON in the univariate analysis. In the multivariate analysis, age and steroid and asparaginase use (HR = 1.99, CI 1.30–3.06) were factors associated with ON. These results suggest that Korean patients with ALL/LBL who used steroids and asparaginase should be closely monitored during follow-up, even among young adult patients.
1. Introduction
Significantly improved survival rates of >90% in children and adolescents with acute lymphoblastic leukaemia (ALL) or lymphoblastic lymphoma (LBL) indicate treatment strategy concerns of long-term side effects of treatments and disease remission [1,2]. Osteonecrosis (ON) is a common and serious side effect that compromises quality of life (QOL) [3,4]. ON incidence varies widely (1.6–17.6%) depending on the study cohort and treatment protocol [5,6,7,8,9,10,11,12,13,14,15]. ON aetiology is multifactorial, with risk factors such as steroid use, sex, body mass index (BMI) [16], genetics [2,7,10,17,18], haematopoietic stem cell transplantation (HSCT) [19] and antileukaemic agents [1,3,11,19,20,21,22,23,24,25,26]. Despite many studies, the results remain debatable [1,21,27], although adolescents aged 10 years and older have the highest risk of developing ON [3,12,22,24,28]. Steroids are essential in the induction phase for ALL treatments; however, high doses of steroids are one of the most important treatment-related risk factors [24,29].
ON treatment slows disease progression and the collapse of major joints, and leads to pain relief and increased range of motion; however, there is no standard treatment for ALL-associated ON [16,29,30,31,32,33]. Some patients complain of symptoms after progression [1,34,35,36]. ON usually occurs in major joints where injury to cartilage is difficult to revitalise [30]. Early diagnosis and prevention of ON are important for preserving QOL after remission in children with malignancies [37]; furthermore, definitive risk factors remain unknown, although some factors such as steroids and antileukaemic agents are critical for survival and remission. There have been prospective randomised control studies and multicentre studies on ON after ALL/LBL treatment [19,26,38,39]; however, there have been no large-scale clinical studies on Korean patients. Because genetic and demographic differences are possible risk factors, we evaluated ON incidence, possible risk factors, and rate of orthopaedic surgery among patients with ALL/LBL based on a national cohort with the Korean National Health Insurance claims database.
2. Materials and Methods
2.1. Data Source
This study was an interdisciplinary (haemato-oncology/orthopaedics/biostatics), nationwide, retrospective, cohort study using the Korean Health Insurance and Review Assessment (HIRA) database. The HIRA database comprises nationwide health insurance claims and covers approximately 98% of the total population; it contains beneficiaries’ general characteristics and also all diagnoses, procedures, treatments and inpatient and outpatient prescriptions [40]. In the HIRA database, all beneficiaries’ and healthcare providers’ identification are encrypted according to the Health Insurance Portability and Accountability Act privacy rules, and diagnoses are coded using the International Classification of Diseases 10th Revision. Both inpatient and outpatient claims from the 2008–2019 database were used. This study was approved by the Care Record Supply Committee of the HIRA (M20210201983) and the Institutional Review Board/Ethics Committee of Severance Hospital, Yonsei University Health System (IRB no. 4-2019-1017), which also waived the need for informed patient consent because of the retrospective study design and use of de-identified data.
2.2. Study Population
This study included individuals aged <40 years diagnosed with ALL/LBL between 2008 and 2019 (n = 8032). Patients with ALL and LBL were defined using ICD-10 codes C91 and C83.5, respectively, with codes for expanding benefit coverage (V-code in Korea). Policies to expand benefit coverage commenced in 2005 and have reduced medical expenses for catastrophic illnesses, including cancer and cardio-cerebrovascular diseases [41]. A 1-year washout period was applied to exclude prevalent cases (n = 2967) (Figure 1). The first ON diagnosis after ALL/LBL diagnosis was the event of interest. ON was defined using ICD-10 code, M87 or M90. Patients with ALL/LBL diagnosed with ON within 30 days after ALL/LBL diagnosis were excluded because they could have ON regardless of ALL/LBL treatment (n = 31). Moreover, we excluded 82 patients with ALL/LBL who did not receive anticancer therapy during the follow-up period. Finally, 4861 patients with ALL/LBL were identified from the database.
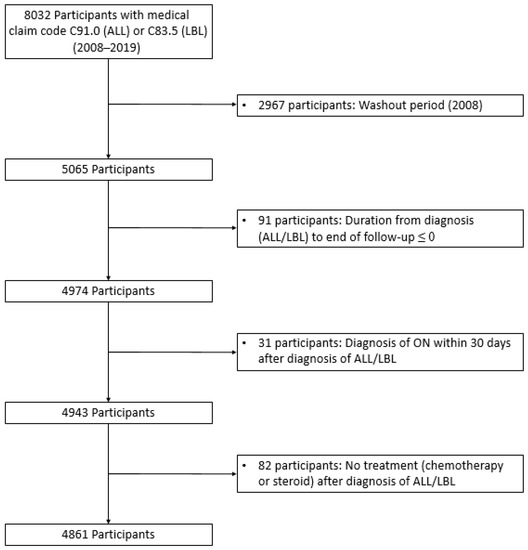
Figure 1.
Participant enrolment flow diagram. Among 8056 participants, 4861 with ALL/LBL were included in the study. A total of 2967 patients were excluded owing to the washout period.
2.3. Possible on Risk Factors
Participants were divided into the paediatric group (<20 years), which was further subdivided into five-year increments and the young adult group (≥20 years). Radiotherapy treatments included total-body and craniospinal irradiation. HSCT included autologous and allogeneic transplants. Steroid usage was analysed to compare different types of steroids used during induction. We reviewed steroid use in the first 60 days after diagnosing ALL/LBL. The dexamethasone group included patients receiving dexamethasone during induction treatment. The prednisone group used prednisone, the dexamethasone + prednisone group used both, and the no steroid group did not use dexamethasone or prednisone during the early treatment phase. L-asparaginase (ASP) is a key drug in the paediatric or paediatric-inspired ALL/LBL regimen; therefore, we identified patients who used L-ASP at least once during the entire treatment period or who never used L-ASP. The use of anthracycline (daunorubicin or doxorubicin) was also analysed during the first 60 days. Anthracycline drugs are usually included in the first-line treatment of adult ALL/LBL and are primarily used in high-risk paediatric ALL during induction.
2.4. Statistical Analysis
Baseline characteristics of the study population are summarised using mean and standard deviation for continuous variables and frequency and percentage for categorical variables. Comparisons between the two groups were conducted using Wilcoxon rank sum test for continuous variables and chi-square tests for categorical variables. Patients with ALL/LBL were followed from the diagnosis date until the date of ON diagnosis (event of interest), death, or end of the study (31 July 2019), whichever came first. The Gray’s method was used to estimate the cumulative incidence (Cuml) of ON according to the age group. A cause-specific hazard regression model was fitted with a Cox proportional hazard regression model to identify risk factors that could affect ON development after ALL diagnosis. Age at ALL/LBL diagnosis, sex, radiotherapy, HSCT, steroid use, ASP use and anthracycline use were considered possible risk factors. Radiotherapy and HSCT were considered time-varying risk factors. Univariate and multivariate analyses were performed to assess the effect of a risk factor without and with adjusting for the effects of other risk factors. The results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical significance was defined as two-sided p-values < 0.05. SAS Enterprise Guide version 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses.
3. Results
3.1. Participants’ Characteristics
Among 4861 participants diagnosed with ALL/LBL, 3344 (68.8%) were aged <20 years. The mean age of all participants was 14.9 years; the paediatric group, 8.3 years, and the young adult group, 29.3 years. Male subjects were more common (2885, 58.4%) in all age groups. Radiotherapy was administered to 995 (20.5%) patients and was the most common among adults (34.3%). HSCT was administered to 54.9% of patients aged >20 years compared with 16.8% in the paediatric group. Prednisone was the most frequently prescribed steroid in the paediatric group (49.8%) during the first 60 days after diagnosis, whereas dexamethasone was predominant in the adult group (37.4%). For patients aged <20 years, prednisone + dexamethasone was used in 23.4%. Neither prednisone nor dexamethasone was used for 10.6% of patients aged <20 years and 12.4% of adults. L-ASP was used in 81.8% of patients aged <20 years and 40.5% of adults. Daunorubicin or doxorubicin was administered to 3149 (64.8%) participants in the early phase of ALL/LBL treatment and was more frequently used in the paediatric group than in the young adult group (p < 0.01). The paediatric group used anthracycline more frequently than the young adult group (1940 vs. 308, p < 0.01) (Table 1).

Table 1.
Demographic characteristics of all the participants.
3.2. Incidence of ON
ON was diagnosed in 158 (3.25%) of 4861 patients with ALL/LBL, with 93 aged <20 years, i.e., 58.9% of all cases, and 65 patients aged ≥ 20 years. The mean age at ALL/LBL diagnosis was 19.41 years for patients who developed ON and 14.73 years for those who did not. In the paediatric group, the mean age at ALL/LBL diagnosis was 13.28 years for patients who developed ON and 8.20 years for those without ON (Figure 2). ON occurred more frequently in the young adult group than in the paediatric group (4.28% vs. 2.78%). However, the Cuml of ON at 10 years was the highest in patients aged 10–20 years (7.34%), especially in the age range of 15–20 years (8.38%) (Figure 3). The mean age at ON diagnosis was 22.0 ± 8.81 years, which is 2.54 ± 1.74 years after ALL/LBL diagnosis in all patients. Time from cancer treatment to ON diagnosis was similar in the paediatric (2.69 ± 1.77 years) and adult groups (2.33 ± 1.68 years) (p = 0.20). Approximately 90% of patients with ON were diagnosed within 5 years of the primary cancer (Figure 4).
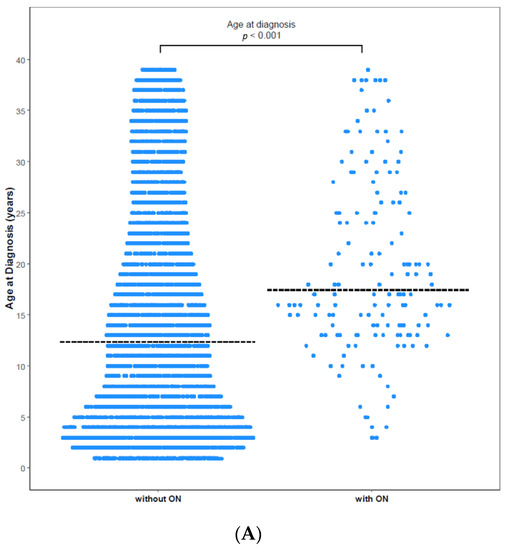
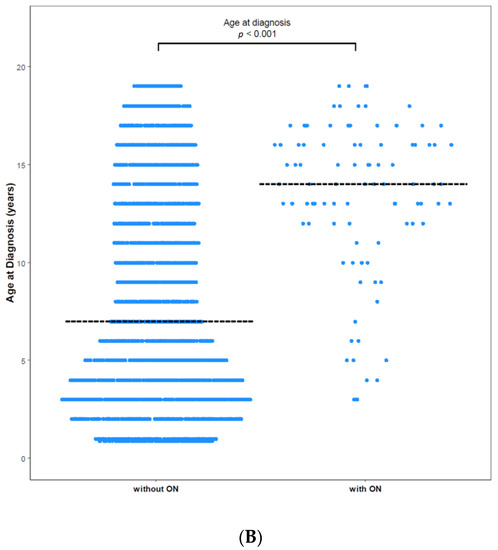
Figure 2.
Age at diagnosis with and without ON among (A) total patients and (B) paediatric patients. (A) The mean age of patients without ON was 14.73 years, and that of patients with ON was 19.41 years (p < 0.0001). (B) The mean age of paediatric patients without ON was 8.20 years, and that of paediatric patients with ON was 13.28 years (p < 0.0001). Dashed horizontal lines reflect the median age at diagnosis of two groups.
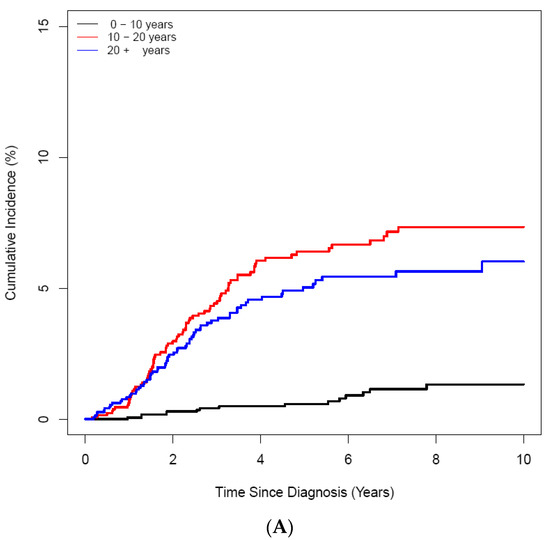
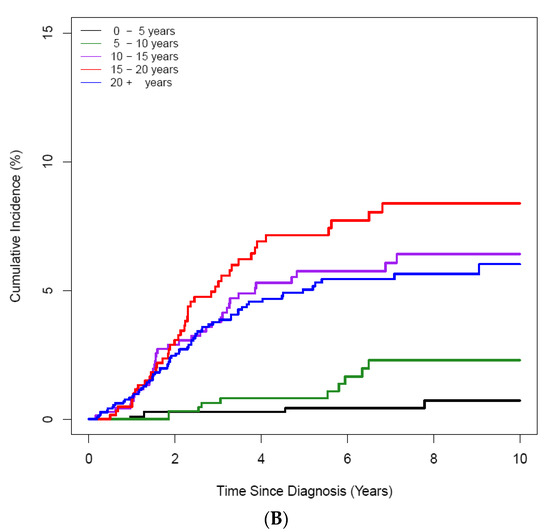
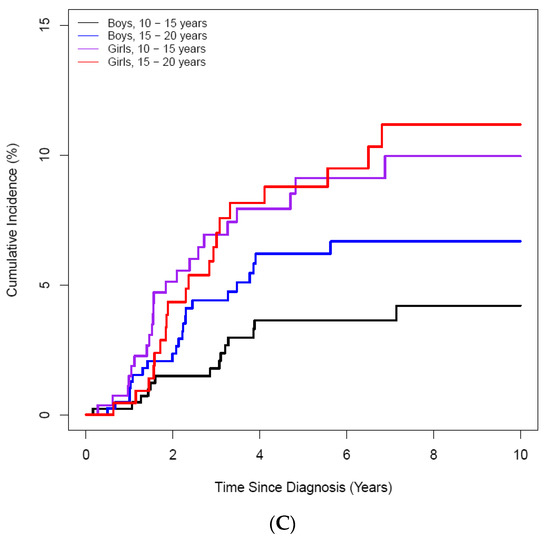
Figure 3.
Cumulative incidence of ON according to age at the diagnosis of ALL/LBL in 4861 patients. (A) A 10-year scale, (B) 5-year scale (C) by age and sex. (A) During 10–20-years, the highest CI was noted, and during 15–20 years, patients had the highest CI (B). Girls showed higher CI than boys (C).
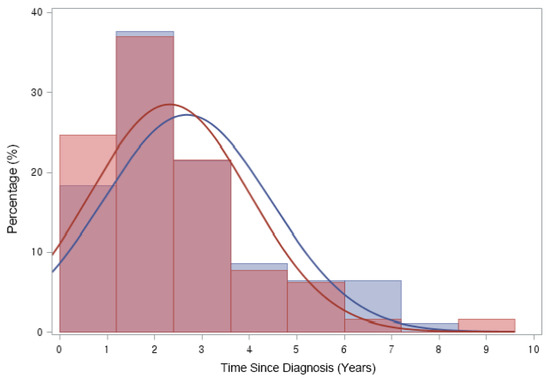
Figure 4.
Time from ALL/LBL diagnosis to ON diagnosis. Time from cancer treatment to ON diagnosis was 2.54 (±1.74) years after ALL or LBL diagnosis in all patients. Approximately 90% of patients were diagnosed as having ON in 5 years after primary cancer. (Blue line: Paediatric group (2.69 ± 1.77 years), Red line: Adult group (2.33 ± 1.68 years)).
3.3. Risk Factors for ON
Age, radiotherapy, HSCT and anthracycline use in the first 60 days after ALL/LBL diagnosis were associated with ON among all patients with ALL/LBL (Table 2). This was evident among paediatric patients, however, these factors were not statistically significantly related to the occurrence of ON in the young adult group (Supplementary Table S1). In the cause-specific regression model, age, radiotherapy, HSCT, steroid use and anthracycline use were significantly associated with ON risk among all patients with ALL/LBL in the univariate analyses. In the multivariate analysis, older age, steroid use and history of L-ASP use were statistically significant risk factors for ON development. Male patients had a 26% lower risk for ON than female patients, although it was not significant (HR = 0.74, 95% CI 0.54–1.01, p = 0.06), this effect was significant in paediatric patients (p < 0.001) (Table 3).

Table 2.
Risk factors of osteonecrosis.

Table 3.
Univariate and multivariate analysis associated with AVN.
In the regression analysis with cause-specific HRs and time-varying covariates, older age, radiotherapy, transplantation, steroid use and anthracycline use were related to ON diagnosis in the univariate analysis. In the multivariate analysis, age, steroid use and history of L-ASP use were factors associated with ON. Males showed lower rates of ON than females, and radiotherapy and transplantation were not associated with ON in the multivariate analysis. Analysis of the paediatric age group showed similar results. Age, steroid use and L-ASP use were related to ON in the multivariate analysis, whereas in the adult group (20–39 years), age, sex, radiotherapy and transplantation were not associated with ON in the univariate analysis (Table 3). No risk factors were statistically significant in the development of ON in young adult ALL/LBL patients from multivariate analysis (Supplementary Table S2).
3.4. Surgical Treatment of ON
Among 158 patients with ON, 23 (14.5%) underwent orthopaedic surgery; 21 patients had ON at the hip joint and 1 knee and 1 shoulder. Among these, most (87%, 20/23) underwent total hip arthroplasty (THR). Three patients underwent arthroscopic debridement: one hip, one knee and one shoulder. The mean age of patients who underwent THR was 28.1 years, with two aged <20 years. An MRI was performed for 48 patients (30.4%, 48/158), and eight patients received surgical treatment (34.8%, 8/23).
4. Discussion
To our knowledge, this is the first study to present the Cuml and risk factors for ON after ALL/LBL treatment in a national cohort comprising Koreans aged <39 years with several important findings.
First, ON was associated with older age in the paediatric group, and young adults (20–39 years) had a higher ON incidence (4.28%) than paediatric patients (2.78%). ON is a critical complication for the QOL of young adults after antileukaemic therapy; however, relatively few studies have been conducted in these age groups [42]. Our study, like others, showed that Cuml is the highest for patients aged 15–20 years, but the very low incidence in those aged <10 years resulted in a lower Cuml in the paediatric group overall than in the young adult group. These results are inconsistent with those of previous studies [43,44,45]. As increasing numbers of young adults receive a paediatric protocol rather than adult protocols with a concomitant higher dose of steroids, our study showed a high incidence in the young adult group [39,46]. Heneghan et al. reported 244/10,729 (2.33%) patients with ALL, which is consistent with our study [47]. Although paediatric-inspired protocol use in adults is increasing, the use of dexamethasone in adults remains higher than that in the paediatric group (37.38% vs. 16.27%, Table 1), which could be related to the relatively high incidence in adults. Second, sex is considered an important risk factor, but there are inconsistent reports [43,44,45]. In our study, female was a risk factor only in the multivariate analysis in the paediatric age group (male, HR = 0.58, p < 0.01). There remains controversy regarding females, and the literature describes that an increase in oestrogen levels in females could have procoagulant effects, but our results suggest that differences in puberty between females and males may contribute to ON development [3,27,48]. Third, the median time to develop ON was 2.54 years, with no difference between the paediatric and young adult groups (2.69 vs. 2.33 years, p = 0.20). This duration was longer than that reported in previous studies [35,45]. Patients in our study could be diagnosed based on progressive symptoms rather than anticipative evaluation or because only clinically symptomatic ON was recorded. Fourth, our study showed that radiotherapy and HSCT were risk factors in the univariate analysis. The subset of patients receiving these treatments is supposed to be exposed to total-body radiation and considerably higher doses of corticosteroids to prevent transplant rejection and combat graft-versus-host disease [8,12,21,49]. Besides these direct risks, patients who require radiotherapy or HSCT generally have severe ALL/LBL. Therefore, patients with ALL/LBL undergoing radiotherapy and HSCT should be closely monitored during follow-up. Finally, besides age and steroid use, L-ASP was a risk factor in the multivariate analysis (HR = 1.99 in all age groups and HR = 2.55 in the paediatric group), but not in the young adult group (Supplementary Table S2). Increasing evidence suggests that L-ASP is associated with an increased ON risk through indirect effects that influence glucocorticoid pharmacokinetics and cause hyperlipidaemia [7,26,33,50].
Previous studies have attempted to define factors that predispose patients to ON. In our multivariate analysis, age, steroid use and L-ASP were significant risk factors (p < 0.05). In our study and other studies, age is the most consistent and significant risk factor, with patients aged ≥ 10 years at the highest risk across treatment regimens and study groups in paediatric age [8,9,10,13,51]. Hormonal changes unique to adolescents may lead to increased ON susceptibility through different mechanisms, including increased local metabolic/perfusion requirements, skeletal maturation, coagulation system, or osseous blood vessel supply [27]. Corticosteroids are key for treating ALL/LBL and can cause adverse effects, including infection, hypertension, diabetes, neuropsychological disorders, and ON [52]. Larsen et al. reported that dexamethasone administered during induction benefited younger children but provided no benefit and was associated with higher ON risk among participants aged ≥ 10 years [24]. Both the steroid type and how steroids are used have influenced ON development [11,53]. In our study, the use of dexamethasone in the paediatric group was lower than that in the prednisone group; however, dexamethasone remains a risk factor for ON development. Similar to steroids, L-Asp is an important drug for treating ALL/LBL, and the intensive use of L-Asp leads to decreased dexamethasone clearance [54]. Steroids and L-ASP are key drugs in paediatric-inspired treatment protocols; therefore, further standard protocols should be considered to suggest the best combination to achieve complete remission of ALL/LBL and prevent ON. Various genetic risk factors for ON development and steroid-induced ON have been identified in large genome-wide studies over decades [7,10,18,55]. Our study is based on claims data; therefore, access to genetic information was unavailable. However, this is the first study related to the treatment of ALL/LBL in a nationwide Korean cohort. Considering the association of genetics and ethnicity with ON, this study will serve as an important basis for further evaluating ON management and prevention in paediatric and young adult patients with ALL/LBL in Koreans.
Our study had some limitations. The cumulative dose of steroids was an important risk factor in previous studies [7,22,24,27,52]; however, it was not available in our national claims data. Methotrexate is also a critical component of ALL/LBL therapy [24]; however, it was not evaluated in our study because of the lack of BMI to determine the total dose. We included ON based on diagnostic codes, but there was no information about its severity based on clinical symptoms, X-ray or MRI. There is no consistent treatment regimen in our study as it was based on national data, including all institutions in Korea. There was no information of ON patients without surgical treatment. Follow-up loss, medical treatment and improvement without treatment are all possible in these patients. Notably, voids in clinical and biological information are imposed by insurance-based data collection, making it impossible to generate risk estimates for ALL/LBL. However, most treatments of ALL/LBL are covered by national insurance; thus, it is valuable to estimate the incidence, clinical characteristics and risk factors in Korean children and young adult patients with ALL/LBL.
Despite its limitations, as this study was based on data from all paediatric, adolescent and young adult patients registered as ALL or LBL in Korea, it is expected to provide important insights into the consideration of ON after treatment of these patients.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/jcm11092489/s1, Table S1: Risk factors of avascular necrosis (young adult group); Table S2: Univariate and multivariate analysis associated with AVN (young adult group).
Author Contributions
Conceptualization, Y.H.K. and I.J.; methodology, M.L.; software, M.L.; validation, S.M.H., M.L. and M.K.; formal analysis, M.L.; investigation, M.L. and E.H.K.; resources, M.L.; data curation, E.H.K. and Y.G.; writing—original draft preparation, S.M.H. and M.L.; writing—review and editing, A.M.A., Y.H.K. and I.J.; visualisation, Y.G.; supervision, A.H.; project administration, Y.H.K.; funding acquisition, Y.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from Yonsei University Medical College (No. 6-2019-0086) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A3A110335 and 2020R1F1A1075564).
Institutional Review Board Statement
This study was approved by the Care Record Supply Committee of the HIRA (M20210201983) and the Institutional Review Board/Ethics Committee of Severance Hospital, Yonsei University Health System (IRB no. 4-2019-1017), which also waived the need for informed patient consent because of the retrospective study design and use of de-identified data.
Informed Consent Statement
Patient consent was waived due to nationwide epidemiological study.
Data Availability Statement
The data were provided in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krull, K.; Kunstreich, M.; Bronsema, A.; Bleckmann, K.; Classen, C.F.; Erdlenbruch, B.; Jorch, N.; Kolb, R.; Leipold, A.; Moser, O.; et al. Osteonecrosis in children with acute lymphoblastic leukemia at initial diagnosis and prior to any chemotherapy. Leuk. Lymphoma 2019, 60, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef]
- Biddeci, G.; Bosco, G.; Varotto, E.; Corradin, M.; Geranio, G.; Tridello, G.; Pillon, M.; Carraro, E.; Garetto, G.; Assadi, R.; et al. Osteonecrosis in Children and Adolescents with Acute Lymphoblastic Leukemia: Early Diagnosis and New Treatment Strategies. Anticancer Res. 2019, 39, 1259–1266. [Google Scholar] [CrossRef]
- Girard, P.; Auquier, P.; Barlogis, V.; Contet, A.; Poiree, M.; Demeocq, F.; Berbis, J.; Herrmann, I.; Villes, V.; Sirvent, N.; et al. Symptomatic osteonecrosis in childhood leukemia survivors: Prevalence, risk factors and impact on quality of life in adulthood. Haematologica 2013, 98, 1089–1097. [Google Scholar] [CrossRef]
- Bond, J.; Adams, S.; Richards, S.; Vora, A.; Mitchell, C.; Goulden, N. Polymorphism in the PAI-1 (SERPINE1) gene and the risk of osteonecrosis in children with acute lymphoblastic leukemia. Blood 2011, 118, 2632–2633. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.S.; Dinu, I.; Wasilewski-Masker, K.; Kaste, S.; Meacham, L.R.; Mahajan, A.; Stovall, M.; Yasui, Y.; Robison, L.L.; Sklar, C.A. Osteonecrosis in adult survivors of childhood cancer: A report from the childhood cancer survivor study. J. Clin. Oncol. 2008, 26, 3038–3045. [Google Scholar] [CrossRef] [PubMed]
- Kawedia, J.D.; Kaste, S.C.; Pei, D.; Panetta, J.C.; Cai, X.; Cheng, C.; Neale, G.; Howard, S.C.; Evans, W.E.; Pui, C.H.; et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood 2011, 117, 2340–2347; quiz 2556. [Google Scholar] [CrossRef]
- Mattano, L.A., Jr.; Sather, H.N.; Trigg, M.E.; Nachman, J.B. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: A report from the Children’s Cancer Group. J. Clin. Oncol. 2000, 18, 3262–3272. [Google Scholar] [CrossRef]
- Niinimäki, R.A.; Harila-Saari, A.H.; Jartti, A.E.; Seuri, R.M.; Riikonen, P.V.; Pääkkö, E.L.; Möttönen, M.I.; Lanning, M. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J. Clin. Oncol. 2007, 25, 1498–1504. [Google Scholar] [CrossRef]
- Relling, M.V.; Yang, W.; Das, S.; Cook, E.H.; Rosner, G.L.; Neel, M.; Howard, S.; Ribeiro, R.; Sandlund, J.T.; Pui, C.H.; et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J. Clin. Oncol. 2004, 22, 3930–3936. [Google Scholar] [CrossRef]
- Mattano, L.A., Jr.; Devidas, M.; Nachman, J.B.; Sather, H.N.; Hunger, S.P.; Steinherz, P.G.; Gaynon, P.S.; Seibel, N.L. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: Results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012, 13, 906–915. [Google Scholar] [CrossRef]
- Padhye, B.; Dalla-Pozza, L.; Little, D.; Munns, C. Incidence and outcome of osteonecrosis in children and adolescents after intensive therapy for acute lymphoblastic leukemia (ALL). Cancer Med. 2016, 5, 960–967. [Google Scholar] [CrossRef] [PubMed]
- te Winkel, M.L.; Pieters, R.; Hop, W.C.; de Groot-Kruseman, H.A.; Lequin, M.H.; van der Sluis, I.M.; Bökkerink, J.P.; Leeuw, J.A.; Bruin, M.C.; Egeler, R.M.; et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. J. Clin. Oncol. 2011, 29, 4143–4150. [Google Scholar] [CrossRef]
- Kuhlen, M.; Moldovan, A.; Krull, K.; Meisel, R.; Borkhardt, A. Osteonecrosis in paediatric patients with acute lymphoblastic leukaemia treated on Co-ALL-07-03 trial: A single centre analysis. Klin. Padiatr. 2014, 226, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yang, S.; Rochester, R.; Britton, L.; Leung, W.H.; Yang, J.; Neel, M.D.; Ness, K.K.; Kaste, S.C. Prevalence of osteonecrosis and associated risk factors in children before allogeneic BMT. Bone Marrow Transplant. 2011, 46, 813–819. [Google Scholar] [CrossRef]
- Vora, A. Management of osteonecrosis in children and young adults with acute lymphoblastic leukaemia. Br. J. Haematol. 2011, 155, 549–560. [Google Scholar] [CrossRef]
- Karol, S.E.; Yang, W.; Van Driest, S.L.; Chang, T.Y.; Kaste, S.; Bowton, E.; Basford, M.; Bastarache, L.; Roden, D.M.; Denny, J.C.; et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood 2015, 126, 1770–1776. [Google Scholar] [CrossRef]
- Karol, S.E.; Mattano, L.A., Jr.; Yang, W.; Maloney, K.W.; Smith, C.; Liu, C.; Ramsey, L.B.; Fernandez, C.A.; Chang, T.Y.; Neale, G.; et al. Genetic risk factors for the development of osteonecrosis in children under age 10 treated for acute lymphoblastic leukemia. Blood 2016, 127, 558–564. [Google Scholar] [CrossRef]
- Kuhlen, M.; Bader, P.; Sauer, M.; Albert, M.H.; Gruhn, B.; Güngör, T.; Kropshofer, G.; Lang, P.; Lawitschka, A.; Metzler, M.; et al. Low incidence of symptomatic osteonecrosis after allogeneic HSCT in children with high-risk or relapsed ALL—Results of the ALL-SCT 2003 trial. Br. J. Haematol. 2018, 183, 104–109. [Google Scholar] [CrossRef]
- Mogensen, P.R.; Grell, K.; Schmiegelow, K.; Overgaard, U.M.; Wolthers, B.O.; Mogensen, S.S.; Vaag, A.; Frandsen, T.L. Dyslipidemia at diagnosis of childhood acute lymphoblastic leukemia. PLoS ONE 2020, 15, e0231209. [Google Scholar] [CrossRef]
- Tanoshima, R.; Carleton, B.C. The incidence of symptomatic osteonecrosis after allogeneic haematopoietic stem cell transplantation in children with acute lymphoblastic leukaemia—Controversy on dexamethasone as a risk factor. Br. J. Haematol. 2019, 185, 958–959. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Gohar, S.; Zaky, I.; Elghoneimy, A.; Youssef, S.; Sameer, G.; Yassin, D.; Salem, S.; Magdi, H.; Sidhom, I. Osteonecrosis in children with acute lymphoblastic leukemia: A report from Children’s Cancer Hospital Egypt (CCHE). Pediatr. Blood Cancer 2019, 66, e27440. [Google Scholar] [CrossRef]
- van den Heuvel-Eibrink, M.M.; Pieters, R. Steroids and risk of osteonecrosis in ALL: Take a break. Lancet Oncol. 2012, 13, 855–857. [Google Scholar] [CrossRef]
- Larsen, E.C.; Devidas, M.; Chen, S.; Salzer, W.L.; Raetz, E.A.; Loh, M.L.; Mattano, L.A., Jr.; Cole, C.; Eicher, A.; Haugan, M.; et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J. Clin. Oncol. 2016, 34, 2380–2388. [Google Scholar] [CrossRef]
- Chen, S.H.; Chang, T.Y.; Jaing, T.H.; Lee, M.S.; Wang, C.J.; Hung, I.J.; Yang, C.P. Incidence, risk factors, and treatment outcome of symptomatic osteonecrosis in Taiwanese children with acute lymphoblastic leukemia: A retrospective cohort study of 245 patients in a single institution. Int. J. Hematol. 2015, 102, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, B.K.; Grell, K.; Abrahamsson, J.; Lund, B.; Vettenranta, K.; Jónsson, Ó.G.; Frandsen, T.L.; Wolthers, B.O.; Heyman, M.; Schmiegelow, K. Intermittent Versus Continuous PEG-Asparaginase to Reduce Asparaginase-Associated Toxicities: A NOPHO ALL2008 Randomized Study. J. Clin. Oncol. 2019, 37, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Kunstreich, M.; Kummer, S.; Laws, H.J.; Borkhardt, A.; Kuhlen, M. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica 2016, 101, 1295–1305. [Google Scholar] [CrossRef]
- Salem, K.H.; Brockert, A.K.; Mertens, R.; Drescher, W. Avascular necrosis after chemotherapy for haematological malignancy in childhood. Bone Jt. J. 2013, 95-B, 1708–1713. [Google Scholar] [CrossRef]
- Kuhlen, M.; Kunstreich, M.; Krull, K.; Meisel, R.; Borkhardt, A. Osteonecrosis in children and adolescents with acute lymphoblastic leukemia: A therapeutic challenge. Blood Adv. 2017, 1, 981–994. [Google Scholar] [CrossRef]
- Karimova, E.J.; Rai, S.N.; Howard, S.C.; Neel, M.; Britton, L.; Pui, C.H.; Kaste, S.C. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J. Clin. Oncol. 2007, 25, 1525–1531. [Google Scholar] [CrossRef]
- Te Winkel, M.L.; Pieters, R.; Wind, E.J.; Bessems, J.H.; van den Heuvel-Eibrink, M.M. Management and treatment of osteonecrosis in children and adolescents with acute lymphoblastic leukemia. Haematologica 2014, 99, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.M.; Lee, A.R.; Wong, S.C. Systematic review of the effects of bisphosphonates on bone density and fracture incidence in childhood acute lymphoblastic leukaemia. Osteoporos. Int. 2020, 31, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, S.S.; Harila-Saari, A.; Frandsen, T.L.; Lähteenmäki, P.; Castor, A.; Kohonen, I.; Schmiegelow, K.; Mäkitie, O. Early presentation of osteonecrosis in acute lymphoblastic leukemia: Two children from the Nordic and Baltic cohort. Pediatr. Blood Cancer 2017, 64, e26624. [Google Scholar] [CrossRef]
- Kaste, S.C.; Karimova, E.J.; Neel, M.D. Osteonecrosis in children after therapy for malignancy. AJR Am. J. Roentgenol. 2011, 196, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Gray, J.; Harvey, N.; Davies, J.H.; Oreffo, R.O.C.; Reading, I.; Clarke, N.M.P.; Aarvold, A. Osteonecrosis following treatment for childhood acute lymphoblastic leukaemia: The Southampton Children’s Hospital experience. J. Child. Orthop. 2017, 11, 440–447. [Google Scholar] [CrossRef]
- Shahnazi, M.; Khatami, A.; Shamsian, B.; Haerizadeh, B.; Mehrafarin, M. Bony lesions in pediatric acute leukemia: Pictorial essay. Iran. J. Radiol. 2012, 9, 50–56. [Google Scholar] [CrossRef]
- Littooij, A.S.; Kwee, T.C.; Enríquez, G.; Verbeke, J.I.; Granata, C.; Beishuizen, A.; de Lange, C.; Zennaro, F.; Bruin, M.C.; Nievelstein, R.A. Whole-body MRI reveals high incidence of osteonecrosis in children treated for Hodgkin lymphoma. Br. J. Haematol. 2017, 176, 637–642. [Google Scholar] [CrossRef]
- Amin, N.; Kinsey, S.; Feltbower, R.; Kraft, J.; Whitehead, E.; Velangi, M.; James, B. British OsteoNEcrosis Study (BONES) protocol: A prospective cohort study to examine the natural history of osteonecrosis in older children, teenagers and young adults with acute lymphoblastic leukaemia and lymphoblastic lymphoma. BMJ Open 2019, 9, e027204. [Google Scholar] [CrossRef]
- Toft, N.; Birgens, H.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Heyman, M.; Klausen, T.W.; Jónsson, Ó.G.; Palk, K.; Pruunsild, K.; et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia 2018, 32, 606–615. [Google Scholar] [CrossRef]
- Kim, J.A.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S. The effect of extension of benefit coverage for cancer patients on health care utilization across different income groups in South Korea. Int. J. Health Care Financ. Econ. 2014, 14, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, M.; Kunstreich, M.; Gokbuget, N. Osteonecrosis in Adults with Acute Lymphoblastic Leukemia: An Unmet Clinical Need. Hemasphere 2021, 5, e544. [Google Scholar] [CrossRef] [PubMed]
- Valtis, Y.K.; Stevenson, K.E.; Place, A.E.; Silverman, L.B.; Vrooman, L.M.; Gotti, G.; Brunner, A.M.; Nauffel, M.; DeAngelo, D.J.; Luskin, M.R. Orthopedic Toxicity Among Adolescents and Young Adults Treated on DFCI ALL Consortium Trials. Blood 2020, 136, 31–32. [Google Scholar] [CrossRef]
- Patel, B.; Richards, S.M.; Rowe, J.M.; Goldstone, A.H.; Fielding, A.K. High incidence of avascular necrosis in adolescents with acute lymphoblastic leukaemia: A UKALL XII analysis. Leukemia 2008, 22, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, S.S.; Harila-Saari, A.; Makitie, O.; Myrberg, I.H.; Niinimaki, R.; Vestli, A.; Hafsteinsdottir, S.; Griskevicius, L.; Saks, K.; Hallbook, H.; et al. Comparing osteonecrosis clinical phenotype, timing, and risk factors in children and young adults treated for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2018, 65, e27300. [Google Scholar] [CrossRef] [PubMed]
- McNeer, J.L.; Bleyer, A. Acute lymphoblastic leukemia and lymphoblastic lymphoma in adolescents and young adults. Pediatr. Blood Cancer 2018, 65, e26989. [Google Scholar] [CrossRef]
- Heneghan, M.B.; Rheingold, S.R.; Li, Y.; Seif, A.E.; Huang, Y.S.; McLeod, L.; Wells, L.; Fisher, B.T.; Aplenc, R. Treatment of Osteonecrosis in Children and Adolescents with Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2016, 16, 223–229.e222. [Google Scholar] [CrossRef]
- te Winkel, M.L.; Appel, I.M.; Pieters, R.; van den Heuvel-Eibrink, M.M. Impaired dexamethasone-related increase of anticoagulants is associated with the development of osteonecrosis in childhood acute lymphoblastic leukemia. Haematologica 2008, 93, 1570–1574. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Brazauskas, R.; Wang, Z.; Al-Seraihy, A.; Baker, K.S.; Cahn, J.Y.; Frangoul, H.A.; Gajewski, J.L.; Hale, G.A.; Hsu, J.W.; et al. Avascular necrosis of bone after allogeneic hematopoietic cell transplantation in children and adolescents. Biol. Blood Marrow Transplant. 2014, 20, 587–592. [Google Scholar] [CrossRef]
- Finch, E.R.; Smith, C.A.; Yang, W.; Liu, Y.; Kornegay, N.M.; Panetta, J.C.; Crews, K.R.; Molinelli, A.R.; Cheng, C.; Pei, D.; et al. Asparaginase formulation impacts hypertriglyceridemia during therapy for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2020, 67, e28040. [Google Scholar] [CrossRef]
- Lackner, H.; Benesch, M.; Moser, A.; Smolle-Jüttner, F.; Linhart, W.; Raith, J.; Urban, C. Aseptic osteonecrosis in children and adolescents treated for hemato-oncologic diseases: A 13-year longitudinal observational study. J. Pediatr. Hematol. Oncol. 2005, 27, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Imamura, T.; Kihira, K.; Suzuki, K.; Ishida, H.; Morita, H.; Kanno, M.; Mori, T.; Hiramatsu, H.; Matsubara, K.; et al. Low Incidence of Osteonecrosis in Childhood Acute Lymphoblastic Leukemia Treated with ALL-97 and ALL-02 Study of Japan Association of Childhood Leukemia Study Group. J. Clin. Oncol. 2018, 36, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Warris, L.T.; van den Heuvel-Eibrink, M.M.; Aarsen, F.K.; Pluijm, S.M.; Bierings, M.B.; van den Bos, C.; Zwaan, C.M.; Thygesen, H.H.; Tissing, W.J.; Veening, M.A.; et al. Hydrocortisone as an Intervention for Dexamethasone-Induced Adverse Effects in Pediatric Patients with Acute Lymphoblastic Leukemia: Results of a Double-Blind, Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Janke, L.J.; Kawedia, J.D.; Ramsey, L.B.; Cai, X.; Mattano, L.A., Jr.; Boyd, K.L.; Funk, A.J.; Relling, M.V. Asparaginase Potentiates Glucocorticoid-Induced Osteonecrosis in a Mouse Model. PLoS ONE 2016, 11, e0151433. [Google Scholar] [CrossRef]
- Asano, T.; Takahashi, K.A.; Fujioka, M.; Inoue, S.; Satomi, Y.; Nishino, H.; Tanaka, T.; Hirota, Y.; Takaoka, K.; Nakajima, S.; et al. Genetic analysis of steroid-induced osteonecrosis of the femoral head. J. Orthop. Sci. 2003, 8, 329–333. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).