Treatment in Latent Tuberculosis Uveitis—Is Immunosuppression Effective or Is Conventional 3- or 4-Drug Antituberculosis Therapy Mandatory?
Abstract
:1. Introduction
2. Patients and Methods
2.1. Treatment
2.2. Statistical Analysis
3. Results
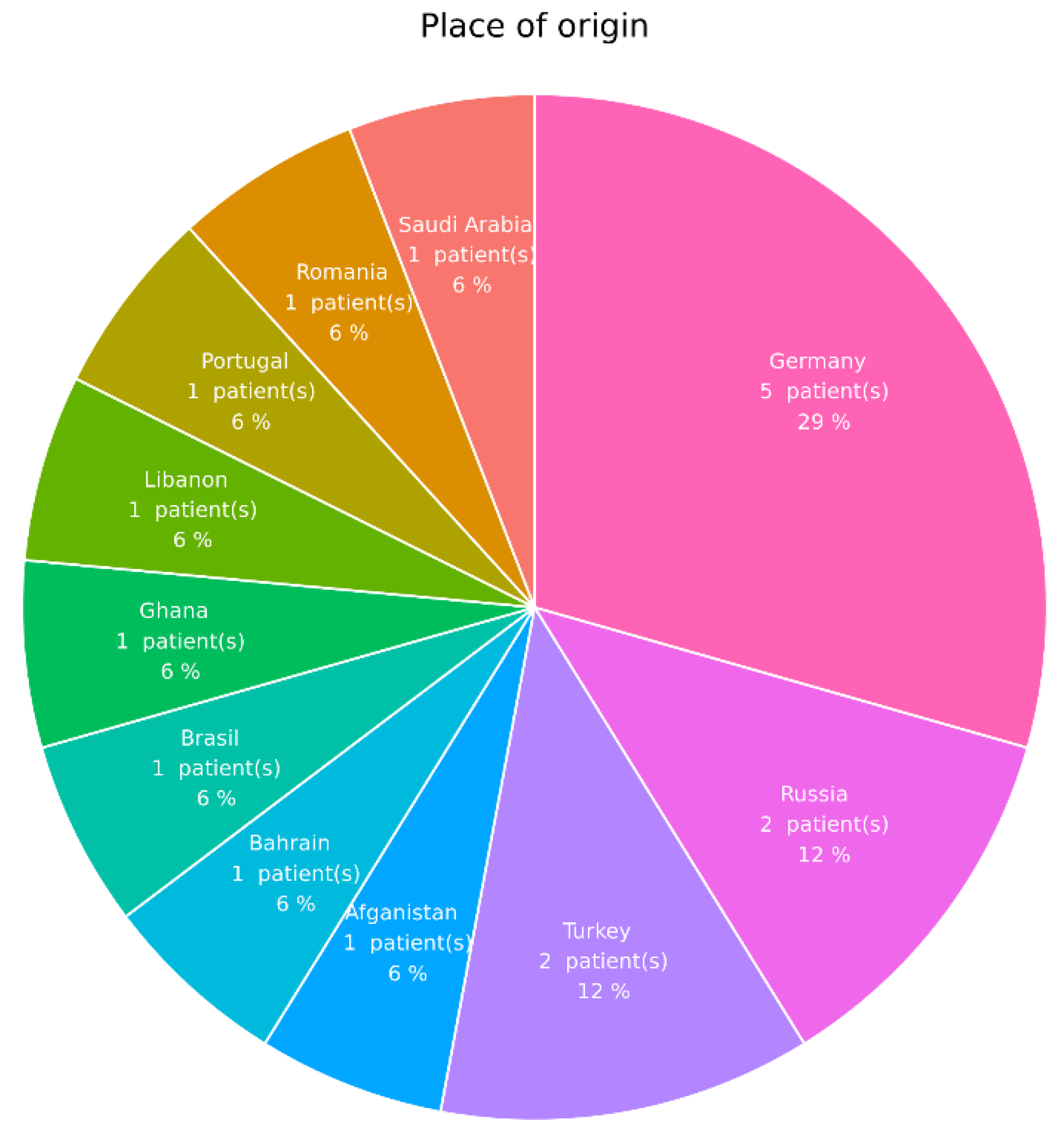
3.1. Ocular Involvement
3.2. Treatment
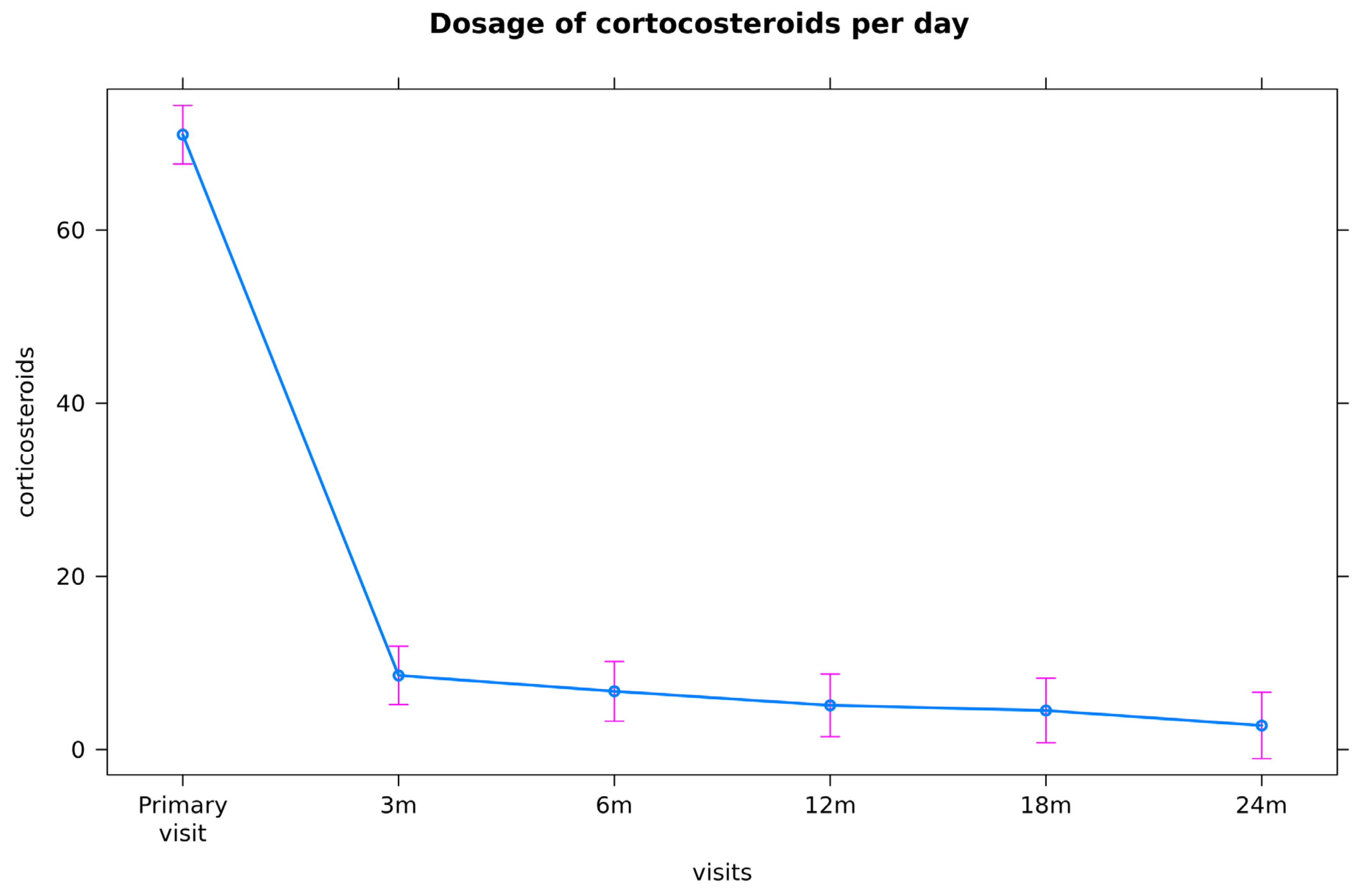
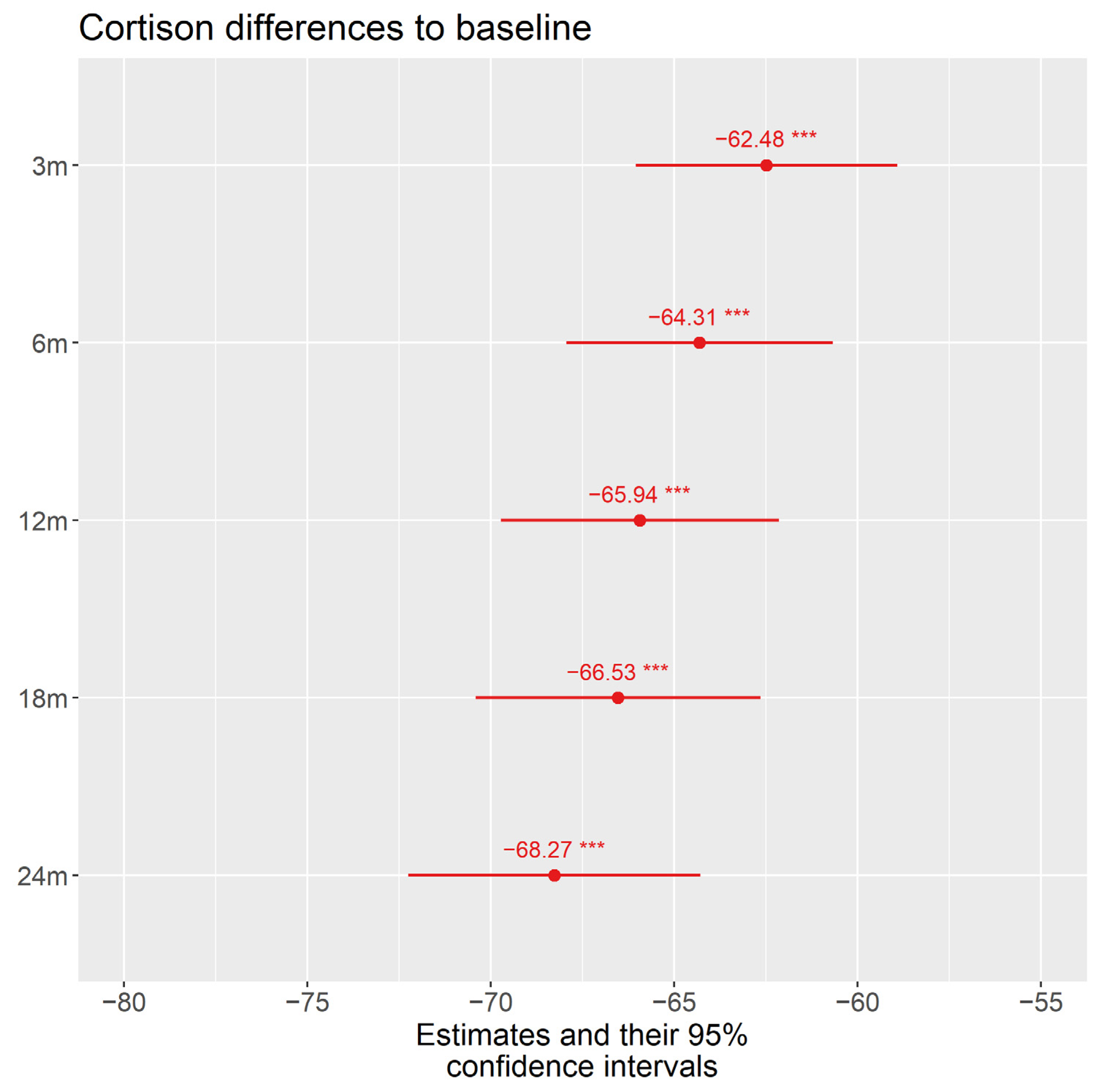
3.2.1. Corticosteroids
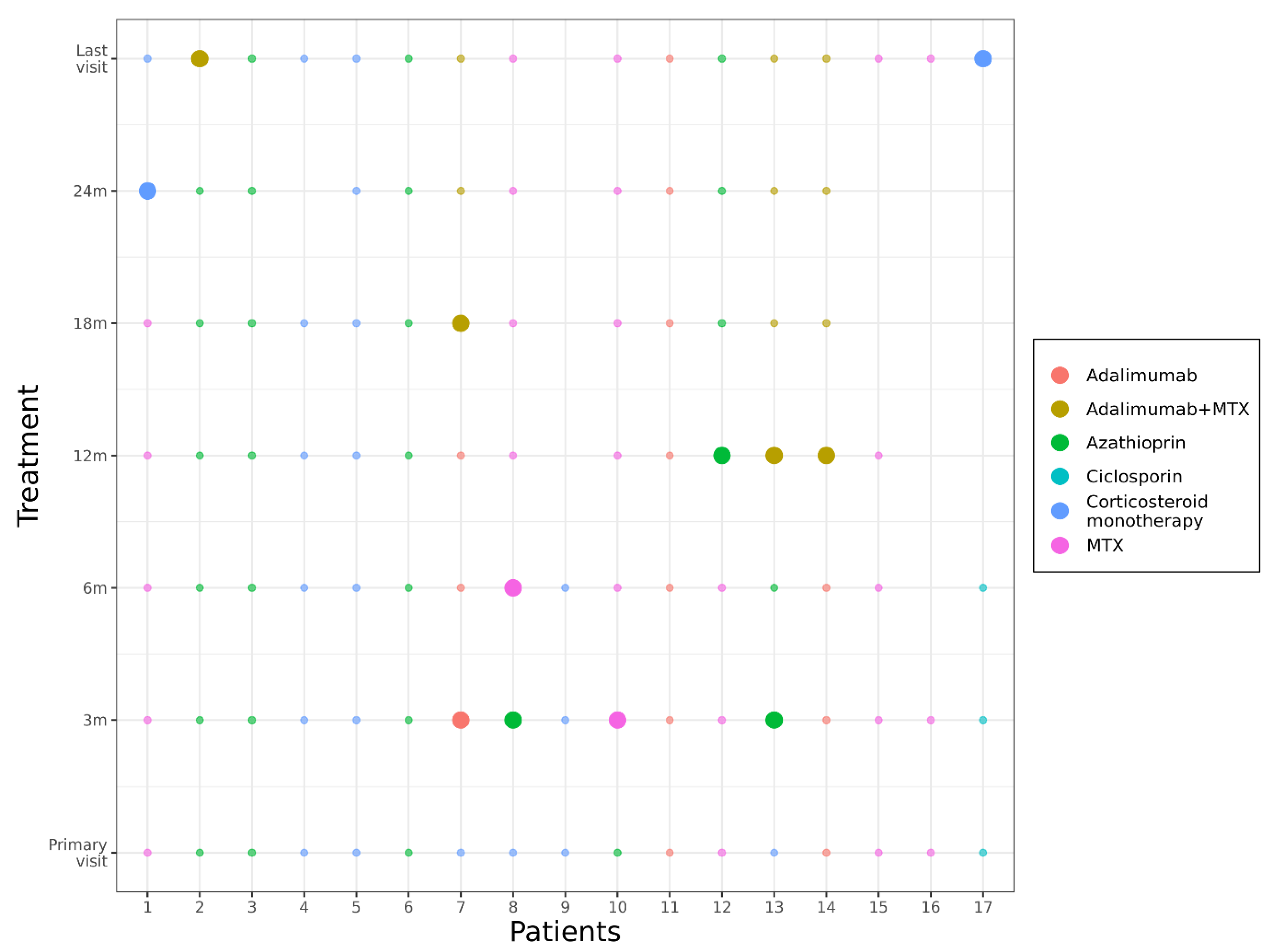
3.2.2. Immunosuppression (cs- and bDMARDs)
3.2.3. Efficacy of Immunosuppression: Relapses of Uveitis/Steroid-Sparing Effect
3.2.4. Isoniazid Prophylaxis
3.2.5. Side Effects of Systemic Therapy
3.2.6. Intravitreal Treatment
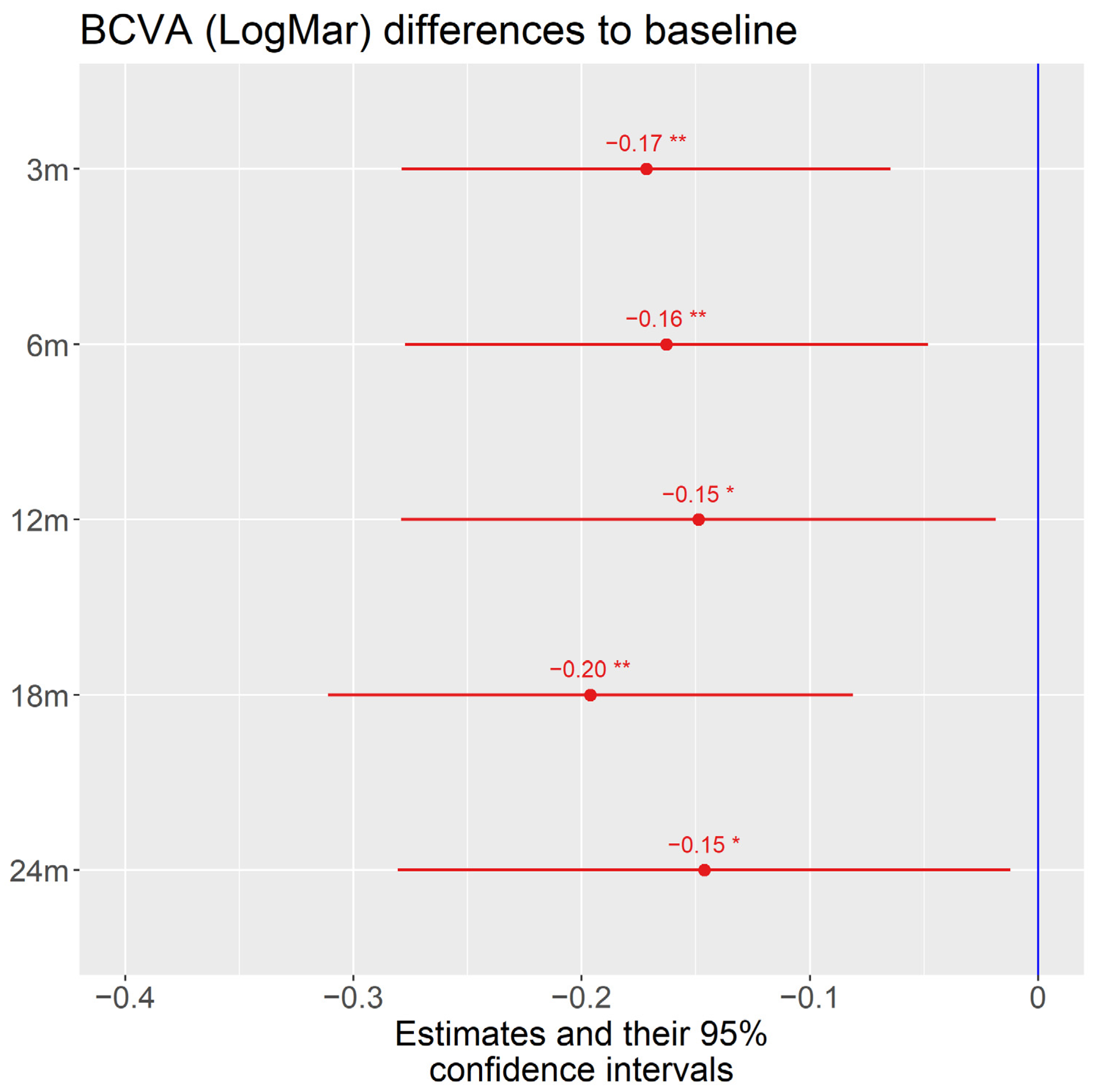
3.3. Visual Acuity
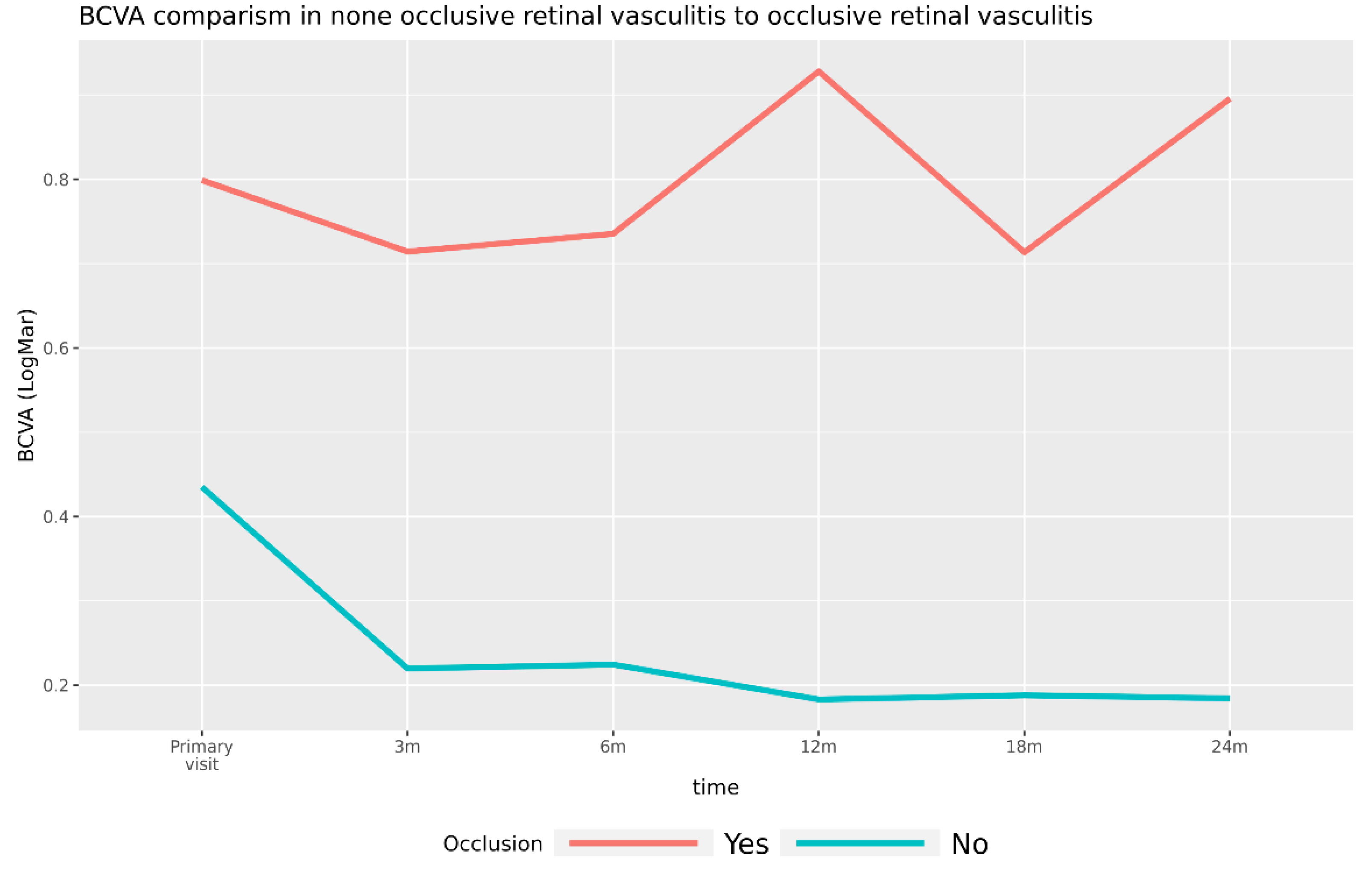
3.3.1. Visual Acuity in Patients with Occlusive Retinal Vasculitis versus Patients with Nonocclusive Retinal Vasculitis
3.3.2. Uveitic Macular Edema
3.3.3. Other Uveitis-Associated Complications
3.3.4. Other Complications
4. Discussion
4.1. Study Design
4.2. Methods of TB Testing
4.3. Recommended Therapy of Latent Tuberculosis
4.4. Efficacy of Preventive Therapy for LTBI
4.5. Efficacy of Immunosuppressive Therapy in Uveitis Patients with LTBI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thompson, M.J.; Albert, D.M. Ocular tuberculosis. Arch. Ophthalmol. 2005, 123, 844–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Tuberculosis Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Nora, R.L.D.; van Velthoven, M.E.; dam-van Loon, N.H.T.; Misotten, T.; Bakker, M.; van Hagen, M.P.; Rothova, A. Clinical Manifestations of Patients With Intraocular Inflammation and Positive QuantiFERON–TB Gold In-Tube Test in a Country Nonendemic for Tuberculosis. Am. J. Ophthalmol. 2014, 157, 754–761. [Google Scholar] [CrossRef]
- Elkington, P.; Tebruegge, M.; Mansour, S. Tuberculosis: An infection-initiated autoimmune disease? Trends Immunol. 2016, 37, 815–818. [Google Scholar] [CrossRef] [Green Version]
- Nora, R.L.D.; Walburg, K.V.; Van Hagen, P.M.; Swagemakers, S.M.A.; Van Der Spek, P.J.; Quinten, E.; Van Velthoven, M.; Ottenhoff, T.H.M.; Dik, W.A.; Haks, M.C. Retinal Pigment Epithelial Cells Control Early Mycobacterium tuberculosis Infection via Interferon Signaling. Investig. Opthalmol. Vis. Sci. 2018, 59, 1384–1395. [Google Scholar] [CrossRef] [Green Version]
- Diel, R.; Goletti, D.; Ferrara, G.; Bothamley, G.; Cirillo, D.; Kampmann, B.; Lange, C.; Losi, M.; Markova, R.; Migliori, G.B.; et al. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 88–99, Erratum in: Eur. Respir. J. 2012, 39, 793. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, G.H.; Jereb, J.; Vernon, A.; LoBue, P.; Goldberg, S.; Castro, K.; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm. Rep. 2010, 59, 1–25. [Google Scholar]
- Nahon-Esteve, S.; Martel, A.; Maschi, C.; Alketbi, M.; Baillif, S.; Tieulie, N. Uveitis associated with latent tuberculosis: A comparative study of the impact of antitubercular therapy combined or not with systemic corticosteroids. Eur. J. Ophthalmol. 2021, 31, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Tomkins-Netzer, O.; Leong, B.C.S.; Zhang, X.; Lightman, S.; McCluskey, P.J. Effect of Antituberculous Therapy on Uveitis Associated With Latent Tuberculosis. Am. J. Ophthalmol. 2018, 190, 164–170. [Google Scholar] [CrossRef]
- Ang, M.; Hedayatfar, A.; Zhang, R.; Chee, S.P. Clinical signs of uveitis associated with latent tuberculosis. Clin. Exp. Ophthalmol. 2012, 40, 689–696. [Google Scholar] [CrossRef]
- Ang, M.; Hedayatfar, A.; Wong, W.; Chee, S.P. Duration of anti-tubercular therapy in uveitis associated with latent tuberculosis: A case-control study. Br. J. Ophthalmol. 2012, 96, 332–336. [Google Scholar] [CrossRef]
- Bansal, R.; Gupta, A.; Gupta, V.; Dogra, M.R.; Bambery, P.; Arora, S.K. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am. J. Ophthalmol. 2008, 146, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Htoon, H.M.; Chee, S.P. Diagnosis of tuberculous uveitis: Clinical application of an interferon-gamma release assay. Ophthalmology 2009, 116, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Albini, T.A.; Karakousis, P.C.; Rao, N.A. Interferon-gamma release assays in the diagnosis of tuberculous uveitis. Am. J. Ophthalmol. 2008, 146, 486–488. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of Uveitis Nomenclature (SUN) Working Group. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Itty, S.; Bakri, S.J.; Pulido, J.S.; Herman, D.C.; Faia, L.J.; Tufty, G.T.; Bennett, S.R.; Falk, N.S. Initial results of QuantiFERON-TB Gold testing in patients with uveitis. Eye 2009, 23, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Kang, Y.A.; Leem, A.Y.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; Kim, S.Y. Active Tuberculosis Incidence and Characteristics in Patients Treated with Tumor Necrosis FactorAntagonists According to Latent Tuberculosis Infection. Sci. Rep. 2017, 7, 6473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Investig. Opthalmology Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. robustlmm: An R package for robust estimation of linear mixed-effects. J. Stat. Softw. 2016, 75, 1–24. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Languuage and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Yee, D.; Valiquette, C.; Pelletier, M.; Parisien, I.; Rocher, I.; Menzies, D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am. J. Respir. Crit. Care Med. 2003, 167, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Nannini, C.; Niccoli, L.; Iannone, F.; Delogu, G.; Garlaschi, G.; Sanduzzi, A.; Matucci, A.; Prignano, F.; Conversano, M.; et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun. Rev. 2015, 14, 503–509. [Google Scholar] [CrossRef]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Den Boon, S.; Borroto Gutierrez, S.M.; Bruchfeld, J.; Burhan, E.; et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 2015, 46, 1563–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, A.T.; Mendes, M.; Freitas, S.; Roxo, P.C. Incidence and risk factors of major toxicity associated to first-line antituberculosis drugs for latent and active tuberculosis during a period of 10 years. Rev. Port. Pneumol. 2015, 21, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ezer, N.; Benedetti, A.; Darvish-Zargar, M.; Menzies, D. Incidence of ethambutol-related visual impairment during treatment of active tuberculosis. Int. J. Tuberc. Lung Dis. 2013, 17, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Garg, R.; Prasad, R.; Mishra, A.K. A prospective study of ocular toxicity in patients receiving ethambutol as a part of directly observed treatment strategy therapy. Lung India 2015, 32, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Chao, C.S.; Vargas, J.H.; Sharp, H.L.; Martín, M.G.; McDiarmid, S.V.; Sinatra, F.R.; Ament, M.E. Isoniazid-related hepatic failure in children: A survey of liver transplantation centers. Transplantation 2007, 84, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Losi, M.; Meacci, M.; Meccugni, B.; Piro, R.; Roversi, P.; Bergamini, B.M.; D’Amico, R.; Marchegiano, P.; Rumpianesi, F.; et al. Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am. J. Respir. Crit. Care Med. 2005, 172, 631–635. [Google Scholar] [CrossRef] [Green Version]
- Colangeli, R.; Arcus, V.L.; Cursons, R.T.; Ruthe, A.; Karalus, N.; Coley, K.; Manning, S.D.; Kim, S.; Marchiano, E.; Alland, D. Whole genome sequencing of Mycobacterium tuberculosis reveals slow growth and low mutation rates during latent infections in humans. PLoS ONE 2014, 9, e91024. [Google Scholar] [CrossRef]
- Menzies, D.; Adjobimey, M.; Ruslami, R.; Trajman, A.; Sow, O.; Kim, H.; Obeng Baah, J.; Marks, G.B.; Long, R.; Hoeppner, V.; et al. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. N. Engl. J. Med. 2018, 379, 440–453. [Google Scholar] [CrossRef]
- Akolo, C.; Adetifa, I.; Shepperd, S.; Volmink, J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst. Rev. 2010, 2010, CD000171. [Google Scholar] [CrossRef]
- Fountain, F.F.; Tolley, E.; Chrisman, C.R.; Self, T.H. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: A 7-year evaluation from a public health tuberculosis clinic. Chest 2005, 128, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Smieja, M.; Marchetti, C.; Cook, D.; Smaill, F.M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst. Rev. 1999, 1999, CD001363. [Google Scholar] [CrossRef] [PubMed]

| Patient | Age (Years) | Sex | Quantiferon Test | Follow Up (Month) | Disease Onset | Type of Retinal Vasculitis | BCVA OD at Begin of Follow Up LogMar | BCVA OD at Begin of Follow Up LogMar | BCVA OD at Last Follow Up LogMar | BCVA OS at Last Follow Up LogMar | CME | CME End of Follow Up | Intravitreal Therapy | Remission State at Last Follow Up | Systemic Therapy (cs-/bDMARDs/Corticosteroids) at Treatment Initiation | Adverse Events | Therapy Change (or Dropout (Reasons) | Systemic Corticosteroids (mg/d) at Last Follow Up | Systemic Therapy at Last Follow Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | m | positive | 33 | 01/18 | Occlusive OD + OS | 0.4 | 1.3 | 0.2 | 0.2 | yes OD < OS | no | Bevacizumab OD 2×; OS 3×, | complete | Cort, MTX | no | Yes (stopped on his own) | 0 | no |
| 2 | 74 | m | positive | 47 | 01/17 | Occlusive OD + OS | 0.3 | 0.6 | 0.4 | 0.4 | yes OD | yes | OD: Triamcinolon parabulbar 2×; Ozurdex 3×, | Incomplete (persisting CME) | Cort, AZA, MTX | no | Yes (persisting macular edema) | 2.5 | ADA, MTX |
| 3 | 49 | m | positive | 25 | 05/17 | Non-occlusive OD + OS | 1.4 | 0.3 | 2.3 | 0.4 | no | no | no | complete | Cort, AZA | no | no | 5 | AZA |
| 4 | 40 | m | positive | 19 | 01/12 | Non-occlusive OD + OS | 0 | 0 | 0 | 0 | yes | no | no | complete | Cort | no | no | 2 | no |
| 5 | 56 | m | positive | 35 | 03/17 | Occlusive OD | 0.4 | 0 | 0.5 | 0.1 | no | no | no | complete | Cort | no | no | 5 | no |
| 6 | 39 | w | positive | 33 | 03/16 | Occlusive OS | 0 | 0 | 0 | 0 | no | no | no | complete | Cort, AZA | Pressure in the throat (once) | no | 0 | AZA |
| 7 | 80 | m | positive | 37 | 03/17 | Occlusive OD >> OS | 0.1 | 1 | 0.1 | 0.4 | yes OS | no | OS Ozurdex 2×, Illuvien 1×, (06/20) | complete | Cort, ADA, MTX | Herpetic infection | no | 0 | ADA, MTX |
| 8 | 74 | w | positive | 32 | 11/18 | Occlusive OS | 0 | 0.3 | 1.4 | 0.1 | yes OS | no | Ranibizumab 9×, | complete | Cort, AZA, MTX | Elevated liver encymes | Yes (adverse events) | 0 | MTX |
| 9 | 37 | w | positive | 8 | 02/18 | Occlusive OD + OS | 0 | 0.2 | 0.1 | 0.1 | yes OS | no | no | complete | Cort | no | no | 5 | no |
| 10 | 58 | m | positive | 32 | 01/17 | Occlusive OD + OS | 0.2 | 0 | 0 | 0 | no | no | no | complete | Cort, AZA, MTX | Hair loss | Yes (adverse events) | 10 | MTX |
| 11 | 34 | w | positive | 31 | 12/17 | Occlusive OD + OS | 0.2 | 0.3 | 0 | 0 | yes OS | no | no | complete | ADA | no | no | 0 | ADA |
| 12 | 41 | w | positive | 55 | 12/15 | Non-occlusive OD + OS | 0 | 1 | 0 | 0.5 | yes OD | no | no | complete | Cort, MTX, AZA | no | Yes (adverse events) | 0 | AZA |
| 13 | 37 | m | positive | 49 | 11/16 | Occlusive OD + OS | 0.4 | 0 | 0.1 | 0 | yes OD | Recurrence OD | OD Ozurdex, Bevacizumab 2×; OS Bevacizumab 4×, | Incomplete (persisting CME) | Cort, AZA, ADA, MTX | no | Yes (inefficacy) | 5 | ADA, MTX |
| no | 36 | w | positive | 23 | 11/18 | Occlusive OS | 0 | 0 | 0 | 0 | no | no | no | complete | Cort, ADA, MTX | no | no | 0 | ADA, MTX |
| 15 | 69 | m | positive | 14 | 07/19 | Occlusive OD + OS | 0.7 | 1.3 | 0.2 | 0.2 | no | no | no | complete | Cort, MTX | no | no | 7.5 | MTX |
| 16 | 83 | m | positive | 6 | 07/20 | Non-occlusive OD + OS | 0.5 | 1.3 | 0.3 | 1.2 | no | no | Bevacizumab OD/OS 2×, | Ongoing (less than 6 month therapy) | Cort, MTX | no | no | 7.5 | MTX |
| 17 | 67 | m | positive | 11 | 05/19 | Non-occlusive OD + OS | 1.6 | 1.3 | 2 | 1.5 | yes | no | Triamcinolon parabulbar | Complete (secondary glaucoma, corneal decompensation) | Cort CyA | no | no | 0 | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigdon, E.; Steinhorst, N.A.; Weissleder, S.; Durchkiv, V.; Stübiger, N. Treatment in Latent Tuberculosis Uveitis—Is Immunosuppression Effective or Is Conventional 3- or 4-Drug Antituberculosis Therapy Mandatory? J. Clin. Med. 2022, 11, 2419. https://doi.org/10.3390/jcm11092419
Bigdon E, Steinhorst NA, Weissleder S, Durchkiv V, Stübiger N. Treatment in Latent Tuberculosis Uveitis—Is Immunosuppression Effective or Is Conventional 3- or 4-Drug Antituberculosis Therapy Mandatory? Journal of Clinical Medicine. 2022; 11(9):2419. https://doi.org/10.3390/jcm11092419
Chicago/Turabian StyleBigdon, Eileen, Nils Alexander Steinhorst, Stephanie Weissleder, Vasyl Durchkiv, and Nicole Stübiger. 2022. "Treatment in Latent Tuberculosis Uveitis—Is Immunosuppression Effective or Is Conventional 3- or 4-Drug Antituberculosis Therapy Mandatory?" Journal of Clinical Medicine 11, no. 9: 2419. https://doi.org/10.3390/jcm11092419
APA StyleBigdon, E., Steinhorst, N. A., Weissleder, S., Durchkiv, V., & Stübiger, N. (2022). Treatment in Latent Tuberculosis Uveitis—Is Immunosuppression Effective or Is Conventional 3- or 4-Drug Antituberculosis Therapy Mandatory? Journal of Clinical Medicine, 11(9), 2419. https://doi.org/10.3390/jcm11092419





