Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis
Abstract
:1. Background
2. Materials and Methods
2.1. Literature Search Criteria
2.2. Patients and Definitions
| Study | Year | Country | Number of Eyes | Follow-Up | Design | Strength of Evidence a |
|---|---|---|---|---|---|---|
| Thebpatiphat et al. [6] | 1996 | USA | 12 | 3 months | A retrospective case series | III |
| Kane et al. [16] | 2007 | Australia | 146 | NA | A retrospective case series | III− |
| Celikkol et al. [20] | 2013 | USA | 2 | 6 weeks | A case report | III |
| Watson et al. [21] | 2009 | UK | 84 | 1–116 months (mean 33 months) | A retrospective case series | III |
| Navas et al. [22] | 2011 | Mexico | 2 | 5 years | A case report | III |
| Visser et al. [23] | 2011 | Netherlands | 3 | 6 months | A case report | III |
| Jaimes et al. [24] | 2012 | Mexico | 19 | 3–31 months (mean 7.89 months) | A retrospective case series | III |
| Nanavaty et al. [25] | 2013 | UK | 12 | mean 9 months | A retrospective case series | III |
| Parikakis et al. [26] | 2014 | Greece | 5 | 18–28 months | A case report | III |
| Alió et al. [27] | 2015 | Spain | 15 | 3–15 months (9.1 months) | A retrospective case series | III |
| Hashemi et al. [28] | 2016 | Iran | 23 | 3 months | A retrospective case series | III |
| Kamiya et al. [29] | 2019 | Japan | 19 | 3 months | A prospective study | II |
| Savini et al. [30] | 2020 | Italy | 41 | One month | A retrospective case series | III |
| Wang et al. [31] | 2020 | USA | 73 | NA | A retrospective case series | III |
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCVA | best-corrected visual acuity |
| CXL | corneal cross-linking |
| D | diopter |
| ELP | effective lens position |
| IOL | intraocular lens |
| KC | keratoconus |
References
- Reeves, S.W.; Ellwein, L.B.; Kim, T.; Lee, P.P. Keratoconus in the medicare population. Cornea 2009, 28, 40–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.B.; Nangia, V.; Matin, A.; Kulkarni, M.; Bhojwani, K. Prevalence and Associations of Keratoconus in Rural Maharashtra in Central India: The Central India Eye and Medical Study. Am. J. Ophthalmol. 2009, 148, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Heydarian, S.; Yekta, A.; Ostadimoghaddam, H.; Aghamirsalim, M.; Derakhshan, A.; Khabazkhoob, M. High prevalence and familial aggregation of keratoconus in an Iranian rural population: A population-based study. Ophthalmic Physiol. Opt. 2018, 38, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Nasser, Q.; Nucci, C.; Angunawela, R.I.; Gatzioufas, Z.; Maurino, V. Cataract Surgery in Patients with Keratoconus: Pearls and Pitfalls. Open Ophthalmol. J. 2017, 11, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaoki, A.; Kojima, T.; Hasegawa, A.; Nakamura, H.; Tanaka, K.; Ichikawa, K. Intraocular lens power calculation in cases with posterior keratoconus Presented as a poster at the ASCRS Symposium on Cataract. J. Cataract Refract. Surg. 2015, 41, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Thebpatiphat, N.; Hammersmith, K.M.; Rapuano, C.J.; Ayres, B.D.; Cohen, E.J. Cataract surgery in keratoconus. Eye Contact Lens. 2007, 33, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Leccisotti, A. Refractive lens exchange in keratoconus. J. Cataract Refract. Surg. 2006, 32, 742–746. [Google Scholar] [CrossRef]
- Aaronson, E.L.; Bates, D.W. National adverse event analysis over time: Current state and future directions. BMJ Qual. Saf. 2021, 307, 529–532. [Google Scholar] [CrossRef]
- Melles, R.B.; Holladay, J.T.; Chang, W.J. Accuracy of Intraocular Lens Calculation Formulas. Ophthalmology 2018, 125, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Fredriksson, A.; Behndig, A. Measurement centration and zone diameter in anterior, posterior and total corneal astigmatism in keratoconus. Acta Ophthalmol. 2017, 95, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Savini, G.; Næser, K.; Schiano-Lomoriello, D.; Mularoni, A. Influence of Posterior Corneal Astigmatism on Total Corneal Astigmatism in Eyes with Keratoconus. Cornea 2016, 35, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Piñero, D.P.; Camps, V.J.; Caravaca-Arens, E.; Pérez-Cambrodí, R.J.; Artola, A. Estimation of the central corneal power in keratoconus: Theoretical and clinical assessment of the error of the keratometric approach. Cornea 2014, 33, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Shimizu, K.; Igarashi, A.; Miyake, T. Assessment of Anterior, Posterior, and Total Central Corneal Astigmatism in Eyes with Keratoconus. Am. J. Ophthalmol. 2015, 160, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Whang, W.J.; Kim, H.S. Analysis of total corneal astigmatism with a rotating Scheimpflug camera in keratoconus. BMC Ophthalmol. 2020, 20, 475. [Google Scholar] [CrossRef] [PubMed]
- Ghiasian, L.; Abolfathzadeh, N.; Manafi, N.; Hadavandkhani, A. Intraocular lens power calculation in keratoconus; A review of literature. J. Curr. Ophthalmol. 2019, 31, 127–134. [Google Scholar] [CrossRef]
- Kane, J.X.; Connell, B.; Yip, H.; McAlister, J.C.; Beckingsale, P.; Snibson, G.R.; Chan, E. Accuracy of Intraocular Lens Power Formulas Modified for Patients with Keratoconus. In Ophthalmology; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 127, pp. 1037–1042. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Keaney, M.; Lorimer, A.R. Auditing the implementation of SIGN clinical guidelines. Int. J. Health Care Qual. Assur. 1999, 12, 314–317. [Google Scholar] [CrossRef]
- Krumeich, J.H.; Daniel, J.; Knalle, A. Live-epikeratophakia for keratoconus. J. Cataract Refract. Surg. 1998, 24, 456–463. [Google Scholar] [CrossRef]
- Celikkol, L.; Ahn, D.; Celikkol, G.; Feldman, S.T. Calculating intraocular lens power in eyes with keratoconus using videokeratography. J. Cataract Refract. Surg. 1996, 22, 497–500. [Google Scholar] [CrossRef]
- Watson, M.P.; Anand, S.; Bhogal, M.; Gore, D.; Moriyama, A.; Pullum, K.; Hau, S.; Tuft, S.J. Cataract surgery outcome in eyes with keratoconus. Br. J. Ophthalmol. 2014, 98, 361–364. [Google Scholar] [CrossRef]
- Navas, A.; Suárez, R. One-year follow-up of toric intraocular lens implantation in forme fruste keratoconus. J. Cataract Refract. Surg. 2009, 35, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.; Gast, S.T.J.M.; Bauer, N.J.C.; Nuijts, R.M.M.A. Cataract surgery with toric intraocular lens implantation in keratoconus: A case report. Cornea 2011, 30, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, M.; Xacur-Garciá, F.; Alvarez-Melloni, D.; Graue-Hernańdez, E.O.; Ramirez-Luquiń, T.; Navas, A. Refractive lens exchange with toric intraocular lenses in keratoconus. J. Refract. Surg. 2011, 27, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Nanavaty, M.A.; Lake, D.B.; Daya, S.M. Outcomes of pseudophakic toric intraocular lens implantation in keratoconic eyes with cataract. J. Refract. Surg. 2012, 28, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Parikakis, E.A.; Chatziralli, I.P.; Peponis, V.G.; David, G.; Chalkiadakis, S.; Mitropoulos, P.G. Toric intraocular lens implantation for correction of astigmatism in cataract patients with Corneal Ectasia. Case Rep. Ophthalmol. 2013, 4, 219–228. [Google Scholar] [CrossRef]
- Alió, J.L.; Peña-García, P.; Guliyeva, F.A.; Soria, F.A.; Zein, G.; Abu-Mustafa, S.K. MICS with toric intraocular lenses in keratoconus: Outcomes and predictability analysis of postoperative refraction. Br. J. Ophthalmol. 2014, 98, 365–370. [Google Scholar] [CrossRef]
- Hashemi, H.; Heidarian, S.; Seyedian, M.A.; Yekta, A.; Khabazkhoob, M. Evaluation of the Results of using toric IOL in the cataract surgery of keratoconus patients. Eye Contact Lens. 2015, 41, 354–358. [Google Scholar] [CrossRef]
- Kamiya, K.; Shimizu, K.; Miyake, T. Changes in astigmatism and corneal higher-order aberrations after phacoemulsification with toric intraocular lens implantation for mild keratoconus with cataract. Jpn. J. Ophthalmol. 2016, 60, 302–308. [Google Scholar] [CrossRef]
- Savini, G.; Abbate, R.; Hoffer, K.J.; Mularoni, A.; Imburgia, A.; Avoni, L.; D’Eliseo, D.; Schiano-Lomoriello, D. Intraocular lens power calculation in eyes with keratoconus. J. Cataract Refract. Surg. 2019, 45, 576–581. [Google Scholar] [CrossRef]
- Wang, K.M.; Jun, A.S.; Ladas, J.G.; Siddiqui, A.A.; Woreta, F.; Srikumaran, D. Accuracy of Intraocular Lens Formulas in Eyes With Keratoconus. Am. J. Ophthalmol. 2020, 212, 26–33. [Google Scholar] [CrossRef]
- Xia, T.; Martinez, C.E.; Tsai, L.M. Update on Intraocular Lens Formulas and Calculations. Asia Pac. J. Ophthalmol. 2020, 9, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.X.; Van Heerden, A.; Atik, A.; Petsoglou, C. Intraocular lens power formula accuracy: Comparison of 7 formulas. J. Cataract Refract. Surg. 2016, 42, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Hoffer, K.J.; Balducci, N.; Barboni, P.; Schiano-Lomoriello, D. Comparison of formula accuracy for intraocular lens power calculation based on measurements by a swept-source optical coherence tomography optical biometer. J. Cataract Refract. Surg. 2020, 46, 27–33. [Google Scholar]
- Cooke, D.L.; Cooke, T.L. Comparison of 9 intraocular lens power calculation formulas. J. Cataract Refract. Surg. 2016, 42, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Shajari, M.; Kolb, C.M.; Petermann, K.; Böhm, M.; Herzog, M.; de’Lorenzo, N.; Schönbrunn, S.; Kohnen, T. Comparison of 9 modern intraocular lens power calculation formulas for a quadrifocal intraocular lens. J. Cataract Refract. Surg. 2018, 44, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Samra, W.A.A.; Awad, E.A.; El Kannishy, A.H. Objective and subjective outcome of clear lensectomy with toric IOL implantation after corneal collagen cross-linking in selected cases of keratoconus. Eye Contact Lens. 2016, 44, S87–S91. [Google Scholar] [CrossRef]
- Moshirfar, M.; Walker, B.D.; Birdsong, O.C. Cataract surgery in eyes with keratoconus: A review of the current literature. Curr. Opin. Ophthalmol. 2018, 29, 75–80. [Google Scholar] [CrossRef]
- Connell, B.J.; Kane, J.X. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019, 4, e000251. [Google Scholar] [CrossRef]
- Barrett, G.D. An improved universal theoretical formula for intraocular lens power prediction. J. Cataract Refract. Surg. 1993, 19, 713–720. [Google Scholar] [CrossRef]
- IOL Power Calculations Which Formula? Available online: https://doctor-hill.com/ (accessed on 13 April 2022).
- Hoffer, K.J. The Hoffer Q formula: A comparison of theoretic and regression formulas. J. Cataract Refract. Surg. 1993, 19, 700–712. [Google Scholar] [CrossRef]
- Holladay, J.T.; Musgrove, K.H.; Prager, T.C.; Lewis, J.W.; Chandler, T.Y.; Ruiz, R.S. A three-part system for refining intraocular lens power calculations. J. Cataract Refract. Surg. 1988, 14, 17–24. [Google Scholar] [CrossRef]
- Németh, J.; Fekete, O.; Pesztenlehrer, N. Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J. Cataract Refract. Surg. 2003, 29, 85–88. [Google Scholar] [CrossRef]
- Retzlaff, J.A.; Sanders, D.R.; Kraff, M.C. Development of the SRK/T intraocular lens implant power calculation formula. J. Cataract Refract. Surg. 1990, 16, 333–340. [Google Scholar] [CrossRef]
- Ryu, S.; Jun, I.; Kim, T.I.; Kim, E.K.; Seo, K.Y. Accuracy of the Kane Formula for Intraocular Lens Power Calculation in Comparison with Existing Formulas: A Retrospective Review. Yonsei Med. J. 2021, 62, 1117. [Google Scholar] [CrossRef]
| Author | No. of Eyes | Year | Formula | Absolute Prediction Error SE (D) | Mean Prediction Error SE (D) | N/(%) of Eyes within 0.5 D | N/(%) of Eyes within 1 D |
|---|---|---|---|---|---|---|---|
| Non-toric IOLs | |||||||
| Celikkol et al. [20] | 1 | 1996 | SRK/T | 1.00 | −1.00 | 0 | 1 (100) |
| Thebpatiphat et al. [6] | 5 | 2007 | SRK II | NA | +1.04 | NA | NA |
| 4 | SRK | NA | +1.42 | NA | NA | ||
| 3 | SRK/T | NA | +1.62 | NA | NA | ||
| Watson et al. [21] | 35 | 2013 | SRK/T | NA | −1.1 | NA | NA |
| Toric IOLs | |||||||
| Navas et al. [22] | 2 | 2009 | SRK II | 0.5 | −0.5 | 2 (100) | 2 (100) |
| Visser et al. [23] | 3 | 2011 | SRK/T | 1.54 | −1.54 | 1 (33) | 2 (67) |
| Jaimes et al. [24] | 15 | 2011 | SRK II | 0.61 | −0.45 | 7 (47) | 14 (93) |
| Nanavaty et al. [25] | 8 | 2012 | Z calc | 0.25 | +0.12 | 7 (88) | 7 (88) |
| Parikakis et al. [26] | 5 | 2013 | SRK/T | 0.95 | −0.95 | 1 (20) | 3 (60) |
| Alió e al. [27] | 6 | 2014 | SRK/T | 0.25 | +0.08 | 6 (100) | 6 (100) |
| 7 | Hoffer Q | 0.93 | −0.86 | 4 (57) | 4 (57) | ||
| Hashemi et al. [28] | 10 | 2015 | SRK/T | 0.81 | NA | NA | NA |
| Holladay I | 0.89 | NA | NA | NA | |||
| SRK II | 0.96 | NA | NA | NA | |||
| Hoffer Q | 1.01 | NA | NA | NA | |||
| Kamiya et al. [29] | 14 | 2016 | SRK/T | 1.63 | −1.63 | 10 (71) | 14 (100) |
| Unknown separation between non-toric and toric IOLs | |||||||
| Savini et al. [30] | 21 | 2019 | SRK/T | 0.43 * | +0.44 | 13 (62) | 17 (81) |
| Haigis | 0.61 * | +0.54 | 8 (38) | 17 (81) | |||
| Barrett | 0.70 * | +0.63 | 9 (43) | 16 (76) | |||
| Holladay I | 0.55 * | +0.75 | 9 (43) | 17 (81) | |||
| Hoffer Q | 0.91 * | +0.90 | 5 (24) | 13 (62) | |||
| Kane et al. [16] | 84 | 2020 | Kane (K*) | 0.49 | −0.18 | 51 (61) | 76 (91) |
| Kane | 0.49 | −0.18 | 51 (61) | 76 (91) | |||
| Barrett | 0.54 | −0.25 | 45 (54) | 75 (89) | |||
| SRK/T | 0.56 | −0.23 | 44 (52) | 74 (88) | |||
| Holladay I | 0.56 | −0.18 | 44 (52) | 72 (86) | |||
| Hoffer | 0.57 | −0.19 | 44 (52) | 72 (86) | |||
| Haigis | 0.58 | −0.26 | 43 (51) | 72 (86) | |||
| Holladay II | 0.62 | −0.38 | 39 (46) | 69 (82) | |||
| Holladay II (K*) | 0.64 | −0.36 | 32 (38) | 68 (81) | |||
| Wang et al. [31] | 46 | 2020 | Haigis | 0.58 * | +0.10 | 18 (39) | 32 (70) |
| SRK/T | 0.62 * | +0.12 | 22 (48) | 33 (72) | |||
| Holladay I | 0.65 * | +0.38 | 18 (39) | 34 (74) | |||
| Barrett | 0.45 * | +0.39 | 24 (52) | 35 (76) | |||
| Holladay II | 0.58 * | +0.56 | 18 (39) | 33 (73) | |||
| Hoffer Q | 0.57 * | +0.65 | 19 (41) | 31 (67) | |||
| Author | No. of Eyes | Year | Formula | Absolute Prediction Error SE (D) | Mean Prediction Error SE (D) | N/(%) of Eyes Within 0.5 D | N/(%) of Eyes Within 1 D |
|---|---|---|---|---|---|---|---|
| Non-toric IOLs | |||||||
| Celikkol et al. [20] | 1 | 1996 | SRK/T | 5.6 | −5.6 | 0 | 0 |
| Watson et al. [21] | 40 | 2013 | SRK/T | NA | −0.6 | NA | NA |
| Toric IOLs | |||||||
| Jaimes et al. [24] | 4 | 2011 | SRK II | 1.84 | −0.46 | 0 | 2 (50) |
| Nanavaty et al. [25] | 4 | 2012 | Z calc | 0.4 | +0.03 | 2 (50) | 4 (100) |
| Alió e al. [27] | 1 | 2014 | SRK/T | 1.25 | −1.25 | 0 | 0 |
| 1 | Hoffer Q | 2.50 | −2.50 | 0 | 0 | ||
| Hashemi et al. [28] | 10 | 2015 | SRK/T | 0.69 | NA | NA | NA |
| Holladay I | 0.78 | NA | NA | NA | |||
| Hoffer Q | 0.80 | NA | NA | NA | |||
| SRK II | 0.84 | NA | NA | NA | |||
| Kamiya et al. [29] | 5 | 2016 | SRK/T | 1.9 | −1.5 | 3 (60) | 4 (80) |
| Unknown separation between non-toric and toric IOLs | |||||||
| Savini et al. [30] | 13 | 2019 | SRK/T | 0.79 * | +0.54 | 4 (31) | 7 (54) |
| Barrett | 1.42 * | +1.32 | 2 (15) | 3 (23) | |||
| Holladay I | 1.23 * | +1.54 | 3 (24) | 5 (38) | |||
| Hoffer Q | 1.55 * | +1.63 | 1 (8) | 4 (31) | |||
| Haigis | 1.57 * | +1.66 | 2 (15) | 2 (15) | |||
| Kane et al. [16] | 37 | 2020 | Kane (K*) | 1.08 | +0.53 | 16 (43) | 21 (57) |
| SRK/T | 1.13 | +0.51 | 11 (30) | 18 (49) | |||
| Barrett | 1.21 | +0.89 | 14 (38) | 20 (54) | |||
| Kane | 1.23 | +1.00 | 14 (38) | 19 (51) | |||
| Holladay II | 1.27 | +1.05 | 13 (35) | 19 (51) | |||
| Holladay I | 1.31 | +1.12 | 14 (38) | 19 (51) | |||
| Haigis | 1.54 | +1.34 | 6 (16) | 16 (43) | |||
| Hoffer Q | 1.58 | +1.47 | 6 (16) | 18 (49) | |||
| Holladay II (K*) | 1.59 | +1.41 | 6 (16) | 15 (41) | |||
| Wang et al. [31] | 22 | 2020 | SRK/T | 0.99 * | +0.36 | 4 (18) | 11 (50) |
| Barrett | 0.45 * | +0.95 | 11 (50) | 13 (59) | |||
| Haigis | 1.45 * | +1.12 | 5 (23) | 8 (36) | |||
| Holladay I | 1.00 * | +1.21 | 9 (41) | 11 (50) | |||
| Holladay II | 1.19 * | +1.49 | 5 (23) | 10 (46) | |||
| Hoffer Q | 1.01 * | +1.70 | 4 (18) | 11 (50) | |||
| Author | No. of Eyes | Year | Formula | Absolute Prediction Error SE (D) | Mean Prediction Error SE (D) | N/(%) of Eyes Within 0.5 D | N/(%) of Eyes Within 1 D |
|---|---|---|---|---|---|---|---|
| Non-toric IOLs | |||||||
| Watson et al. [21] | 9 | 2013 | SRK/T | NA | −0.6 | NA | NA |
| Toric IOLs | |||||||
| Hashemi et al. [28] | 3 | 2015 | SRK/T | 0.39 | NA | NA | NA |
| SRK II | 0.40 | NA | NA | NA | |||
| Holladay I | 1.14 | NA | NA | NA | |||
| Hoffer Q | 1.30 | NA | NA | NA | |||
| Unknown separation between non-toric and toric IOLs | |||||||
| Savini et al. [30] | 7 | 2019 | Barrett | 2.65 * | +2.64 | 0 | 1 (14) |
| SRK/T | 3.99 * | +3.01 | 1 (14) | 1 (14) | |||
| Haigis | 2.75 * | +3.26 | 0 | 1 (14) | |||
| Hoffer Q | 4.04 * | +3.46 | 1 (14) | 1 (14) | |||
| Holladay I | 4.09 * | +3.77 | 0 | 0 | |||
| Kane et al. [16] | 25 | 2020 | Kane (K*) | 1.44 | +0.02 | 6 (24) | 12 (48) |
| SRK/T | 2.32 | +1.86 | 3 (12) | 5 (20) | |||
| Barrett | 2.45 | +1.72 | 1 (4) | 4 (16) | |||
| Kane | 2.64 | +2.22 | 1 (4) | 3 (12) | |||
| Haigis | 2.88 | +2.43 | 1 (4) | 4 (16) | |||
| Holladay II | 3.01 | +2.62 | 2 (8) | 3 (12) | |||
| Holladay I | 3.07 | +2.43 | 1 (4) | 3 (12) | |||
| Holladay II (K*) | 3.19 | +2.88 | 2 (8) | 4 (16) | |||
| Hoffer Q | 3.36 | +3.02 | 0 | 3 (12) | |||
| Wang et al. [31] | 5 | 2020 | Haigis | 1.18 * | +1.90 | 2 (40) | 2 (40) |
| SRK/T | 1.74 * | +2.51 | 0 | 0 | |||
| Holladay I | 1.96 * | +2.99 | 0 | 0 | |||
| Hoffer Q | 4.22 * | +4.00 | 0 | 0 | |||
| Holladay II | 4.52 * | +4.38 | 0 | 0 | |||
| Barrett | NA | NA | NA | NA | |||
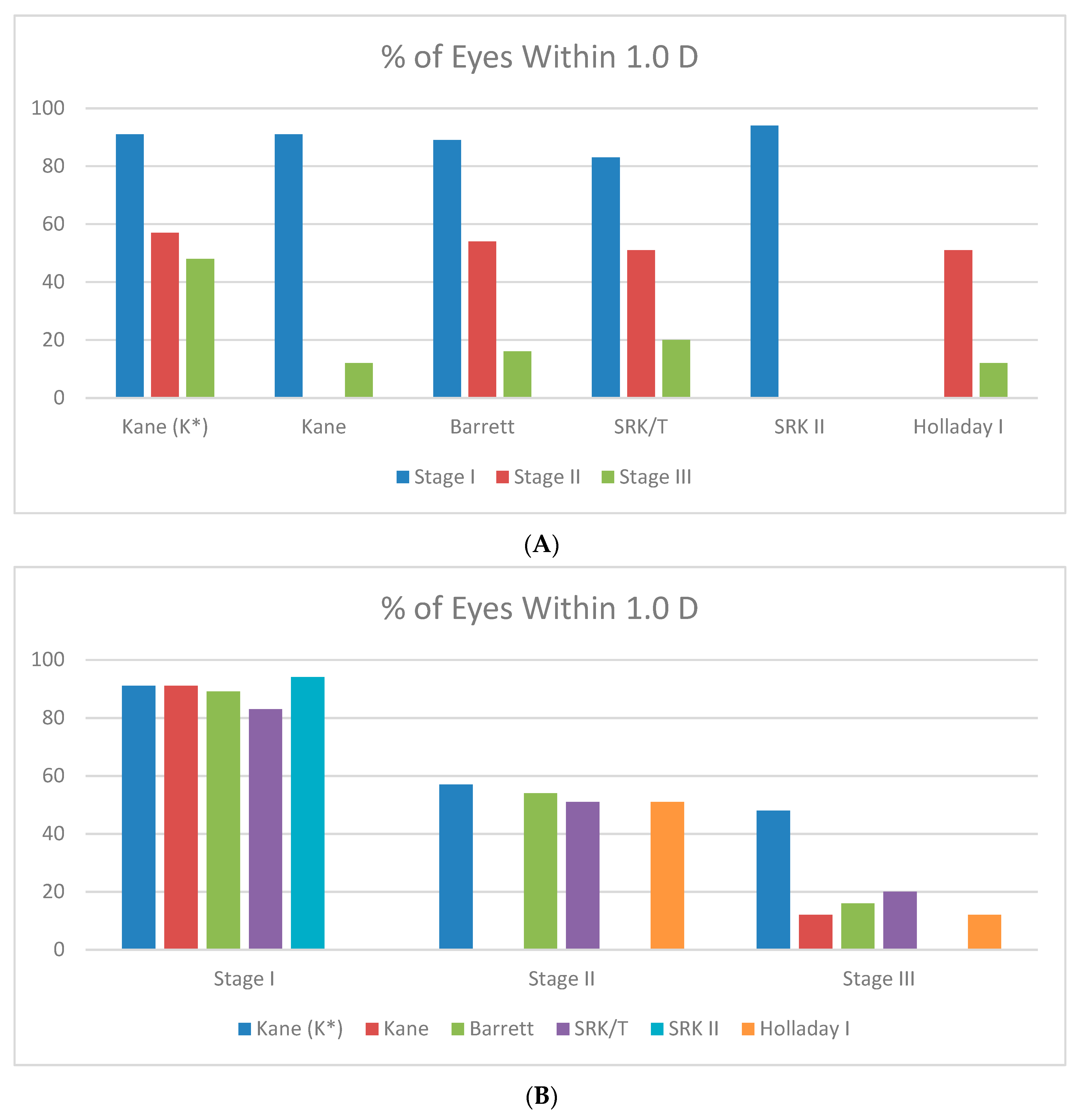
| Formula | Absolute Prediction Error SE (D) | % of Eyes Within 0.5 D | % of Eyes Within 1 D | |
|---|---|---|---|---|
| Stage I | ||||
| Kane (K*) | 0.49 | 61 | 91 | |
| Kane | 0.49 | 61 | 91 | |
| Barrett | 0.54 | 54 | 89 | |
| SRK/T | 0.73 | 54 | 83 | |
| SRK II | 0.73 | 53 | 94 | |
| Stage II | ||||
| Kane (K*) | 1.08 | 43 | 57 | |
| SRK II | 1.13 | NA | NA | |
| Holladay I | 1.20 | 38 | 51 | |
| SRK/T | 1.20 | 28 | 51 | |
| Barrett | 1.21 | 38 | 54 | |
| Stage III | ||||
| Kane (K*) | 1.44 | 24 | 48 | |
| SRK/T | 2.11 | 12 | 20 | |
| Barrett | 2.45 | 4 | 16 | |
| Kane | 2.64 | 4 | 12 | |
| Holladay I | 2.86 | 4 | 12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahalomi, T.; Achiron, A.; Hecht, I.; Arnon, R.; Levinger, E.; Pikkel, J.; Tuuminen, R. Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2456. https://doi.org/10.3390/jcm11092456
Yahalomi T, Achiron A, Hecht I, Arnon R, Levinger E, Pikkel J, Tuuminen R. Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(9):2456. https://doi.org/10.3390/jcm11092456
Chicago/Turabian StyleYahalomi, Tal, Asaf Achiron, Idan Hecht, Roee Arnon, Eliya Levinger, Joseph Pikkel, and Raimo Tuuminen. 2022. "Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 9: 2456. https://doi.org/10.3390/jcm11092456
APA StyleYahalomi, T., Achiron, A., Hecht, I., Arnon, R., Levinger, E., Pikkel, J., & Tuuminen, R. (2022). Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(9), 2456. https://doi.org/10.3390/jcm11092456






