Mid-Term Mortality in Older Anemic Patients with Type 2 Myocardial Infarction: Does Blood Transfusion sImprove Prognosis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Patients
2.4. Data Collection
2.5. Biological Data
2.6. Statistical Analysis
2.6.1. Missing Values
2.6.2. Description of Covariates
2.6.3. Propensity Score
3. Results
3.1. Population
3.2. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Lanier, J.B.; Park, J.J.; Callahan, R.C. Anemia in Older Adults. Am. Fam. Physician 2018, 98, 437–442. [Google Scholar] [PubMed]
- Putot, A.; Jeanmichel, M.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Type 2 Myocardial Infarction: A Geriatric Population-based Model of Pathogenesis. Aging Dis. 2020, 11, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curcio, F.; Gerundo, G.; Sasso, G.; Panicara, V.; Liguori, I.; Testa, G.; Della-Morte, D.; Gargiulo, G.; Galizia, G.; Ungar, A.; et al. Type 2 myocardial infarction: Is it a geriatric syndrome? Aging Clin. Exp. Res. 2020, 32, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, X.; Du, R.; Chen, Y.; Zhang, Q. Impact of red blood cell transfusion on acute coronary syndrome: A meta-analysis. Intern. Emerg. Med. 2018, 13, 231–241. [Google Scholar] [CrossRef]
- Mueller, M.M.; Van Remoortel, H.; Meybohm, P.; Aranko, K.; Aubron, C.; Burger, R. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA 2019, 321, 983. [Google Scholar] [CrossRef]
- Ducrocq, G.; Gonzalez-Juanatey, J.R.; Puymirat, E.; Lemesle, G.; Cachanado, M.; Durand-Zaleski, I. Effect of a Restrictive vs Liberal Blood Transfusion Strategy on Major Cardiovascular Events Among Patients With Acute Myocardial Infarction and Anemia: The REALITY Randomized Clinical Trial. JAMA 2021, 325, 552–560. [Google Scholar] [CrossRef]
- Putot, A.; Zeller, M.; Perrin, S.; Beer, J.-C.; Ravisy, J.; Guenancia, C.; Robert, R.; Manckoundia, P.; Cottin, Y. Blood Transfusion in Elderly Patients with Acute Myocardial Infarction: Data from the RICO Survey. Am. J. Med. 2018, 131, 422–429.e4. [Google Scholar] [CrossRef]
- Zeller, M.; Steg, P.G.; Ravisy, J.; Lorgis, L.; Laurent, Y.; Sicard, P.; Janin-Manificat, L.; Beer, J.-C.; Makki, H.; Lagrost, A.C.; et al. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation 2008, 118, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Joint, E.S.C.; ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef] [Green Version]
- Killip, T.; Kimball, J.T. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am. J. Cardiol. 1967, 20, 457–464. [Google Scholar] [CrossRef]
- American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease); Society of Cardiovascular Anesthesiologists; Bonow, R.O.; Carabello, B.A.; Chatterjee, K.; de Leon, A.C., Jr.; Faxon, D.P.; Freed, M.D.; Gaasch, W.H.; et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2006, 48, e1–e148. [Google Scholar]
- Hayati Rezvan, P.; Lee, K.J.; Simpson, J.A. The rise of multiple imputation: A review of the reporting and implementation of the method in medical research. BMC Med. Res. Methodol. 2015, 15, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyrat, C.; Seaman, S.R.; White, I.R.; Douglas, I.; Smeeth, L.; Kim, J.; Resche-Rigon, M.; Carpenter, J.R.; Williamson, E.J. Propensity score analysis with partially observed covariates: How should multiple imputation be used? Stat. Methods Med. Res. 2019, 28, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010, 13, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.R.; Hernan, M.A. Constructing Inverse Probability Weights for Marginal Structural Models. Am. J. Epidemiol. 2008, 168, 656–664. [Google Scholar] [CrossRef]
- Cole, S.R.; Hernán, M.A. Adjusted survival curves with inverse probability weights. Comput. Methods Programs Biomed. 2004, 75, 45–49. [Google Scholar] [CrossRef]
- Salisbury, A.C.; Alexander, K.P.; Reid, K.J.; Masoudi, F.A.; Rathore, S.S.; Wang, T.Y. Incidence, correlates, and outcomes of acute, hospital-acquired anemia in patients with acute myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Putot, A.; Derrida, S.B.; Zeller, M.; Avondo, A.; Ray, P.; Manckoundia, P.; Cottin, Y. Short-Term Prognosis of Myocardial Injury, Type 1, and Type 2 Myocardial Infarction in the Emergency Unit. Am. J. Med. 2018, 131, 1209–1219. [Google Scholar] [CrossRef]
- Goodnough, L.T. Blood management: Transfusion medicine comes of age. Lancet 2013, 381, 1791–1792. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z.; Huang, Y.; Ye, Q.; Xie, N.; Zeng, L.; Lian, X.; Dai, Y.; Chen, J.; He, P.; et al. Restrictive vs. Liberal Red Blood Cell Transfusion Strategy in Patients With Acute Myocardial Infarction and Anemia: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 736163. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, M.; Lawler, P.R.; Filion, K.B.; Eisenberg, M.J. Red blood cell transfusion and mortality among patients hospitalized for acute coronary syndromes: A systematic review. Int. J. Cardiol. 2013, 164, 151–157. [Google Scholar] [CrossRef]
- Hill, S.R.; Carless, P.A.; Henry, D.A.; Carson, J.L.; Hebert, P.C.; McClelland, D.B.; Henderson, K.M. Transfusion Thresholds and Other Strategies for Guiding Allogeneic Red Blood Cell Transfusion. Cochrane Database Syst. Rev. 2002, 2, CD002042. [Google Scholar] [CrossRef]
- Carson, J.L.; Sieber, F.; Cook, D.R.; Hoover, D.R.; Noveck, H.; Chaitman, B.R.; Fleisher, L.; Beaupre, L.; Macaulay, W.; Rhoads, G.G.; et al. Liberal versus Restrictive Blood Transfusion Strategy: 3-Year Survival and Cause of Death Results from the FOCUS Randomised Controlled Trial. Lancet 2015, 385, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, Y.; Jaffe, A.S. Type 2 Myocardial Infarction: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1846–1860. [Google Scholar] [CrossRef]
- Chapman, A.R.; Shah, A.S.V.; Lee, K.K.; Anand, A.; Francis, O.; Adamson, P.; McAllister, D.A.; Strachan, F.E.; Newby, D.E.; Mills, N.L. Long-Term Outcomes in Patients With Type 2 Myocardial Infarction and Myocardial Injury. Circulation 2018, 137, 1236–1245. [Google Scholar] [CrossRef]
- Mohee, K.; Aldalati, O.; Dworakowski, R.; Haboubi, H. Aortic stenosis and anemia with an update on approaches to managing angiodysplasia in 2018. Cardiol. J. 2020, 27, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Nagao, K.; Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; et al. Anemia in Patients with Severe Aortic Stenosis. Sci. Rep. 2019, 9, 1924. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.S.; von Lueder, T.G.; Zannad, F.; Rossignol, P.; Duarte, K.; Chouihed, T.; Dickstein, K.; Atar, D.; Agewall, S.; Girerd, N.; et al. Relationship between left ventricular ejection fraction and mortality after myocardial infarction complicated by heart failure or left ventricular dysfunction. Int. J. Cardiol. 2018, 272, 260–266. [Google Scholar] [CrossRef]
- Zhou, Y.; Mo, T.; Bao, Y.P.; Wang, L.; Zhong, R.R.; Tian, J.L. Clinical features and prognosis of patients with different types of heart failure in relation to coronary artery disease. Zhonghua Yi Xue Za Zhi 2020, 100, 1142–1147. [Google Scholar]

| No Transfusion (n = 92) | Transfusion (n = 86) | p Value | |
|---|---|---|---|
| Demographic data | |||
| Age (year) | 81.5 (76,75–86) | 83 (79–86) | 0.22 |
| Age > 80 (year) | 51 (55.4) | 60 (69.8) | 0.07 |
| Female | 52 (56.5) | 48 (55.8) | 1.00 |
| BMI (kg/m2) (n = 176) | 25 (22–29) | 24 (23–27) | 0.15 |
| Obesity (n = 176) | 18 (19.6) | 9 (10.5) | 0.14 |
| CV risk factors | |||
| Hypertension (n = 178) | 73 (79.3) | 72 (83.7) | 0.58 |
| Diabetes (n = 178) | 30 (32.6) | 27 (31.4) | 0.99 |
| Dyslipidemia (n = 177) | 52 (56.5) | 47 (55.3) | 0.99 |
| Family history of CAD (n = 167) | 13 (14.9) | 16 (20.0) | 0.511 |
| Smoking (n = 168) | 7 (7.6) | 5 (5.8) | 0.859 |
| Medical history | |||
| Vascular history (n = 178) | 22 (23.9) | 27 (31.4) | 0.343 |
| Myocardial infarction (n = 178) | 22 (23.9) | 16 (18.6) | 0.496 |
| Coronary artery bypass graft (n = 178) | 9 (9.8) | 8 (9.3) | 1.000 |
| Kidney disease (n = 174) | 16 (17.6) | 19 (22.9) | 0.494 |
| Thrombo-embolic event (n = 176) | 12 (13.2) | 7(8.2) | 0.415 |
| Atrial fibrillation (n = 166) | 23 (26.7) | 21 (26.2) | 1.000 |
| Aortic stenosis (n = 178) | 14 (15.2) | 26 (30.2) | 0.027 |
| Neurocognitive disorder (n = 172) | 5 (5.6) | 6 (7.3) | 0.873 |
| Neoplasia (n = 173) | 27 (30.0) | 24 (28.9) | 1.000 |
| Chronic treatments | |||
| Aspirin (n = 178) | 32 (34.8) | 34 (39.5) | 0.617 |
| Other antiplatelet (n = 178) | 15 (16.3) | 19 (22.1) | 0.429 |
| Vitamin K inhibitor (n = 178) | 28 (30.4) | 17 (19.8) | 0.143 |
| Oral anticoagulant (n = 178) | 0 (0.0) | 1 (1.2) | 0.973 |
| Calcium inhibitor (n = 178) | 24 (26.1) | 26 (30.2) | 0.654 |
| Angiotensin Receptor Blocker (n = 178) | 32 (34.8) | 21 (24.4) | 0.178 |
| Angiotensin Converting Enzyme inhibitor (n = 178) | 19 (20.7) | 23 (26.7) | 0.435 |
| Clinical data on admission | |||
| Heart rate (b/min) (n = 162) | 86 (72–100) | 82 (70–100) | 0.504 |
| SBP (mmHg) (n = 163) | 140 (119–156) | 123 (120–144) | 0.005 |
| DBP (mmHg) (n = 163) | 72 (63–81.5) | 64 (55–74) | <0.001 |
| Heart failure (n = 178) | 51 (55.4) | 44 (51.8) | 0.73 |
| LVEF (%) (n = 177) | 45 (35–60) | 50 (40–60) | 0.039 |
| LVEF > 40% (n = 177) | 63 (68.5) | 69 (80.2) | 0.105 |
| Biological data | |||
| Hemoglobin at admission (g/dL) (n = 178) | 10.75 (9.9–12) | 9.9 (8.7–11.4) | 0.001 |
| Nadir hemoglobin level (g/dL) (n = 178) | 9.3 (8.9–9.7) | 7.8 (7.3–8.3) | <0.001 |
| Drop in hemoglobin (n = 178) | 42 (45.7) | 77 (89.5) | <0.001 |
| Creatinine (µmol/L) (n = 176) | 102 (72–139) | 112 (81–147) | 0.410 |
| e-GFR (CKD-EPI) < 60 mL/min/1.73 m2 (n = 176) | 56 (60.9) | 55 (64.0) | 0.787 |
| C reactive protein > 3 mg/L (n = 175) | 74 (82.2) | 73 (85.9) | 0.650 |
| NT-proBNP (pg/mL) (n = 169) | 6632 (2018–15,993) | 6068 (3131–13,824) | 0.709 |
| Troponin Ic peak (ng/mL) (n = 175) | 3.1 (0.96–9.77) | 9.8 (2.8–22) | 0.008 |
| Coronary angiography (n = 178) | 87 (94.6) | 71 (82.6) | 0.022 |
| No Transfusion | Transfusion | ||
|---|---|---|---|
| ICU stay duration (d) | 4.0 (3.0–7.0) | 5.0 (3.0–6.5) | p = 0.69 |
| 30-day | |||
| All-cause death | 7 (7.6) | 11 (12.8) | p = 0.37 |
| CV death | 7 (7.6) | 8 (9.3) | p = 0.89 |
| 1-year | |||
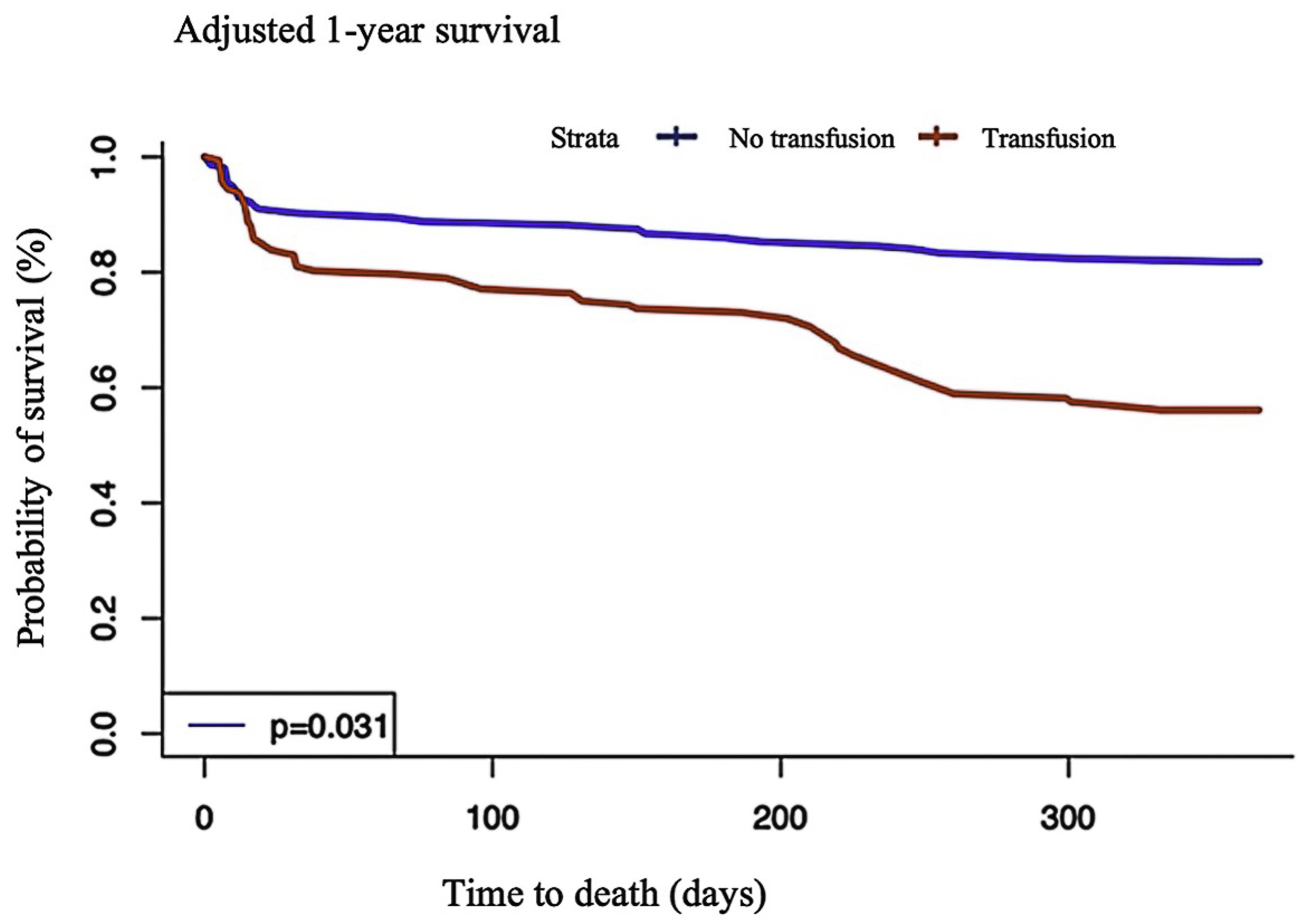
| All-cause death | 23 (25.0) | 37 (43.0) | p = 0.02 |
| CV death | 13 (14.1) | 22 (25.6) | p = 0.08 |
| Recurrent MI | 3 (3.3) | 3 (3.5) | p = 1.0 |
| Re-hospitalization for heart failure | 67 (72.8) | 51 (59.3) | p = 0.08 |
| 30-Day Mortality | 1-Year Mortality | |||
|---|---|---|---|---|
| HR [95% CI] | p | HR [95% CI] | p | |
| Unadjusted | 1.39 (0.61–3.18) | 0.42 | 1.89 (1.12–3.19) | 0.02 |
| SIPW-adjusted | ||||
| All patients | 1.59 (0.55–4.56) | 0.38 | 2.47 (1.22–4.97) | 0.01 |
| Stratified on age | ||||
| ≤80 y | 1.70 (0.37–7.81) | 0.49 | 2.30 (0.74–7.23) | 0.14 |
| >80 y | 1.55 (0.38–6.33) | 0.53 | 1.94 (0.76–4.98) | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacquin, A.; Putot, A.; Chague, F.; Manckoundia, P.; Cottin, Y.; Zeller, M. Mid-Term Mortality in Older Anemic Patients with Type 2 Myocardial Infarction: Does Blood Transfusion sImprove Prognosis? J. Clin. Med. 2022, 11, 2423. https://doi.org/10.3390/jcm11092423
Hacquin A, Putot A, Chague F, Manckoundia P, Cottin Y, Zeller M. Mid-Term Mortality in Older Anemic Patients with Type 2 Myocardial Infarction: Does Blood Transfusion sImprove Prognosis? Journal of Clinical Medicine. 2022; 11(9):2423. https://doi.org/10.3390/jcm11092423
Chicago/Turabian StyleHacquin, Arthur, Alain Putot, Frederic Chague, Patrick Manckoundia, Yves Cottin, and Marianne Zeller. 2022. "Mid-Term Mortality in Older Anemic Patients with Type 2 Myocardial Infarction: Does Blood Transfusion sImprove Prognosis?" Journal of Clinical Medicine 11, no. 9: 2423. https://doi.org/10.3390/jcm11092423
APA StyleHacquin, A., Putot, A., Chague, F., Manckoundia, P., Cottin, Y., & Zeller, M. (2022). Mid-Term Mortality in Older Anemic Patients with Type 2 Myocardial Infarction: Does Blood Transfusion sImprove Prognosis? Journal of Clinical Medicine, 11(9), 2423. https://doi.org/10.3390/jcm11092423








