The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children
Abstract
1. Introduction
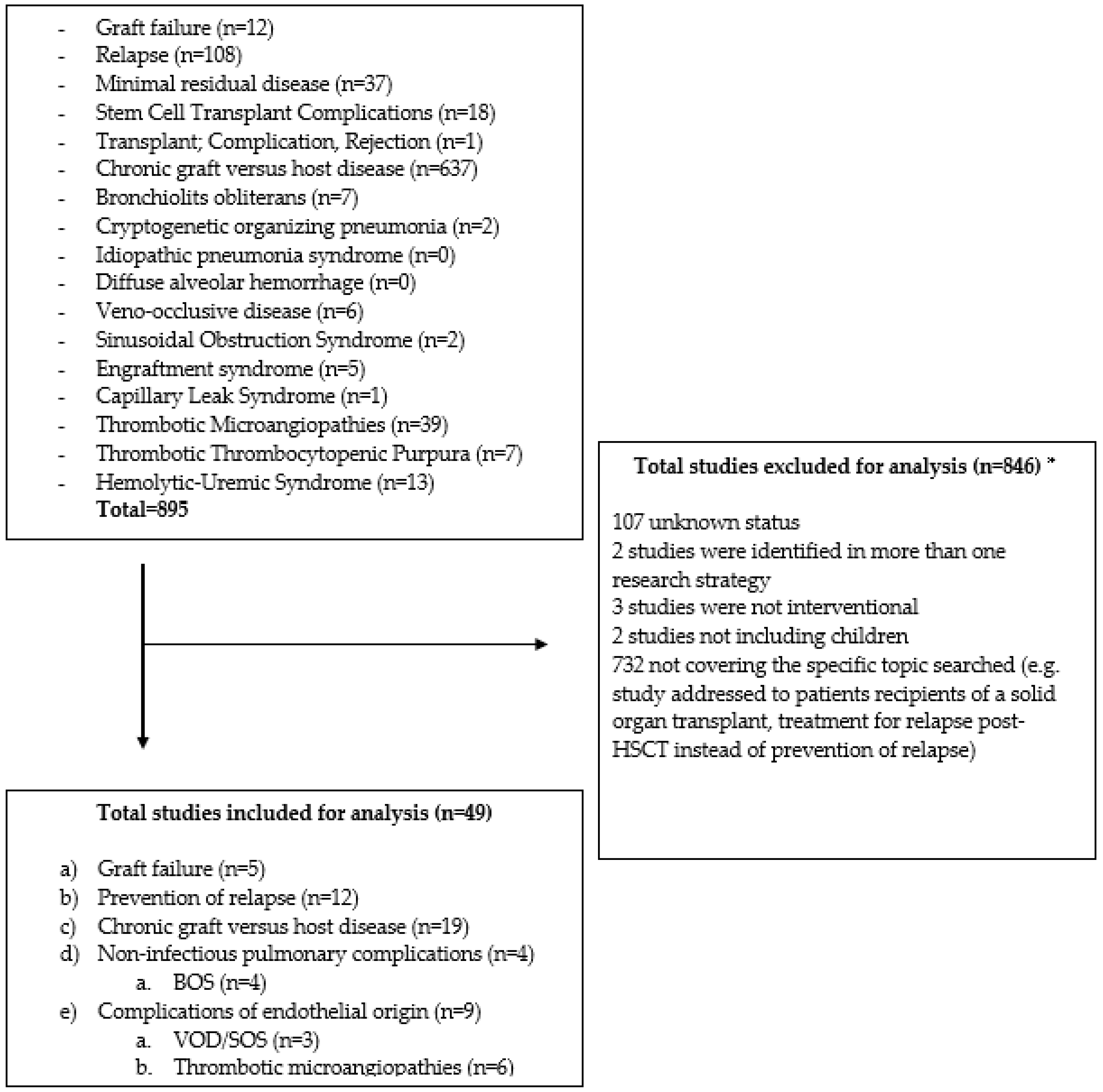
2. Materials and Methods
3. Results
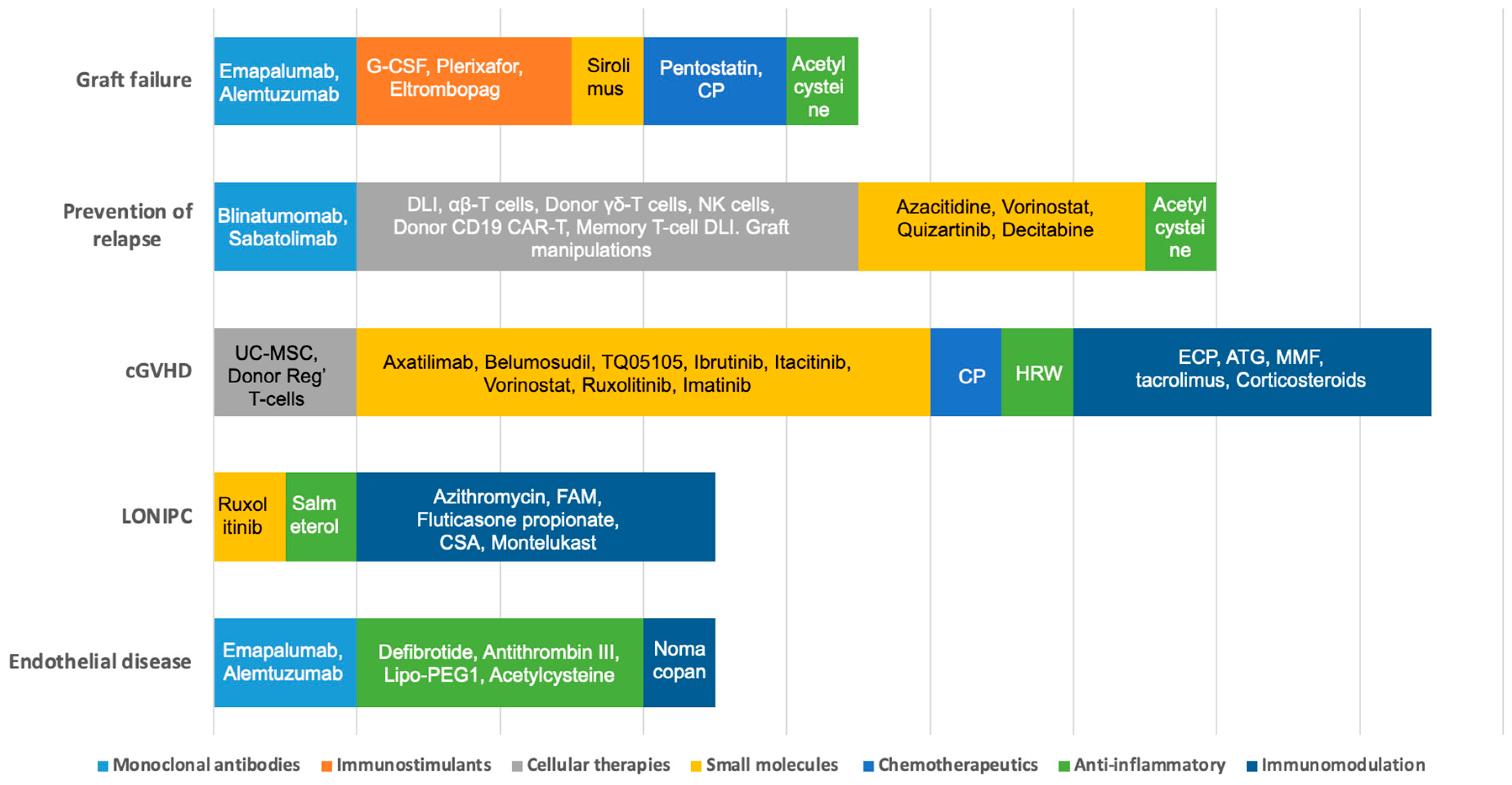
3.1. Graft Failure
3.2. Prevention of Relapse
3.2.1. Cellular Therapies
3.2.2. Hypomethylating Agents (HMA)
3.2.3. Targeted Therapy
Tyrosine Kinase Inhibition
Monoclonal Antibodies/Bispecific T-Cell Engagers
3.3. Chronic Graft vs. Host Disease (cGVHD)
3.3.1. Cellular Therapies
3.3.2. Targeted Therapies
3.3.3. Chemotherapeutics
3.3.4. Other Therapies
3.4. Non-Infectious Pulmonary Complications
3.4.1. Bronchiolitis Obliterans Syndrome (BOS)
3.4.2. Cryptogenetic Organizing Pneumonia (COP)
3.4.3. Idiopathic Pneumonia Syndrome (IPS)
3.4.4. Diffuse Alveolar Hemorrhage (DAH)
3.5. Complications of Endothelial Origin
3.5.1. Veno-Occlusive Disease (VOD) or Sinusoidal Obstruction Syndrome (SOS)
3.5.2. Engraftment Syndrome (ES)
3.5.3. Capillary Leak Syndrome (CLS)
3.5.4. Transplant-Associated Thrombotic Microangiopathies (TA-TMAs)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valcarcel, D.; Sureda, A. Graft Failure. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Carreras, E., Dufour, C., Mohty, M., Kroger, N., Eds.; Springer: Cham, Switzerland, 2019; pp. 307–313. [Google Scholar] [CrossRef]
- Olsson, R.F.; Logan, B.R.; Chaudhury, S.; Zhu, X.; Akpek, G.; Bolwell, B.J.; Bredeson, C.N.; Dvorak, C.C.; Gupta, V.; Ho, V.T.; et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia 2015, 29, 1754–1762. [Google Scholar] [CrossRef]
- Balashov, D.; Laberko, A.; Shcherbina, A.; Trakhtman, P.; Abramov, D.; Gutovskaya, E.; Kozlovskaya, S.; Shelikhova, L.; Novichkova, G.; Maschan, M.; et al. A Conditioning Regimen with Plerixafor Is Safe and Improves the Outcome of TCRαβ(+) and CD19(+) Cell-Depleted Stem Cell Transplantation in Patients with Wiskott-Aldrich Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 1432–1440. [Google Scholar] [CrossRef]
- Pietro, M.; Ignazio, C.; Rita De, V.; Luisa, S.; Gerrit, W.; Francesca Del, B.; Vanessa, B.; Paolo, M.; Maria Giuseppina, C.; Angela, P.; et al. Role of interferon-γ in immune-mediated graft failure after allogeneic hematopoietic stem cell transplantation. Haematologica 2019, 104, 2314–2323. [Google Scholar] [CrossRef]
- Ahmed, S.; Bashir, Q.; Bassett, R.; Poon, M.C.; Valdez, B.; Konoplev, S.; Alousi, A.M.; Andersson, B.S.; Ciurea, S.; Hosing, C.; et al. Eltrombopag for Post-Transplantation Thrombocytopenia: Results of Phase II Randomized, Double-Blind, Placebo-Controlled Trial. Transplant. Cell. Ther. 2021, 27, 430.e1–430.e7. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, Y.; Zhang, Y.Y.; Shi, M.M.; Mo, X.D.; Sun, Y.Q.; Chang, Y.J.; Xu, L.P.; Zhang, X.H.; Liu, K.Y.; et al. Prophylactic oral NAC reduced poor hematopoietic reconstitution by improving endothelial cells after haploidentical transplantation. Blood Adv. 2019, 3, 1303–1317. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Fan, Q.; Huang, F.; Zhang, Y.; Wang, Y.; Chen, X.Y.; Fan, Z.P.; Zhou, H.S.; Xiao, Y.; Xu, X.J.; et al. Mesenchymal stem cells versus mesenchymal stem cells combined with cord blood for engraftment failure after autologous hematopoietic stem cell transplantation: A pilot prospective, open-label, randomized trial. Biol. Blood Marrow Transpl. 2014, 20, 236–242. [Google Scholar] [CrossRef]
- Delgado, J.; Thomson, K.; Russell, N.; Ewing, J.; Stewart, W.; Cook, G.; Devereux, S.; Lovell, R.; Chopra, R.; Marks, D.I.; et al. Results of alemtuzumab-based reduced-intensity allogeneic transplantation for chronic lymphocytic leukemia: A British Society of Blood and Marrow Transplantation Study. Blood 2006, 107, 1724–1730. [Google Scholar] [CrossRef]
- Lankester, A.C.; Locatelli, F.; Bader, P.; Rettinger, E.; Egeler, M.; Katewa, S.; Pulsipher, M.A.; Nierkens, S.; Schultz, K.; Handgretinger, R.; et al. Will post-transplantation cell therapies for pediatric patients become standard of care? Biol. Blood Marrow Transpl. 2015, 21, 402–411. [Google Scholar] [CrossRef]
- Soiffer, R.J.; Chen, Y.-B. Pharmacologic agents to prevent and treat relapse after allogeneic hematopoietic cell transplantation. Blood Adv. 2017, 1, 2473–2482. [Google Scholar] [CrossRef]
- Liga, M.; Triantafyllou, E.; Tiniakou, M.; Lambropoulou, P.; Karakantza, M.; Zoumbos, N.C.; Spyridonidis, A. High alloreactivity of low-dose prophylactic donor lymphocyte infusion in patients with acute leukemia undergoing allogeneic hematopoietic cell transplantation with an alemtuzumab-containing conditioning regimen. Biol. Blood Marrow Transpl. 2013, 19, 75–81. [Google Scholar] [CrossRef]
- Wahlstrom, J.T.; Horn, B.N.; Fraser-Browne, C.; Hoeweler, R.; Lu, Y.; Melton, A.; Willert, J.; Dvorak, C.C. Azacitidine Administration Following Hematopoietic Stem Cell Transplantation Is Safe and Feasible in Children with Acute Leukemia. Blood 2016, 128, 4805. [Google Scholar] [CrossRef]
- Yu, S.; Huang, F.; Fan, Z.; Xuan, L.; Nie, D.; Xu, Y.; Yang, T.; Wang, S.; Jiang, Z.; Xu, N.; et al. Haploidentical versus HLA-matched sibling transplantation for refractory acute leukemia undergoing sequential intensified conditioning followed by DLI: An analysis from two prospective data. J. Hematol. Oncol. 2020, 13, 18. [Google Scholar] [CrossRef]
- Hallett, W.H.D.; Ames, E.; Alvarez, M.; Barao, I.; Taylor, P.A.; Blazar, B.R.; Murphy, W.J. Combination therapy using IL-2 and anti-CD25 results in augmented natural killer cell-mediated antitumor responses. Biol. Blood Marrow Transpl. 2008, 14, 1088–1099. [Google Scholar] [CrossRef]
- de Lima, M.; Oran, B.; Champlin, R.E.; Papadopoulos, E.B.; Giralt, S.A.; Scott, B.L.; William, B.M.; Hetzer, J.; Laille, E.; Hubbell, B.; et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol. Blood Marrow Transpl. 2018, 24, 2017–2024. [Google Scholar] [CrossRef]
- Carpenter, P.A.; Snyder, D.S.; Flowers, M.E.; Sanders, J.E.; Gooley, T.A.; Martin, P.J.; Appelbaum, F.R.; Radich, J.P. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood 2007, 109, 2791–2793. [Google Scholar] [CrossRef]
- Pfeifer, H.; Wassmann, B.; Bethge, W.; Dengler, J.; Bornhäuser, M.; Stadler, M.; Beelen, D.; Vucinic, V.; Burmeister, T.; Stelljes, M.; et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR–ABL1-positive acute lymphoblastic leukemia. Leukemia 2013, 27, 1254–1262. [Google Scholar] [CrossRef]
- Gaballa, M.R.; Banerjee, P.; Milton, D.R.; Jiang, X.; Ganesh, C.; Khazal, S.; Nandivada, V.; Islam, S.; Kaplan, M.; Daher, M.; et al. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia. Blood 2022, 139, 1908–1919. [Google Scholar] [CrossRef]
- Oshikawa, G.; Kakihana, K.; Saito, M.; Aoki, J.; Najima, Y.; Kobayashi, T.; Doki, N.; Sakamaki, H.; Ohashi, K. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br. J. Haematol. 2015, 169, 756–759. [Google Scholar] [CrossRef]
- Schwartz, S.; Patel, N.; Longmire, T.; Jayaraman, P.; Jiang, X.; Lu, H.; Baker, L.; Velez, J.; Ramesh, R.; Wavreille, A.S.; et al. Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor. Immunother. Adv. 2022, 2, ltac019. [Google Scholar] [CrossRef]
- Grube, M.; Holler, E.; Weber, D.; Holler, B.; Herr, W.; Wolff, D. Risk Factors and Outcome of Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation-Results from a Single-Center Observational Study. Biol. Blood Marrow Transpl. 2016, 22, 1781–1791. [Google Scholar] [CrossRef]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transpl. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Wolff, D.; Lawitschka, A. Chronic Graft-Versus-Host Disease. In The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Carreras, E., Dufour, C., Mohty, M., Kroger, N., Eds.; Springer: Cham, Switzerland, 2019; pp. 331–345. [Google Scholar] [CrossRef]
- Baird, K.; Cooke, K.; Schultz, K.R. Chronic graft-versus-host disease (GVHD) in children. Pediatr. Clin. N. Am. 2010, 57, 297–322. [Google Scholar] [CrossRef]
- Launspach, M.; Temel, D.; Ohlendorf, E.; Zirngibl, F.; Materne, B.; Oevermann, L.; Deubzer, H.E.; Henssen, A.G.; Kunkele, A.; Hundsdorfer, P.; et al. Rituximab therapy after pediatric hematopoietic stem cell transplantation can cause prolonged B cell impairment and increases the risk for infections—A retrospective matched cohort study. Haematologica 2022, 108, 267–272. [Google Scholar] [CrossRef]
- Morata-Tarifa, C.; Macias-Sanchez, M.D.M.; Gutierrez-Pizarraya, A.; Sanchez-Pernaute, R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease-a meta-analysis. Stem Cell Res. Ther. 2020, 11, 64. [Google Scholar] [CrossRef]
- Vadakekolathu, J.; Rutella, S. T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation. Biomedicines 2017, 5, 33. [Google Scholar] [CrossRef]
- Guo, W.W.; Su, X.H.; Wang, M.Y.; Han, M.Z.; Feng, X.M.; Jiang, E.L. Regulatory T Cells in GVHD Therapy. Front. Immunol. 2021, 12, 697854. [Google Scholar] [CrossRef]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Miklos, D.; Abu Zaid, M.I. Ibrutinib vs Placebo in Combination with Corticosteroids in Patients with New-Onset Chronic Graft-Versus-Host Disease (Cgvhd): Results From the Randomized, Double-Blind Phase 3 Integrate Study; EHA Library: Ultimo NSW, Australia, 2021. [Google Scholar]
- Abboud, R.; Choi, J.; Ruminski, P.; Schroeder, M.A.; Kim, S.; Abboud, C.N.; DiPersio, J.F. Insights into the role of the JAK/STAT signaling pathway in graft-versus-host disease. Ther. Adv. Hematol 2020, 11, 2040620720914489. [Google Scholar] [CrossRef]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef]
- Cutler, C.; Lee, S.J.; Arai, S.; Rotta, M.; Zoghi, B.; Lazaryan, A.; Ramakrishnan, A.; DeFilipp, Z.; Salhotra, A.; Chai-Ho, W.; et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar Study. Blood 2021, 138, 2278–2289. [Google Scholar] [CrossRef]
- Arora, M.; Jagasia, M.; Di Stasi, A.; Meyers, M.L.; Quaranto, C.; Schmitt, A.; Sankoh, S.; Abu Zaid, M.I.; Hill, G.R.; Weisdorf, D.J.; et al. Phase 1 Study of Axatilimab (SNDX-6352), a CSF-1R Humanized Antibody, for Chronic Graft-Versus-Host Disease after 2 or More Lines of Systemic Treatment. Blood 2020, 136, 1–2. [Google Scholar] [CrossRef]
- Fang, S.; Meng, X.; Zhang, Z.; Wang, Y.; Liu, Y.; You, C.; Yan, H. Vorinostat Modulates the Imbalance of T Cell Subsets, Suppresses Macrophage Activity, and Ameliorates Experimental Autoimmune Uveoretinitis. Neuromolecular Med. 2016, 18, 134–145. [Google Scholar] [CrossRef]
- Edelson, R.; Wu, Y.; Schneiderman, J. American council on ECP (ACE): Why now? J. Clin. Apher. 2018, 33, 464–468. [Google Scholar] [CrossRef]
- Bergeron, A.; Chevret, S.; Peffault de Latour, R.; Chagnon, K.; de Margerie-Mellon, C.; Rivière, F.; Robin, M.; Mani, J.; Lorillon, G.; Socié, G.; et al. Noninfectious lung complications after allogeneic haematopoietic stem cell transplantation. Eur. Respir. J. 2018, 51, 1702617. [Google Scholar] [CrossRef]
- Carreras, E.; Dufour, C.; Mohty, M.; Kroger, N. (Eds.) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Yanik, G.A.; Mineishi, S.; Levine, J.E.; Kitko, C.L.; White, E.S.; Vander Lugt, M.T.; Harris, A.C.; Braun, T.; Cooke, K.R. Soluble tumor necrosis factor receptor: Enbrel (etanercept) for subacute pulmonary dysfunction following allogeneic stem cell transplantation. Biol. Blood Marrow Transpl. 2012, 18, 1044–1054. [Google Scholar] [CrossRef]
- Yanik, G.A.; Grupp, S.A.; Pulsipher, M.A.; Levine, J.E.; Schultz, K.R.; Wall, D.A.; Langholz, B.; Dvorak, C.C.; Alangaden, K.; Goyal, R.K.; et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children’s Oncology Group Study (ASCT0521). Biol. Blood Marrow Transpl. 2015, 21, 67–73. [Google Scholar] [CrossRef]
- Barker, A.F.; Bergeron, A.; Rom, W.N.; Hertz, M.I. Obliterative bronchiolitis. N. Engl. J. Med. 2014, 370, 1820–1828. [Google Scholar] [CrossRef]
- Yadav, H.; Peters, S.G.; Keogh, K.A.; Hogan, W.J.; Erwin, P.J.; West, C.P.; Kennedy, C.C. Azithromycin for the Treatment of Obliterative Bronchiolitis after Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transpl. 2016, 22, 2264–2269. [Google Scholar] [CrossRef]
- Glanville, A.R.; Benden, C.; Bergeron, A.; Cheng, G.-S.; Gottlieb, J.; Lease, E.D.; Perch, M.; Todd, J.L.; Williams, K.M.; Verleden, G.M. Bronchiolitis obliterans syndrome after lung or haematopoietic stem cell transplantation: Current management and future directions. ERJ Open Res. 2022, 8, 00185–02022. [Google Scholar] [CrossRef]
- Zeiser, R.; Polverelli, N.; Ram, R.; Hashmi, S.K.; Chakraverty, R.; Middeke, J.M.; Musso, M.; Giebel, S.; Uzay, A.; Langmuir, P.; et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2021, 385, 228–238. [Google Scholar] [CrossRef]
- Streiler, C.; Shaikh, F.; Davis, C.; Abhyankar, S.; Brownback, K.R. Ruxolitinib is an effective steroid sparing agent in bronchiolitis obliterans due to chronic graft-versus-host-disease. Bone Marrow Transpl. 2020, 55, 1194–1196. [Google Scholar] [CrossRef]
- Olivieri, J.; Coluzzi, S.; Attolico, I.; Olivieri, A. Tirosin kinase inhibitors in chronic graft versus host disease: From bench to bedside. Sci. World J. 2011, 11, 1908–1931. [Google Scholar] [CrossRef]
- Olivieri, A.; Cimminiello, M.; Locatelli, F.; Zecca, M.; Corradini, P.; Patriarca, F.; Mordini, N.; Iacopino, P.; Donelli, A.; Selleri, C.; et al. Imatinib Is Safe and Effective In Patients with Refractory Chronic Graft Versus Host Disease: Analysis of Two Consecutive Prospective GITMO* Studies.*Gruppo Italiano Trapianto Midollo Osseo. Blood 2010, 116, 246. [Google Scholar] [CrossRef]
- Bergeron, A.; Chevret, S.; Chagnon, K.; Godet, C.; Bergot, E.; Peffault de Latour, R.; Dominique, S.; de Revel, T.; Juvin, K.; Maillard, N.; et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 1242–1249. [Google Scholar] [CrossRef]
- Williams, K.M.; Cheng, G.S.; Pusic, I.; Jagasia, M.; Burns, L.; Ho, V.T.; Pidala, J.; Palmer, J.; Johnston, L.; Mayer, S.; et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transpl. 2016, 22, 710–716. [Google Scholar] [CrossRef]
- Athale, J.; Gormley, N.J.; Reger, R.; Alsaaty, S.; Reda, D.; Worthy, T.; Saxena, A.; Tian, X.; Childs, R.W.; Suffredini, A.F. Effect of Cyclosporine Inhalation Solution (CIS) on Lung Function and Inflammatory Biomarkers in Patients with Hematopoietic Stem Cell Transplant (HSCT) Associated Bronchiolitis Obliterans Syndrome (BOS). Blood 2019, 134, 4552. [Google Scholar] [CrossRef]
- Raghu, G.; Meyer, K.C. Cryptogenic organising pneumonia: Current understanding of an enigmatic lung disease. Eur. Respir. Rev. 2021, 30, 210094. [Google Scholar] [CrossRef]
- Cooke, K.R.; Yanik, G.A. Lung Injury Following Hematopoietic Cell Transplantation. In Thomas’ Hematopoietic Cell Transplantation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1156–1172. [Google Scholar] [CrossRef]
- Varelias, A.; Gartlan, K.H.; Kreijveld, E.; Olver, S.D.; Lor, M.; Kuns, R.D.; Lineburg, K.E.; Teal, B.E.; Raffelt, N.C.; Cheong, M.; et al. Lung parenchyma-derived IL-6 promotes IL-17A-dependent acute lung injury after allogeneic stem cell transplantation. Blood 2015, 125, 2435–2444. [Google Scholar] [CrossRef]
- Rathi, N.K.; Tanner, A.R.; Dinh, A.; Dong, W.; Feng, L.; Ensor, J.; Wallace, S.K.; Haque, S.A.; Rondon, G.; Price, K.J.; et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transpl. 2015, 50, 420–426. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transpl. 2018, 53, 138–145. [Google Scholar] [CrossRef]
- Schechter, T.; Perez-Albuerne, E.; Lin, T.F.; Irwin, M.S.; Essa, M.; Desai, A.V.; Frangoul, H.; Yanik, G.; Dupuis, L.L.; Jacobsohn, D.; et al. Veno-occlusive disease after high-dose busulfan–melphalan in neuroblastoma. Bone Marrow Transpl. 2020, 55, 531–537. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, M.H.; Lee, H.; Kim, H.S.; Kim, K.; Kim, W.S.; Jung, C.W.; Im, Y.H.; Yoon, S.S.; Kang, W.K.; et al. A randomized trial of heparin plus ursodiol vs. heparin alone to prevent hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2002, 29, 137–143. [Google Scholar] [CrossRef]
- Rosenthal, J.; Sender, L.; Secola, R.; Killen, R.; Millerick, M.; Murphy, L.; Cairo, M.S. Phase II trial of heparin prophylaxis for veno-occlusive disease of the liver in children undergoing bone marrow transplantation. Bone Marrow Transpl. 1996, 18, 185–191. [Google Scholar]
- Corbacioglu, S.; Cesaro, S.; Faraci, M.; Valteau-Couanet, D.; Gruhn, B.; Rovelli, A.; Boelens, J.J.; Hewitt, A.; Schrum, J.; Schulz, A.S.; et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: An open-label, phase 3, randomised controlled trial. Lancet 2012, 379, 1301–1309. [Google Scholar] [CrossRef]
- Richardson, P.G.; Riches, M.L.; Kernan, N.A.; Brochstein, J.A.; Mineishi, S.; Termuhlen, A.M.; Arai, S.; Grupp, S.A.; Guinan, E.C.; Martin, P.L.; et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 2016, 127, 1656–1665. [Google Scholar] [CrossRef]
- Grupp, S.A.; Corbacioglu, S.; Kang, H.J.; Teshima, T.; Zanette, M.; Lopez, P.; Amber, V.; Pagliuca, A.; Richardson, P.G. A Phase 3, Randomized, Adaptive Study of Defibrotide (DF) Vs Best Supportive Care (BSC) for the Prevention of Hepatic Veno-Occlusive Disease/Sinusoidal Obstruction Syndrome (VOD/SOS) in Patients (pts) Undergoing Hematopoietic Cell Transplantation (HCT): Preliminary Results. Blood 2021, 138, 749. [Google Scholar] [CrossRef]
- Morris, J.D.; Harris, R.E.; Hashmi, R.; Sambrano, J.E.; Gruppo, R.A.; Becker, A.T.; Morris, C.L. Antithrombin-III for the treatment of chemotherapy-induced organ dysfunction following bone marrow transplantation. Bone Marrow Transpl. 1997, 20, 871–878. [Google Scholar] [CrossRef]
- Gluckman, E.; Jolivet, I.; Scrobohaci, M.L.; Devergie, A.; Traineau, R.; Bourdeau-Espérou, H.; Lehn, P.; Faure, P.; Drouet, L. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br. J. Haematol. 1990, 74, 277–281. [Google Scholar] [CrossRef]
- Morio, S.; Oh, H.; Kogure, K.; Ishii, H.; Ishii, A.; Nakaseko, C.; Ikegami, T.; Kawano, E.; Matsuura, Y.; Nishimura, M.; et al. A trial use of prostaglandin E1 for prevention of hepatic veno-occlusive disease after allogeneic bone marrow transplantation. Rinsho Ketsueki 1994, 35, 846–852. [Google Scholar]
- Cornell, R.F.; Hari, P.; Drobyski, W.R. Engraftment Syndrome after Autologous Stem Cell Transplantation: An Update Unifying the Definition and Management Approach. Biol. Blood Marrow Transpl. 2015, 21, 2061–2068. [Google Scholar] [CrossRef]
- Jin, L.; Sun, Z.; Liu, H.; Zhu, X.; Zhou, Y.; Fu, B.; Zheng, X.; Song, K.; Tang, B.; Wu, Y.; et al. Inflammatory monocytes promote pre-engraftment syndrome and tocilizumab can therapeutically limit pathology in patients. Nat. Commun. 2021, 12, 4137. [Google Scholar] [CrossRef]
- Lucchini, G.; Willasch, A.M.; Daniel, J.; Soerensen, J.; Jarisch, A.; Bakhtiar, S.; Rettinger, E.; Brandt, J.; Klingebiel, T.; Bader, P. Epidemiology, risk factors, and prognosis of capillary leak syndrome in pediatric recipients of stem cell transplants: A retrospective single-center cohort study. Pediatr. Transpl. 2016, 20, 1132–1136. [Google Scholar] [CrossRef]
- Yabe, H.; Yabe, M.; Koike, T.; Shimizu, T.; Morimoto, T.; Kato, S. Rapid improvement of life-threatening capillary leak syndrome after stem cell transplantation by bevacizumab. Blood 2010, 115, 2723–2724. [Google Scholar] [CrossRef]
- Rosenthal, J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: A review of pathophysiology, diagnosis, and treatment. J. Blood Med. 2016, 7, 181–186. [Google Scholar] [CrossRef]
- Epperla, N.; Li, A.; Logan, B.; Fretham, C.; Chhabra, S.; Aljurf, M.; Chee, L.; Copelan, E.; Freytes, C.O.; Hematti, P.; et al. Incidence, Risk Factors for and Outcomes of Transplant-Associated Thrombotic Microangiopathy. Br. J. Haematol. 2020, 189, 1171–1181. [Google Scholar] [CrossRef]
- Jodele, S.; Zhang, K.; Zou, F.; Laskin, B.; Dandoy, C.E.; Myers, K.C.; Lane, A.; Meller, J.; Medvedovic, M.; Chen, J.; et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 2016, 127, 989–996. [Google Scholar] [CrossRef]
- Higham, C.S.; Shimano, K.A.; Melton, A.; Kharbanda, S.; Chu, J.; Dara, J.; Winestone, L.E.; Hermiston, M.L.; Huang, J.N.; Dvorak, C.C. A pilot trial of prophylactic defibrotide to prevent serious thrombotic microangiopathy in high-risk pediatric patients. Pediatr. Blood Cancer 2022, 69, e29641. [Google Scholar] [CrossRef]
- Jodele, S.; Dandoy, C.E.; Lane, A.; Laskin, B.L.; Teusink-Cross, A.; Myers, K.C.; Wallace, G.; Nelson, A.; Bleesing, J.; Chima, R.S.; et al. Complement blockade for TA-TMA: Lessons learned from a large pediatric cohort treated with eculizumab. Blood 2020, 135, 1049–1057. [Google Scholar] [CrossRef]
- Syed, Y.Y. Ravulizumab: A Review in Atypical Haemolytic Uraemic Syndrome. Drugs 2021, 81, 587–594. [Google Scholar] [CrossRef]
- Khaled, S.K.; Claes, K.; Goh, Y.T.; Kwong, Y.L.; Leung, N.; Mendrek, W.; Nakamura, R.; Sathar, J.; Ng, E.; Nangia, N.; et al. Narsoplimab, a Mannan-Binding Lectin-Associated Serine Protease-2 Inhibitor, for the Treatment of Adult Hematopoietic Stem-Cell Transplantation-Associated Thrombotic Microangiopathy. J. Clin. Oncol. 2022, 40, 2447–2457. [Google Scholar] [CrossRef]
- Lankester, A.C.; Albert, M.H.; Booth, C.; Gennery, A.R.; Güngör, T.; Hönig, M.; Morris, E.C.; Moshous, D.; Neven, B.; Schulz, A.; et al. EBMT/ESID inborn errors working party guidelines for hematopoietic stem cell transplantation for inborn errors of immunity. Bone Marrow Transpl. 2021, 56, 2052–2062. [Google Scholar] [CrossRef]
- Olsson, R.; Remberger, M.; Schaffer, M.; Berggren, D.M.; Svahn, B.M.; Mattsson, J.; Ringden, O. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transpl. 2013, 48, 537–543. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-H.; Lee, J.-H.; Park, H.-S.; Choi, E.-J.; Kang, Y.-A.; Kang, H.; Woo, J.M.; Lee, Y.-S.; Jeon, M.; et al. Incidence, Management, and Prognosis of Graft Failure and Autologous Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. J. Korean Med. Sci. 2021, 36, e151. [Google Scholar] [CrossRef]
- Pasvolsky, O.; Shimony, S.; Yeshurun, M.; Shargian, L.; Wolach, O.; Raanani, P.; Gafter-Gvili, A.; Gurion, R. Maintenance therapy after allogeneic hematopoietic transplant for acute myeloid leukemia: A systematic review and meta-analysis. Acta Oncol. 2021, 60, 1335–1341. [Google Scholar] [CrossRef]
- Barone, A.; Casey, D.; McKee, A.E.; Reaman, G. Cancer drugs approved for use in children: Impact of legislative initiatives and future opportunities. Pediatr. Blood Cancer 2019, 66, e27809. [Google Scholar] [CrossRef]
- Neel, D.V.; Shulman, D.S.; DuBois, S.G. Timing of first-in-child trials of FDA-approved oncology drugs. Eur. J. Cancer 2019, 112, 49–56. [Google Scholar] [CrossRef]
- Blackmon, A.; Aldoss, I.; Ball, B.J. FLT3 Inhibitors as Maintenance Therapy after Allogeneic Stem-Cell Transplantation. Blood Lymphat. Cancer 2022, 12, 137–147. [Google Scholar] [CrossRef]
| Disease of Interest (If Specified) | Ages | Phase | Investigational Agent(s) | Intervention | Trial Number | Estimated Enrollment (Number of Participants) | Status | |
|---|---|---|---|---|---|---|---|---|
| Graft failure | ||||||||
| Sickle cell disease and β-thalassemia | 4 Years and Older | Phase 2 | Alemtuzumab, low dose radiation, oral cyclophosphamide, pentostatin, and sirolimus | N/A | NCT02105766 | 162 | Active, recruiting | |
| All hematological diseases | 3 Years to 70 Years | Phase 2 | Cyclophosphamide (CP) | Post-transplant preventive CP at 50 mg/kg/day at D + 3 and D + 4 | NCT05126186 | 35 | Active, recruiting | |
| Chronic granulomatous disease | 1 Month to 24 Years | Phase 2 | Plerixafor/G-CSF | Both agents are administered before transplant | NCT03547830 | 17 | Active, recruiting | |
| Non-malignant diseases, leukemia, and lymphoma | 1 Year and Older | Phase 2 | Emapalumab | Given IV from day 0 every 3–4 days for 15 doses or engraftment evidence at 6 mg/kg (first dose) and 3 mg/kg (subsequent doses) | NCT04731298 | 250 | Terminated | |
| Leukemia and patients with poor graft function criteria | 6 Years and Older | Phase 2 | Eltrombopag | Eltrombopag OD at 50 mg/day starting not earlier than D60 post-HSCT. In the absence of response, dose can be increased up to 150 mg/day in blocks of 50 mg | NCT03948529 | 25 | Active, recruiting | |
| Acute leukemia patients | 15 Years to 60 Years | Phase 2 | *N-acetyl-L-cysteine | N-acetyl-L-cysteine given orally at dosages of 400 mg three times per day from 14 days pre-allotransplant to 2 months after allotransplant | NCT03236220 | 35 | Completed | |
| Prevention of relapse including early interventions after detection of MRD positivity following HSCT in ALL and AML | ||||||||
| Cellular therapy | ||||||||
| ALL, AML | 10 Years to 65 Years | Phase 1 | Donor γδT cell infusion | One infusion of 0.5 × 106–8 × 107 γδT/kg | NCT04439721 | 5 | Active, recruiting | |
| AML, MDS, JMML | 1 Year and Older | Phase 1 | Cytokine-induced memory-like natural killer (CIML-NK) cells | CIML-NK given IV on day 0 with IL-2, three doses of fludarabine from day −5 to −3 and two doses CP on day −4 and −4 | NCT04024761 | 50 | Active, recruiting | |
| CD19 positive B-ALL/B-CLL/NHL | All | Phase 1 | CD19/CD28 CAR T cells derived from donor | Maximum of six doses, 4 to 6 weeks apart, of CAR-T | NCT02050347 | 40 | Active, recruiting | |
| Leukemia, lymphoma, and myeloproliferative diseases | Up to 21 Years | Phase 2 | * TCRαβ-depleted progenitor cell graft with additional memory T cell DLI and blinatumomab | CD45RA-depleted DLI two weeks after engraftment of TCRα/β+ and CD19+-depleted, for CD19 pos; Blinatumomab will be given at least one-week post-DLI | NCT03849651 | 140 | Active, recruiting | |
| ALL, AML, JMML, MDS | Up to 29 Years | Phase 2 | Azacitidine and DLI | Up to seven cycles of low dose azacitidine (40 mg/m2 IV/SC daily × 4 days) at six weekly intervals and additional cycles according to risk levels | NCT02458235 | 17 | Completed | |
| Acute leukemia | 14 Years to 65 Years | Phase 2/3 | DLI | DLI administered at a median dose of 1.0 (range 0.7–1.4) × 108 mononuclear cells/kg once by day +60 and further based on MRD and GVHD status | NCT02673008 | 206 | Completed | |
| Hypomethylating agents (HMA) | ||||||||
| AML, MDS, JMML, MPAL | 1 Year to 21 Years | Phase 1 | Vorinostat/azacitidine | Two cycles of standard post-transplant azacitidine followed by vorinostat orally | NCT03843528 | 15 | Active, recruiting | |
| AML, MDS | 1 Year to 75 Years | Phase 2 | Low dose of azacitidine | Azacitidine at 32 mg/m2 SC for 5 days every 28 days. At 60–120 days post-T-cell depletion of allo-HSCT | NCT01995578 | 32 | Active, not recruiting | |
| All hematological diseases | 10 Years to 70 Years | Phase 3 | Decitabine and acetylcysteine | Decitabine (20 mg/m2/d) on days −10 to −8 and acetylcysteine from day −10 to +365 | NCT04945096 | 100 | Active, not yet recruiting | |
| Targeted therapy | ||||||||
| AML | 12 Years to 99 Years | Phase 1/2 | Sabatolimab | Sabatolimab will be given every 4 weeks in combination with azacitidine | NCT04623216 | 59 | Active, recruiting | |
| AML FLT3 ITD | 1 Month to 21 Years | Phase 1/2 | * Quizartinib | Quizartinib once daily starting on day 6 and continuing through to day 28 | NCT03793478 | 65 | Active, recruiting | |
| B-ALL | Up to 25 Years | Phase 2 | αβ T cell- and B cell-depleted allogeneic hematopoietic cell transplantation (HCT) followed by blinatumomab | Twenty-eight-day continuous infusion of blinatumomab starting on day 100 post-transplant | NCT04746209 | 25 | Active, recruiting | |
| Leukemia, lymphoma and myeloproliferative diseases | Up to 21 Years | Phase 2 | TCRαβ- and CD45RA-depleted haploidentical donor progenitor cell transplantation followed by post-HSCT Blinatumomab | Continuous IV blinatumomab infusion at least 2 weeks post-engraftment for CD19-positive patients | NCT02790515 | 52 | Active, recruiting | |
| B-ALL | 6 Months to 21 Years | Phase 2 | * Blinatumomab | Blinatumomab over a 28-day cycle. MRD-positive patients before HSCT start between day +60–+100 and for patients, who become MRD positive post-HSCT between day +60–+360 | NCT04785547 | 32 | Active, recruiting | |
| B-ALL | 1 Year and Older | Phase 2 | Blinatumomab | Blinatumomab for 6 weeks (4 weeks followed by a 2-week treatment-free period) for up to four cycles | NCT04044560 | 8 | Terminated | |
| Chronic GVHD | ||||||||
| Cellular therapy | ||||||||
| All | Phase 1 | Donor regulatory T cells | CD25hi regulatory T cells from CD8 and/or CD19 pre-depleted leukapheresis products will be given in three dose levels | NCT03683498 | 16 | Completed | ||
| 14 Years to 70 Years | Phase 1/2 | Umbilical cord mesenchymal stem cells | N/A | NCT05152160 | 10 | Active, recruiting | ||
| All | Phase 2 | Donor regulatory T cells | 2 × 106 cells/kg dose of regulatory T cell-enriched infusion | NCT05095649 | 15 | Active, recruiting | ||
| Targeted therapy | ||||||||
| Up to 18 Years | Phase 1 | Ruxolitinib | Ruxolitinib BID daily | NCT05121142 | 28 | Active, recruiting | ||
| 1 Year to 21 Years | Phase 1/2 | Ibrutinib | Ibrutinib orally once daily | NCT03790332 | 59 | Active, not recruiting | ||
| ALL, AML, CML, MDS, mature B-cell malignancies | 3 Years to 39 Years | Phase 1/2 | Vorinostat | Vorinostat at 30, 45, or 60 mg/m2 BID orally from day −10 days, until day +30 post-transplant.Haploidentical BMT recipients: same intervention but starting from day +5 | NCT03842696 | 49 | Active, recruiting | |
| 6 Years and Older | Phase 1/2 | Axatilimab (SNDX-6352) | SNDX-6352 IV will be given at a dose of 0.15–3 mg/kg | NCT03604692 | 40 | Active, not recruiting | ||
| 12 Years and Older | Phase1/2 | TQ05105 | TQ05105 10 mg given orally, twice daily in 28 day-cycle | NCT04944043 | 97 | Active, recruiting | ||
| 28 Days to 18 Years | Phase 2 | Ruxolitinib | Ruxolitinib 5 mg BID | NCT03774082 | 46 | Active, not recruiting | ||
| ALL, AML, CML, MDS, myelofibrosis | Up to 80 Years | Phase 2 | Itacitinib | CP IV QD on days 3 and 4, itacitinib PO QD on days 5–100, and tacrolimus IV or PO on days 6–65 | NCT05364762 | 50 | Active, not yet recruiting | |
| 12 Years and Older | Phase 2 | Belumosudil | Belumosudil 200 mg once or twice daily according to randomization | NCT03640481 | 175 | Active, recruiting | ||
| 12 Years and Older | Phase 2 | Belumosudil | Belumosudil orally, OD, or BID (if taking CYP3A4 inhibitors or proton pump inhibitors) | NCT05567406 | 12 | Active, not yet recruiting | ||
| 2 Years and Older | Phase 2 | Axatilimab (SNDX- 6352) | Axatilimab 0.3–3 mg/kg IV every 2 weeks for up to 2 years | NCT04710576 | 210 | Active, not recruiting | ||
| Up to 21 Years | Phase 2 | Mycophenolate mofetil (MMF) and imatinib | MMF 15–20 mg/kg BID and imatinib QD 260 mg/m2/d | NCT01898377 | 9 | Terminated | ||
| 12 Years and Older | Phase 3 | Ibrutinib in combination with corticosteroids | Ibrutinib 420 mg is given orally OD starting on day 1 until cGVHD progression; in addition, 1 mg/kg/d prednisone OD until unacceptable toxicity or until participant is successfully tapered from the prednisone | NCT02959944 | 193 | Completed | ||
| Chemotherapeutics | ||||||||
| AML, MDS | 16 Years to 70 Years | Phase 2 | Cyclophosphamide | ATG, 4.5 mg/kg IV on days −2, −1, and +1 and CP at 50 mg/kg IV daily on days +3 and +4 vs. ATG alone | NCT04202835 | 80 | Active, recruiting | |
| ALL, AML, CML, MDS, CLL, Lymphoma | 5 Years to 75 Years | Phase 2 | Tacrolimus, high dose cyclophosphamide, and MMF | CP on days 3–4, mycophenolate mofetil TID on day 5 and stopping on day 35 if no severe GVHD is present. Tacrolimus IV continuously on days 5–180 with a taper beginning on day 90 in the absence of disease progression or unacceptable toxicity | NCT03128359 | 38 | Active, not recruiting | |
| Other | ||||||||
| All | Phase 2 | Extracorporeal photopheresis | Six cycles of extracorporeal photopheresis every 2 weeks | NCT03083574 | 100 | Active, recruiting | ||
| up to 65 Years | Phase 2 | Hydrogen-rich water | Hydrogen-rich water 4 mL/kg orally TID one day | NCT02918188 | 21 | Active, recruiting | ||
| Noninfectious pulmonary complications | ||||||||
| 5 Years to 25 Years | Phase 2 | Ruxolitinib | Ruxolitinib orally twice daily for 24 weeks plus standard fluticasone/montelukast and steroids | NCT04908735 | 40 | Active, recruiting | ||
| 6 Years to 99 Years | Phase 2 | Fluticasone propionate, azithromycin, and montelukast sodium (FAM) | Fluticasone propionate inhaled PO BID, azithromycin PO 3 days a week, and montelukast sodium PO QD for 6 months | NCT01307462 | 36 | Completed | ||
| 10 Years to 80 Years | Phase 2 | Cyclosporine inhalation solution | Cyclosporine inhalation solution (CIS) 150 mg three times weekly during weeks 1–5. Dose escalated to 300 mg three times weekly from weeks 6–8 until week 19 | NCT01287078 | 25 | Completed | ||
| 6 Years to 17 Years | Phase 3 | Fluticasone propionate and salmeterol | Inhaled fluticasone propionate 50 or 125 μg and 25 μg salmeterol, BID from randomization and until 6 months | NCT04655508 | 243 | Active, recruiting | ||
| Complications of endothelial origin | ||||||||
| VOD/SOS | ||||||||
| up to 65 Years | Phase 2 | Antithrombin-III | AT-III at units required (IU)/kg = 50 + [(desired-baseline AT-III level) × weight (kg)/1.4] | NCT01886248 | 32 | Active, not recruiting | ||
| All | Phase 2/3 | Lipoprostaglandin E1 | Dose of 1.5 mcg/kg/day, continuous infusion | NCT02338440 | 30 | Active, not recruiting | ||
| 1 Month and Older | Phase 3 | Defibrotide | Defibrotide 25 mg/kg/day IV in addition to best supportive care, day before the first day of the conditioning for a recommended minimum of 21 days | NCT02851407 | 372 | Completed | ||
| TA-TMA | ||||||||
| All | Phase 2 | Eculizumab | Eculizumab IV for 24 weeks | NCT03518203 | 23 | Active, recruiting | ||
| up to 30 Years | Phase 2 | Defibrotide | Defibrotide 6.25 mg/kg administered intravenously for 28–35 days | NCT03384693 | 25 | Completed | ||
| All | Phase 3 | N-Acetylcysteine | 50 mg/kg orally | NCT03252925 | 170 | Completed | ||
| 12 Years and Older | Phase 3 | Ravulizumab | Weight-based doses of ravulizumab will be administered intravenously as loading dose regimen followed by maintenance dosing every 8 weeks plus best supportive care (BSC) vs. placebo | NCT04543591 | 184 | Active, not recruiting | ||
| 28 Days to 17 Years | Phase 3 | Ravulizumab | Weight-based doses of ravulizumab administered IV as a loading dose regimen followed by maintenance every 4 or 8 weeks plus best supportive care | NCT04557735 | 40 | Active, recruiting | ||
| 6 Months to 18 Years | Phase 3 | Nomacopan | NA | NCT04784455 | 50 | Active, recruiting | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilan, U.; Brivio, E.; Algeri, M.; Balduzzi, A.; Gonzalez-Vincent, M.; Locatelli, F.; Zwaan, C.M.; Baruchel, A.; Lindemans, C.; Bautista, F. The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children. J. Clin. Med. 2023, 12, 2149. https://doi.org/10.3390/jcm12062149
Ilan U, Brivio E, Algeri M, Balduzzi A, Gonzalez-Vincent M, Locatelli F, Zwaan CM, Baruchel A, Lindemans C, Bautista F. The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children. Journal of Clinical Medicine. 2023; 12(6):2149. https://doi.org/10.3390/jcm12062149
Chicago/Turabian StyleIlan, Uri, Erica Brivio, Mattia Algeri, Adriana Balduzzi, Marta Gonzalez-Vincent, Franco Locatelli, Christian Michel Zwaan, Andre Baruchel, Caroline Lindemans, and Francisco Bautista. 2023. "The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children" Journal of Clinical Medicine 12, no. 6: 2149. https://doi.org/10.3390/jcm12062149
APA StyleIlan, U., Brivio, E., Algeri, M., Balduzzi, A., Gonzalez-Vincent, M., Locatelli, F., Zwaan, C. M., Baruchel, A., Lindemans, C., & Bautista, F. (2023). The Development of New Agents for Post-Hematopoietic Stem Cell Transplantation Non-Infectious Complications in Children. Journal of Clinical Medicine, 12(6), 2149. https://doi.org/10.3390/jcm12062149







