Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Variables
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
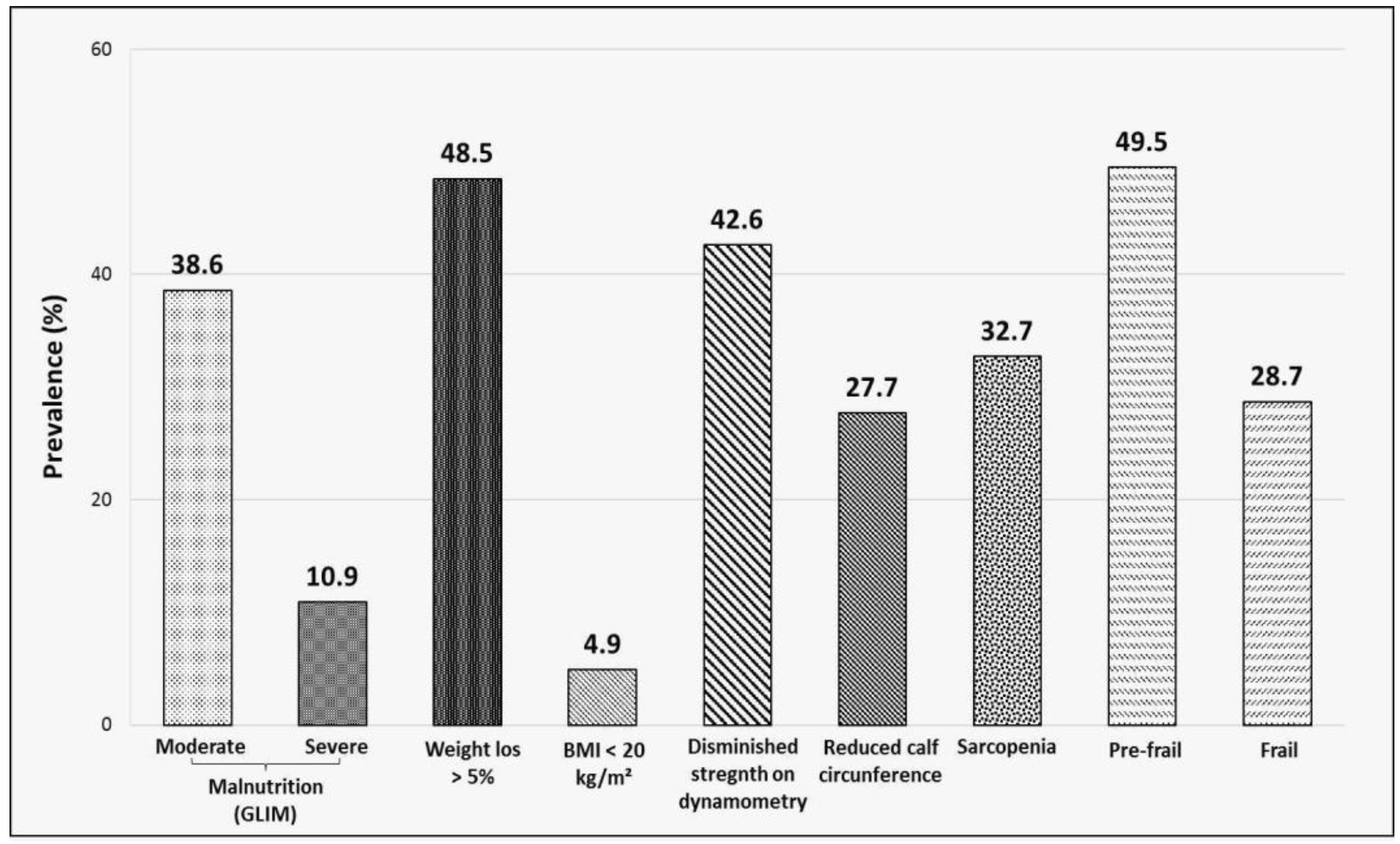
3.2. Prevalence of Malnutrition, Sarcopenia and Frailty
3.3. Determinants of Malnutrition, Sarcopenia and Frailty
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Gozalo, P.L.; Pop-Vicas, A.; Feng, Z.; Gravenstein, S.; Mor, V. Effect of Influenza on Functional Decline. J. Am. Geriatr. Soc. 2012, 60, 1260–1267. [Google Scholar] [CrossRef] [Green Version]
- Bartley, J.M.; Pan, S.J.; Keilich, S.; Hopkins, J.W.; Al-Naggar, I.M.; Kuchel, G.; Haynes, L. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging 2016, 8, 620–635. [Google Scholar] [CrossRef] [Green Version]
- Simpson, R.; Robinson, L. Rehabilitation After Critical Illness in People With COVID-19 Infection. Am. J. Phys. Med. Rehabil. 2020, 99, 470–474. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Ali, Z.; E Armitage, A.; Bonell, A.; Cerami, C.; Drakesmith, H.; Jobe, M.; Jones, K.S.; Liew, Z.; Moore, S.; et al. The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review. J. Nutr. 2021, 151, 1854–1878. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Li, Y.; Zhang, Y.; Zhong, M.; Meng, X. Evaluation of the nutritional status in patients with COVID-19. J. Clin. Biochem. Nutr. 2020, 67, 116–121. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S.; Ravaioli, F.; Baracco, B.; Battaiola, C.; Bocedi, G.; Brodosi, L.; Leoni, L.; Mari, G.A.; Musio, A. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin. Nutr. 2021, 40, 1330–1337. [Google Scholar] [CrossRef]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Czapla, M.; Juárez-Vela, R.; Gea-Caballero, V.; Zieliński, S.; Zielińska, M. The Association between Nutritional Status and In-Hospital Mortality of COVID-19 in Critically-Ill Patients in the ICU. Nutrients 2021, 13, 3302. [Google Scholar] [CrossRef]
- Shahbazi, S.; Hajimohammadebrahim-Ketabforoush, M.; Shariatpanahi, M.V.; Shahbazi, E.; Shariatpanahi, Z.V. The validity of the global leadership initiative on malnutrition criteria for diagnosing malnutrition in critically ill patients with COVID-19: A prospective cohort study. Clin. Nutr. ESPEN 2021, 43, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riesgo, H.; Castro, A.; del Amo, S.; Ceferino, M.J.S.; Izaola, O.; Primo, D.; Hoyos, E.G.; Gómez, J.J.L.; de Luis, D.A. Prevalence of Risk of Malnutrition and Risk of Sarcopenia in a Reference Hospital for COVID-19: Relationship with Mortality. Ann. Nutr. Metab. 2021, 77, 324–329. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Hou, L.; Yang, X.; Huang, Z.; Yang, X.; Zhao, N.; He, M.; Shi, Y.; Kang, Y.; Yue, J.; et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: A prospective cohort study. BMC Med. 2020, 18, 274. [Google Scholar] [CrossRef]
- Aw, D.; Woodrow, L.; Ogliari, G.; Harwood, R. Association of frailty with mortality in older inpatients with COVID-19: A cohort study. Age Ageing 2020, 49, 915–922. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index: A simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md State Med. J. 1965, 14, 61–65. [Google Scholar]
- Fine, M.J.; Auble, T.E.; Yealy, D.M.; Hanusa, B.H.; Weissfeld, L.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Kapoor, W.N. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. N. Engl. J. Med. 1997, 336, 243–250. [Google Scholar] [CrossRef]
- Lim, W.S. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003, 58, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torralvo, F.J.S.; Porras, N.; Fernández, J.A.; Torres, F.G.; Tapia, M.J.; Lima, F.; Soriguer, F.; Gonzalo, M.; Martínez, G.R.; Olveira, G. Normative reference values for hand grip dynamometry in Spain. Association with lean mass. Nutr. Hosp. 2018, 35, 98–103. [Google Scholar]
- Cuervo, M.; Ansorena, D.; García, A.; González Martínez, M.A.; Astiasarán, I.; Martínez, J.A. Assessment of calf circumference as an indicator of the risk for hyponutrition in the elderly. Nutr. Hosp. 2009, 24, 63–67. [Google Scholar] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, G.A.; Rolland, Y.M.; Morley, J.E.; Vellas, B. Frailty: Toward a Clinical Definition. J. Am. Med. Dir. Assoc. 2008, 9, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Bedock, D.; Lassen, P.B.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Rouget, A.; Vardon-Bounes, F.; Lorber, P.; Vavasseur, A.; Marion, O.; Marcheix, B.; Lairez, O.; Balardy, L.; Fourcade, O.; Conil, J.-M.; et al. Prevalence of malnutrition in coronavirus disease 19: The NUTRICOV study. Br. J. Nutr. 2021, 126, 1296–1303. [Google Scholar] [CrossRef]
- Larrazabal, R.B.; Perez, B.M.B.; Masamayor, E.M.I.; Chiu, H.H.C.; Palileo-Villanueva, L.A.M. The prevalence of malnutrition and analysis of related factors among adult patients with the Coronavirus Disease 2019 (COVID 19) in a tertiary government hospital: The MalnutriCoV study. Clin. Nutr. ESPEN 2021, 42, 98–104. [Google Scholar] [CrossRef]
- Wall, B.T.; van Loon, L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; Kruizenga, H.M.; Konings, L.A.; Krebbers, D.; Jorissen, J.R.; Joosten, M.-H.I.; van Aken, L.H.; Tan, F.M.; van Bodegraven, A.A.; Soeters, M.R.; et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin. Nutr. ESPEN 2021, 43, 369–376. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, J.; Wang, Y.; Hou, H.; Feng, H.; Yang, H. An updated meta-analysis on the relationship between obesity and COVID-19 mortality. Metabolism 2021, 122, 154820. [Google Scholar] [CrossRef] [PubMed]
- Cava, E.; Yeat, N.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. Int. Rev. J. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Total (n = 101) n (% col) | Malnutrition (n = 50) n (% row) | p * | Sarcopenia (n = 33) n (% row) | p * | Frailty (n = 29) n (% row) | p * |
|---|---|---|---|---|---|---|---|
| Age in years, mean 66.3 (95% CI 50.8–81.8) | |||||||
| ≤60 years | 38 (37.6) | 12 (31.6) | 0.014 | 6 (15.8) | <0.001 | 4 (10.5) | <0.001 |
| 61–74 years | 32 (31.7) | 21 (65.7) | 8 (25.0) | 7 (21.9) | |||
| ≥75 years | 31 (30.7) | 17 (54.8) | 19 (61.3) | 18 (58.1) | |||
| Sex | |||||||
| Male | 68 (67.3) | 35 (51.5) | 0.57 | 20 (29.4) | 0.32 | 17 (25) | 0.236 |
| Female | 33 (32.7) | 15 (45.5) | 13 (39.4) | 12 (36.4) | |||
| Comorbidities | |||||||
| Hypertension | 59 (58.4) | 27 (45.8) | 0.37 | 23 (39) | 0.11 | 22 (37.3) | 0.024 |
| Dyslipidemia | 35 (35.6) | 20 (55.6) | 0.37 | 15 (41.7) | 0.15 | 13 (36.1) | 0.22 |
| Obesity | 31 (30.7) | 11 (35.5) | 0.061 | 6 (19.4) | 0.058 | 9 (36.1) | 0.96 |
| Diabetes mellitus | 20 (19.8) | 12 (60) | 0.30 | 10 (50) | 0.065 | 11 (55) | 0.004 |
| COPD | 15 (14.9) | 9 (60) | 0.38 | 6 (40) | 0.51 | 7 (46.7) | 0.096 |
| Chronic kidney disease | 10 (9.9) | 6 (60) | 0.48 | 6 (60) | 0.052 | 6 (60) | 0.021 |
| Heart failure | 10 (9.9) | 7 (70) | 0.17 | 6 (60) | 0.052 | 6 (60) | 0.021 |
| Asthma | 6 (5.9) | 3 (50) | 0.98 | 1 (16.7) | 0.39 | 1 (16.7) | 0.30 |
| Immunocompromised | 4 (3.9) | 1 (25) | 0.32 | 0 | 0.16 | 0 | 0.20 |
| Liver disease | 3 (2.9) | 1 (33) | 0.57 | 1 (33) | 0.98 | 1 (33) | 0.86 |
| HIV | 2 (1.9) | 0 | 0.37 | 0 | 0.28 | 1 (50) | 0.24 |
| Barthel index, median 100 points (IQR 87.5–100) | |||||||
| ≤60 points (dependent) | 21 (20.8) | 14 (66.7) | 0.077 | 18 (85.7) | <0.001 | 17 (81.0) | <0.001 |
| >60 points (autonomous) | 80 (79.2) | 36 (45.0) | 15 (18.7) | 12 (15.0) | |||
| Length of hospital stay, median 16 days (IQR 8–26.5) | |||||||
| <30 days | 83 (82.2) | 35 (42.2) | 0.002 | 25 (30.1) | 0.12 | 20 (24.1) | 0.028 |
| ≥30 days | 18 (17.2) | 15 (83.3) | 8 (44.4) | 9 (50) | |||
| Admission to ICU | |||||||
| Yes | 25 (24.8) | 18 (72.0) | 0.010 | 8 (32.0) | 0.93 | 7 (28.0) | 0.33 |
| No | 76 (75.2) | 32 (42.1) | 25 (32.9) | 22 (28.9) | |||
| FINE-Pneumonia Severity Index † on admission | |||||||
| I | 11 (10.9) | 3 (27.3) | 0.042 | 0 (0) | 0.004 | 0 (0) | 0.010 |
| II | 32 (31.7) | 13 (40.6) | 6 (18.8) | 5 (15.6) | |||
| III | 29 (28.7) | 13 (44.8) | 11 (37.9) | 8 (27.6) | |||
| IV | 23 (22.8) | 16 (69.6) | 13 (56.5) | 13 (56.5) | |||
| V | 6 (5.9) | 5 (83.3) | 3 (50.0) | 3 (50.0) | |||
| CURB-65 ‡ pneumonia severity score on admission (points) | |||||||
| 0 | 29 (28.7) | 7 (24.1) | 0.002 | 3 (10.3) | 0.001 | 2 (6.9) | 0.001 |
| 1 | 36 (35.6) | 17 (47.2) | 10 (27.8) | 9 (25.0) | |||
| 2 | 28 (27.2) | 20 (71.4) | 14 50.0) | 13 (46.4) | |||
| 3 | 8 (7.9) | 6 (75.0) | 6 (66.7) | 5 (62.5) | |||
| ARDS on admission | |||||||
| Yes | 39 (38.6) | 24 (61.5) | 0.055 | 10 (25.6) | 0.196 | 11 (28.2) | 0.59 |
| No | 62 (61.4) | 26 (41.9) | 23 (37.1) | 18 (29.5) | |||
| Malnutrition GLIM (n = 50) n (%) | Sarcopenia SARC-F (n = 33) n (%) | Frailty FRAIL (n = 29) n (%) | |
|---|---|---|---|
| Malnutrition GLIM | 24 (72.7) | 21 (72.4) | |
| Moderate | - | 17 (51.5) | 13 (44.8) |
| Severe | - | 7 (21.2) | 8 (27.6) |
| Weight loss > 5% | - | 18 (54.5) | 14 (48.3) |
| BMI < 20 kg/m2 | - | 3 (9.1) | 2 (6.9) |
| Sarcopenia | |||
| SARC-F | 24 (48.0) | - | 23 (79.3) |
| Diminished strength on dynamometry | 26 (52.0) | 27 (81.8) | 24 (82.6) |
| Reduced calf circumference | 26 (52.0) | 20 (60.6) | 19 (65.5) |
| Frailty (FRAIL) | |||
| Pre-frail | 26 (52.0) | 10 (30.3) | - |
| Frail | 21 (42.0) | 23 (69.7) | - |
| Variable | Malnutrition | Sarcopenia | Frailty | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.25 (0.52–3.03) | 0.62 | 1.21 (0.48–3.06) | 0.63 | 2.37 (0.76–7.36) | 0.17 |
| Sex | * | * | * | |||
| Hypertension | * | * | 1.74 (0.30–9.97) | 0.53 | ||
| Dyslipidemia | * | * | * | |||
| Obesity | 0.38 (0.13–1.06) | 0.064 | 0.20 (0.04–0.90) | 0.036 | * | |
| Diabetes | * | 1.65 (0.34–8.09) | 0.54 | 1.30 (0.24–7.05) | 0.76 | |
| COPD | * | * | 0.34 (0.06–1.88) | 0.22 | ||
| Chronic kidney disease | * | * | 0.19 (0.01–2.73) | 0.22 | ||
| Heart failure | * | 2.82 (0.40–20.00) | 0.30 | 1.53 (0.11–22.29) | 0.76 | |
| Asthma | * | * | * | |||
| Immunocompromised | * | * | * | |||
| Liver disease | * | * | * | |||
| HIV | * | * | * | |||
| Barthel index ≤ 60 points | 1.90 (0.47–7.72) | 0.37 | 29.52 (4.51–193.16) | <0.001 | 32.27 (4.53–229.93) | 0.001 |
| Length of stay ≥ 30 days | 3.27 (0.53–20.34) | 0.20 | * | 9.11 (1.80–46.02) | 0.008 | |
| ICU admission | 5.23 (0.71–38.61) | 0.11 | * | * | ||
| FINE-PSI on admission | 0.48 (0.03–8.22) | 0.61 | 1.45 (0.15–14.38) | 0.751 | 0.85 (0.51–14.01) | >0.99 |
| CURB-65 on admission | 2.61 (1.06–6.41) | 0.036 | * | 0.62 (0.19–2.04) | 0.43 | |
| ARDS on admission | 1.25 (0.52–3.03) | 0.62 | 1.13 (0.43–2.97) | 0.069 | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Uranga, A.; Guzmán-Martínez, J.; Esteve-Atiénzar, P.J.; Wikman-Jorgensen, P.; Núñez-Cruz, J.M.; Espinosa-del-Barrio, L.; Hernández-Isasi, I.; Pomares-Gómez, F.J.; Perelló-Camacho, E.; Fernández-García, N.; et al. Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients. J. Clin. Med. 2022, 11, 2424. https://doi.org/10.3390/jcm11092424
Gómez-Uranga A, Guzmán-Martínez J, Esteve-Atiénzar PJ, Wikman-Jorgensen P, Núñez-Cruz JM, Espinosa-del-Barrio L, Hernández-Isasi I, Pomares-Gómez FJ, Perelló-Camacho E, Fernández-García N, et al. Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients. Journal of Clinical Medicine. 2022; 11(9):2424. https://doi.org/10.3390/jcm11092424
Chicago/Turabian StyleGómez-Uranga, Angie, Javier Guzmán-Martínez, Pedro Jesús Esteve-Atiénzar, Philip Wikman-Jorgensen, Juan Manuel Núñez-Cruz, Leticia Espinosa-del-Barrio, Isidro Hernández-Isasi, Francisco J. Pomares-Gómez, Eva Perelló-Camacho, Nuria Fernández-García, and et al. 2022. "Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients" Journal of Clinical Medicine 11, no. 9: 2424. https://doi.org/10.3390/jcm11092424
APA StyleGómez-Uranga, A., Guzmán-Martínez, J., Esteve-Atiénzar, P. J., Wikman-Jorgensen, P., Núñez-Cruz, J. M., Espinosa-del-Barrio, L., Hernández-Isasi, I., Pomares-Gómez, F. J., Perelló-Camacho, E., Fernández-García, N., Sánchez-Miralles, Á., & Giner-Galvañ, V. (2022). Nutritional and Functional Impact of Acute SARS-CoV-2 Infection in Hospitalized Patients. Journal of Clinical Medicine, 11(9), 2424. https://doi.org/10.3390/jcm11092424





