Non-Invasive Tests of Liver Fibrosis Help in Predicting the Development of Hepatocellular Carcinoma among Patients with NAFLD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Patients
2.3. Collected Variables
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Sample
3.2. Baseline and Clinical Characteristics
3.3. Incidence and Characteristics of HCC
3.4. Liver Events and Death
3.5. Predictors of HCC
4. Discussion
4.1. Major Findings
4.2. Incidence of HCC
4.3. Decompensations, Liver Transplant, and Mortality
4.4. Predictors of HCC
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Henry, L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021, 3, 100305. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orci, L.A.; Sanduzzi-Zamparelli, M.; Caballol, B.; Sapena, V.; Colucci, N.; Torres, F.; Bruix, J.; Reig, M.; Toso, C. Incidence of Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin. Gastroenterol. Hepatol. 2022, 20, 283–292.e10. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.A.; Singal, A.G.; Kum, H.C.; Lee, Y.-T.; Park, S.; Rich, N.E.; Noureddin, M.; Yang, J.D. Clinical Characteristics and Outcomes of Nonalcoholic Fatty Liver Disease-Associated Hepatocellular Carcinoma In the United States. Clin. Gastroenterol. Hepatol. 2022. In press. [Google Scholar] [CrossRef]
- Castellana, M.; Donghia, R.; Lampignano, L.; Castellana, F.; Zupo, R.; Sardone, R.; Pergola, G.; Giannelli, G. Prevalence of the Absence of Cirrhosis in Subjects with NAFLD-Associated Hepatocellular Carcinoma. J. Clin. Med. 2021, 10, 4638. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Petta, S.; Sebastiani, G.; Viganò, M.; Ampuero, J.; Wai-Sun Wong, V.; Boursier, J.; Berzigotti, A.; Bugianesi, E.; Fracanzani, A.L.; Cammà, C.; et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin. Gastroenterol. Hepatol. 2021, 1, 806–815. [Google Scholar] [CrossRef]
- Boursier, J.; Vergniol, J.; Guillet, A.; Hiriart, J.B.; Lannes, A.; Le Bail, B.; Michalak, S.; Chermak, F.; Bertrais, S.; Foucher, J.; et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 570–578. [Google Scholar] [CrossRef]
- Shili-Masmoudi, S.; Wong, G.L.; Hiriart, J.B.; Liu, K.; Chermak, F.; Shu, S.S.; Foucher, J.; Tse, Y.K.; Bernard, P.H.; Yip, T.C.; et al. Liver stiffness measurement predicts long-term survival and complications in non-alcoholic fatty liver disease. Liver Int. 2020, 40, 581–589. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Kostev, K.; Keitel, V.; Tacke, F.; Roderburg, C.; Luedde, T. An elevated FIB-4 score predicts liver cancer development: A longitudinal analysis from 29,999 patients with NAFLD. J. Hepatol. 2022, 76, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2017, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Caviglia, G.P.; Govaere, O.; Rosso, C.; Armandi, A.; Sanavia, T.; Pennisi, G.; Liguori, A.; Francione, P.; Gallego-Durán, R.; et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 786–794. [Google Scholar] [CrossRef]
- Aoki, T.; Iijima, H.; Tada, T.; Kumada, T.; Nishimura, T.; Nakano, C.; Kishino, K.; Shimono, Y.; Yoh, K.; Takata, R.; et al. Prediction of development of hepatocellular carcinoma using a new scoring system involving virtual touch quantification in patients with chronic liver diseases. J. Gastroenterol. 2017, 52, 104–112. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef] [Green Version]
- Shearer, J.E.; Jones, R.; Parker, R.; Ferguson, J.; Rowe, I.A. The natural history of advanced chronic liver disease defined by transient elastography. Clin. Gastroenterol. Hepatol. 2022. In press. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Hayward, K.L.; Patel, P.; Horsfall, L.U.; Cheah, A.E.Z.; Irvine, K.M.; Russell, A.W.; Stuart, K.A.; Williams, S.; Hartel, G.; et al. Predicting Liver-Related Outcomes in People With Nonalcoholic Fatty Liver Disease: The Prognostic Value of Noninvasive Fibrosis Tests. Hepatol. Commun. 2022, 6, 728–739. [Google Scholar] [CrossRef]
- Lee, J.S.; Sinn, D.H.; Park, S.Y.; Shin, H.J.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Oh, J.H.; et al. Liver Stiffness-Based Risk Prediction Model for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancers 2021, 13, 4567. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Sakai, Y.; Terashima, T.; Shimode, T.; Seki, A.; Orita, N.; Takeshita, Y.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Decline in serum albumin concentration is a predictor of serious events in nonalcoholic fatty liver disease. Medicine 2021, 100, e26835. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.; Kerr, K.F.; Berry, K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J. Hepatol. 2019, 71, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.; Lowy, E.; Mun, E.J.; Berry, K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS ONE 2018, 13, e0204412. [Google Scholar] [CrossRef] [Green Version]

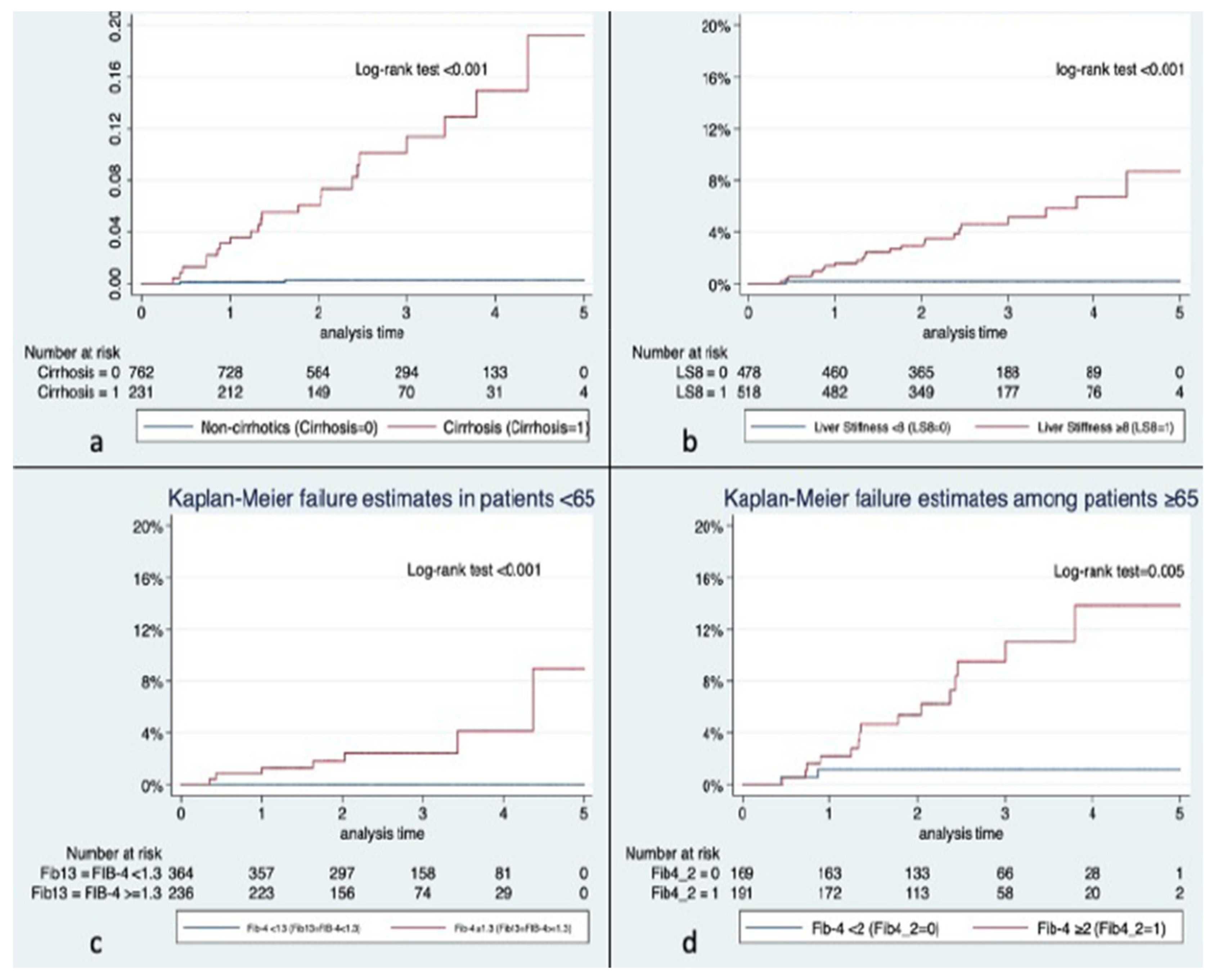
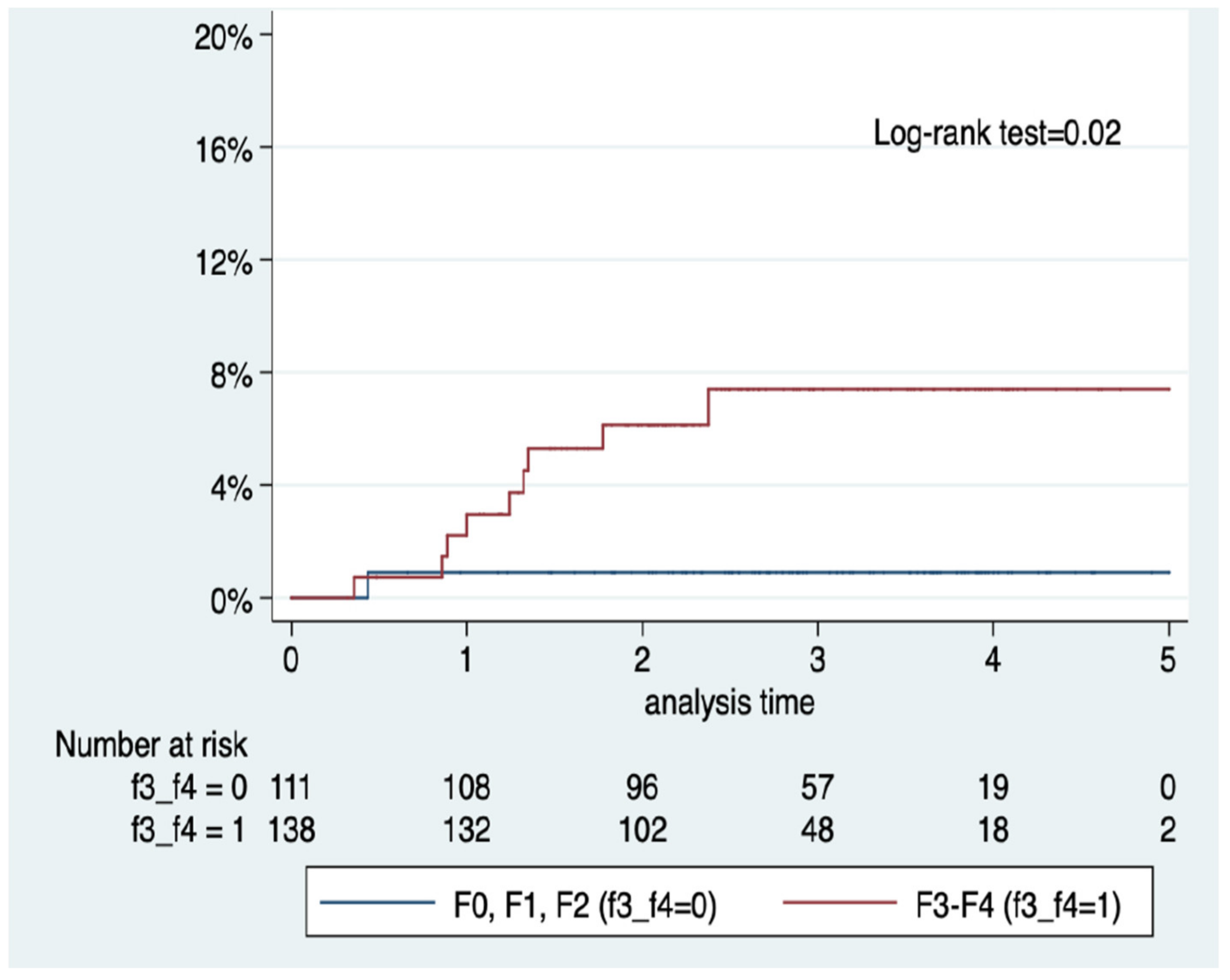
| No HCC n = 970 | HCC n = 26 | p | |
|---|---|---|---|
| Female, n (%) | 505 (52.1) | 2 (7.6) | <0.001 |
| Age, median (IQR) | 60 (51–68) | 69 (60–72) | 0.001 |
| Arterial hypertension, n (%) | 530 (54.6) | 10 (38.5) | 0.102 |
| Dyslipidemia, n (%) | 595 (61.3) | 8 (30.8) | 0.002 |
| Type 2 diabetes mellitus, n (%) | 396 (40.8) | 19 (73.1) | 0.001 |
| Body mass index, median kg/m2 (IQR) | 30.9 (27.7–34.7) | 29.8 (27.5–35) | 0.4 |
| Metabolic syndrome, n (%) | 187 (19.3) | 6 (23) | 0.6 |
| Hb1Ac, median g/L (IQR) | 5.8 (5.5–6.7) | 5.8 (5–6.5) | 0.2 |
| Platelets, median × 109/L (IQR) | 228 (176–278) | 102 (72–139) | <0.001 |
| Bilirubin, median mg/dL (IQR) | 0.61 (0.49–0.85) | 1.1 (0.86–1.83) | <0.001 |
| AST, median U/L (IQR) | 32 (24–45) | 48 (33–57) | 0.002 |
| ALT, median U/L (IQR) | 36 (24–54) | 40 (28–45) | 0.9 |
| Alkaline phosphatase, median U/L (IQR) | 90 (72–115) | 125 (96–189) | <0.001 |
| GGT, median U/L (IQR) | 63 (36–124) | 187 (91–323) | <0.001 |
| Albumin, median g/L (IQR) | 4.3 (4.1–4.5) | 3.8 (3.3–4.2) | <0.001 |
| Total cholesterol, median mg/dL (IQR) | 197 (173–228) | 175 (145–200) | <0.001 |
| c-HDL median mg/dL (IQR) | 48 (42–58) | 45 (41–59) | 0.7 |
| c-LDL median mg/dL (IQR) | 117 (95–142) | 100 (85–130) | 0.09 |
| Triglycerides median mg/dL (IQR) | 137 (100–191) | 91 (74–117) | <0.001 |
| C Peptide, median U/L (IQR) | 2.45 (1.81–3.22) | 2.74 (2.23–2.83) | 0.6 |
| FIB-4, median (IQR) | 1.3 (0.9–2.1) | 5.5 (2.6–7.5) | <0.001 |
| Liver stiffness, median kPa (IQR) | 7.8 (5.4–12.6) | 32 (17.8–58.2) | 0.001 |
| Control attenuation parameter, median dB/m (IQR) | 319 (273–359) | 297 (250–350) | 0.3 |
| Steatosis in abdominal US, n (%) | 778 (82.8) | 8 (30.8) | <0.001 |
| Fibrosis in patients with available liver biopsy, n (%) | n = 238 | n = 11 | 0.009 |
| F0 | 29 (12.1) | 1 (9.1) | |
| F1 | 47 (19.8) | 0 | |
| F2 | 34 (14.3) | 0 | |
| F3 | 43 (18.1) | 0 | |
| F4 | 85 (35.7) | 10 (90.9) | |
| Death, n (%) | 43 (4.4) | 8 (30.8) | <0.001 |
| Causes of death | 0.005 | ||
| Liver Failure, n (%) | 12 (28%) | 1 (12%) | |
| HCC, n (%) | 0 | 2 (25%) | |
| Cardiovascular, n (%) | 6 (14%) | 0 | |
| Other neoplasms, n (%) | 8 (18%) | 4 (50%) | |
| Infectious, n (%) | 12 (27.9%) | 1 (12.5%) | |
| Other causes, n (%) | 5 (11.6) | 0 | |
| Follow-up, median years (IQR) | 2.5 (1.9–3.6) | 1.4 (0.85–2.4) | <0.001 |
| No HCC n = 207 | HCC n = 24 | p | |
|---|---|---|---|
| % over total cohort | 21.3 | 92.3 | <0.001 |
| Female, n (%) | 98 (47.3) | 2 (8.3) | <0.001 |
| Median age, years (IQR) | 65 (58–73) | 69 (60–71) | 0.3 |
| Arterial hypertension, n (%) | 145 (70) | 10 (41.6) | 0.01 |
| Dyslipidemia, n (%) | 122 (58.9) | 8 (33.3) | 0.02 |
| T2DM, n (%) | 148 (71.5) | 17 (70.8) | 0.9 |
| HbA1c, % | 6.2 (5.5–7.3) | 5.8 (5–6.5) | 0.07 |
| BMI, kg/m2 | 31.9 (28.5–35.2) | 30 (27.9–35.1) | 0.2 |
| Metabolic syndrome, n (%) | 70 (33.8) | 6 (25) | 0.3 |
| Platelets, ×109/L, median (IQR) | 139 (94–208) | 99.5 (71.5–126) | 0.01 |
| Bilirubin, median mg/dL (IQR) | 0.8 (0.6–1.1) | 1.2 (0.8–1.9) | <0.001 |
| AST, median IU/L (IQR) | 43 (30–57) | 51 (33.5–58.5) | 0.3 |
| ALT, median IU/L (IQR) | 36 (22–58) | 40 (28.5–45) | 0.7 |
| Alkaline phosphatase, median IU/L (IQR) | 103 (76–137) | 129 (98–191) | 0.02 |
| GGT, median UI/L (IQR) | 102 (55–223) | 171 (87–326) | 0.01 |
| Albumin, median UI/L (IQR) | 4.1 (3.8–4.4) | 3.7 (3.2–4.1) | 0.001 |
| FIB-4, median (IQR) | 3.3 (1.9–5.2) | 6.09 (3.1–7.8) | 0.054 |
| Steatosis in US, n (%) | 116 (56.5) | 7 (29.1) | 0.01 |
| CAP, median dB/m (IQR) | 310.5 (262–360) | 308 (242.5–363.5) | 0.8 |
| Liver stiffness, median kPa (IQR) | 27.3 (17.9) | 38.3 (22.3) | 0.031 |
| Liver stiffness > 15 kPa, n (%) | 137 (73.6%) | 11 (84.6%) | 0.3 |
| Median HVPG, mmHg (IQR) * | 10.5 (6.5–13) | 8 (8–8) | 0.5 |
| Portal hypertension signs in abdominal US, n (%) | 115 (56.1) | 18 (78.2) | 0.04 |
| Varices, n (%) ** | 75 (45.1) | 14 (60.8) | 0.18 |
| Child score, n (%) | 0.001 | ||
| A | 157 (90.8) | 15 (62.5) | |
| B | 15 (8.6) | 8 (33.3) | |
| C | 1 (0.5) | 1 (4.1) | |
| Child–Pugh score ≥6, n (%) | 37 (17.8) | 13 (54.1) | <0.001 |
| MELD | 7.7 (6.7–9.4) | 9.4 (7.5–12) | 0.004 |
| Hepatic decompensation during follow-up, n (%) | 59 (29.6) | 15 (71.4) | <0.001 |
| Ascites, n (%) | 48 (31.1) | 9 (37.5) | 0.5 |
| Hepatic encephalopathy, n (%) | 26 (16.8) | 10 (41.6) | 0.005 |
| Upper digestive bleeding, n (%) | 22 (14.2) | 5 (20.8) | 0.4 |
| Liver transplant, n (%) | 1 (0.4) | 9 (37.5) | <0.001 |
| Death | 37 (17.8) | 8 (33.3) | 0.09 |
| Median follow-up, years (IQR) | 2.2 (1.8–3.5) | 1.5 (0.8–2.7) | 0.02 |
| Total Number of HCC | 26 (100) |
|---|---|
| HCC BCLC stage, n (%) | 0: 6 (23) |
| A: 15 (57.6) | |
| B: 2 (7.6) | |
| C: 1 (3.8) | |
| D: 1 (3.8) | |
| Not classified 1 (3.8) | |
| HCC size, median (IQR) | 30 (16–35) |
| HCC type of treatment, n (%) | Curative: 21 (80.7) |
| Surgical 6 (23) | |
| TACE: 7 (27) | |
| Radiofrequency Ablation: 10 (38.4) | |
| Systemic 2 (7.6) | |
| HCC relapses, n (%) | 8 (30.7) |
| HR | 95% Confidence Interval | p | ||
|---|---|---|---|---|
| Model 1 (overall cohort) | ||||
| Type 2 diabetes mellitus | 1.51 | 0.58 | 3.89 | 0.394 |
| Body mass index (×1 kg/m2) | 0.99 | 0.91 | 1.09 | 0.930 |
| Age (×1 year) | 1.06 | 1.01 | 1.11 | 0.025 |
| FIB-4 ≥ 1.3 | 8.46 | 1.06 | 67.37 | 0.044 |
| Model 2 (cirrhotics) | ||||
| Platelets (x + 10 × 109/L) | 0.98 | 0.98 | 0.99 | 0.001 |
| Albumin (x + 1 IU/L) | 0.34 | 0.13 | 0.87 | 0.024 |
| Liver stiffness (x + 1 kPa) | 1.03 | 1.00 | 1.06 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons, M.; Rivera-Esteban, J.; Manzano, R.; Bañares, J.; Bermúdez, M.; Vargas, V.; Salcedo-Allende, M.T.; Castells, L.; Augustin, S.; Mínguez, B.; et al. Non-Invasive Tests of Liver Fibrosis Help in Predicting the Development of Hepatocellular Carcinoma among Patients with NAFLD. J. Clin. Med. 2022, 11, 2466. https://doi.org/10.3390/jcm11092466
Pons M, Rivera-Esteban J, Manzano R, Bañares J, Bermúdez M, Vargas V, Salcedo-Allende MT, Castells L, Augustin S, Mínguez B, et al. Non-Invasive Tests of Liver Fibrosis Help in Predicting the Development of Hepatocellular Carcinoma among Patients with NAFLD. Journal of Clinical Medicine. 2022; 11(9):2466. https://doi.org/10.3390/jcm11092466
Chicago/Turabian StylePons, Mònica, Jesús Rivera-Esteban, Ramiro Manzano, Juan Bañares, María Bermúdez, Víctor Vargas, Maria Teresa Salcedo-Allende, Lluís Castells, Salvador Augustin, Beatriz Mínguez, and et al. 2022. "Non-Invasive Tests of Liver Fibrosis Help in Predicting the Development of Hepatocellular Carcinoma among Patients with NAFLD" Journal of Clinical Medicine 11, no. 9: 2466. https://doi.org/10.3390/jcm11092466
APA StylePons, M., Rivera-Esteban, J., Manzano, R., Bañares, J., Bermúdez, M., Vargas, V., Salcedo-Allende, M. T., Castells, L., Augustin, S., Mínguez, B., & Pericàs, J. M. (2022). Non-Invasive Tests of Liver Fibrosis Help in Predicting the Development of Hepatocellular Carcinoma among Patients with NAFLD. Journal of Clinical Medicine, 11(9), 2466. https://doi.org/10.3390/jcm11092466







