Dermatochalasis Aggravates Meibomian Gland Dysfunction Related Dry Eyes
Abstract
:1. Introduction
2. Materials and Methods
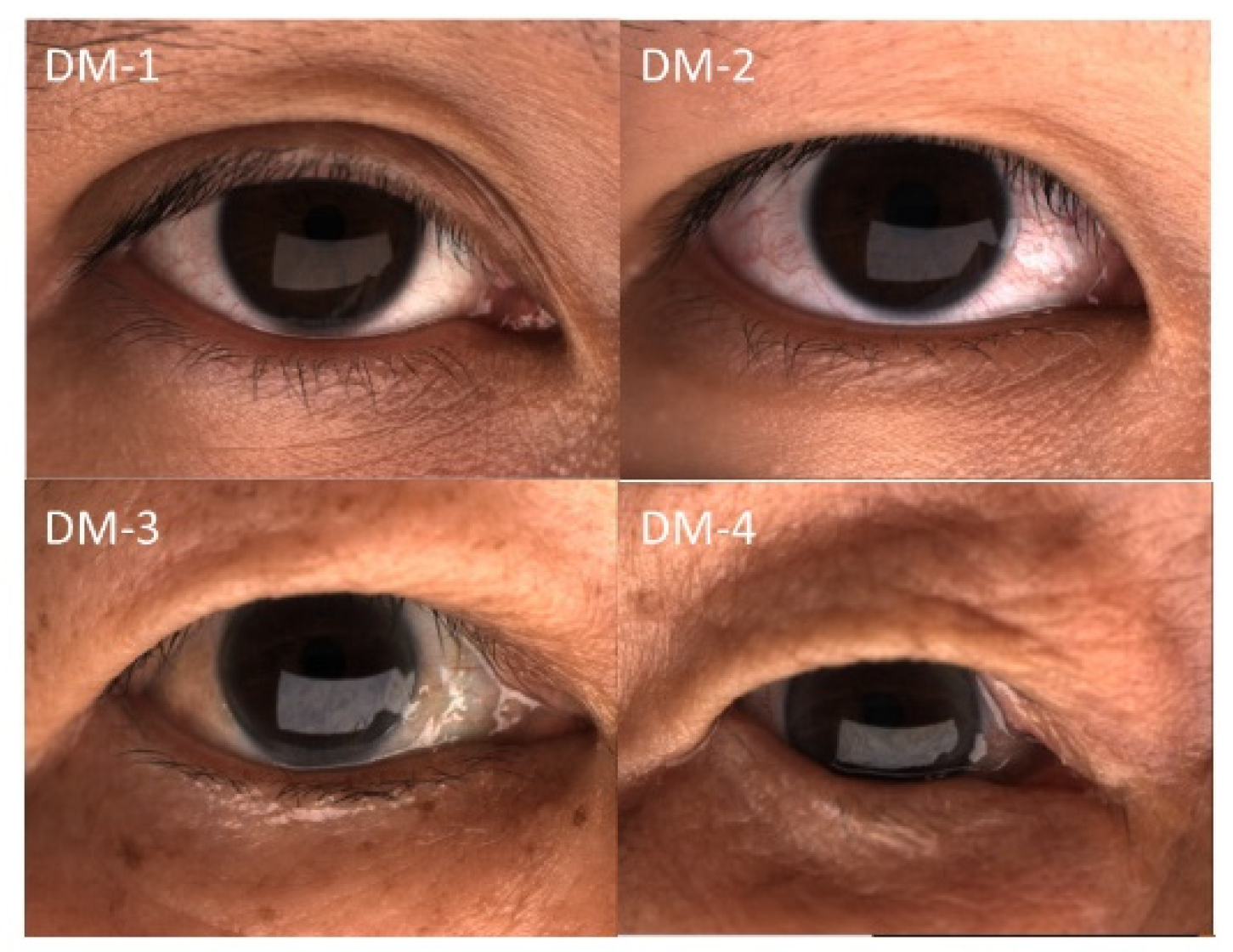
2.1. Patients
2.2. Dry-Eye Examination Protocol
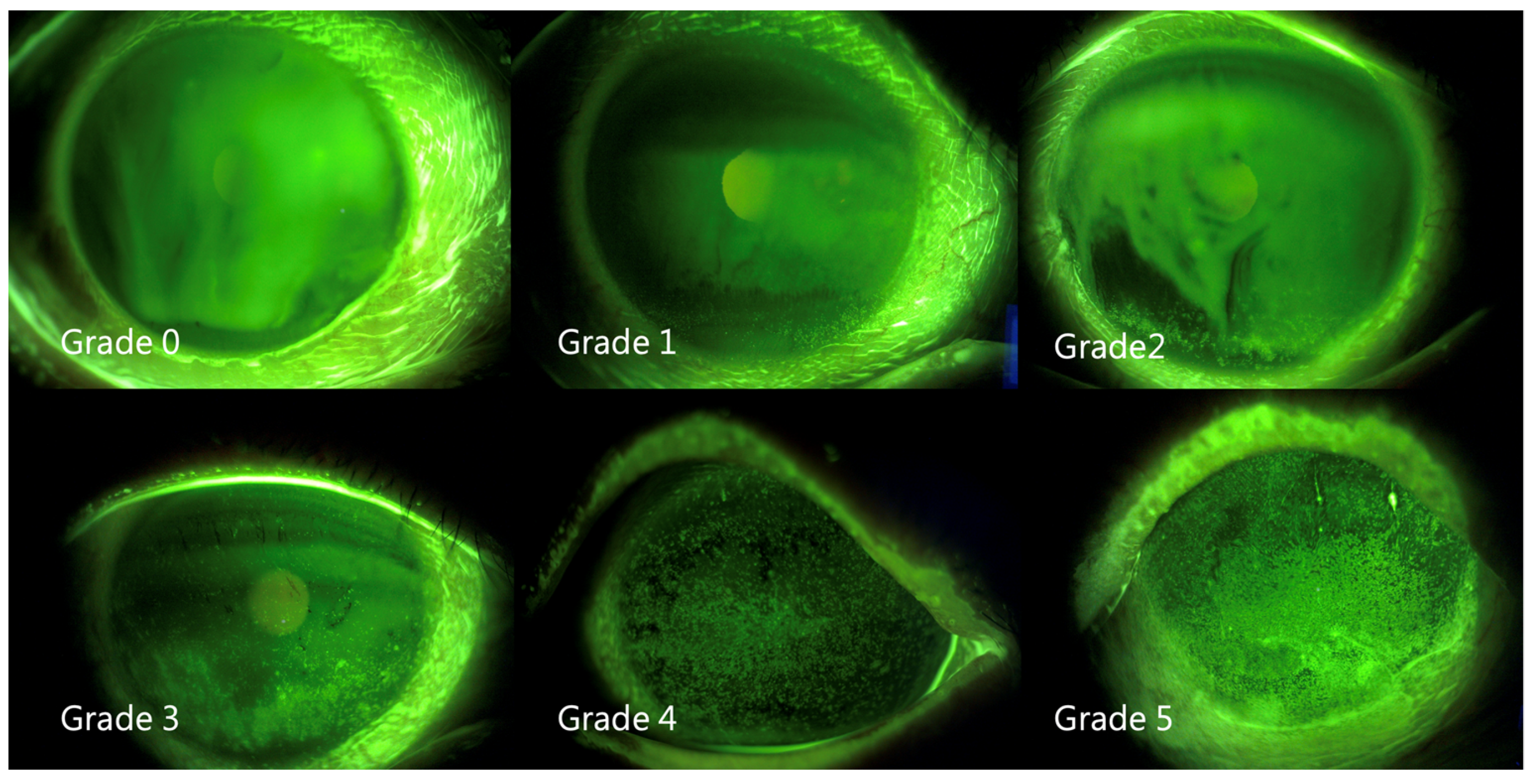
2.3. Measurement of Fluorescein Tear-Film Break-Up Time (FTBUT) and Grading of Superficial Punctate Keratitis (SPK)
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Dermatochalasis Severity and Subjective Symptoms
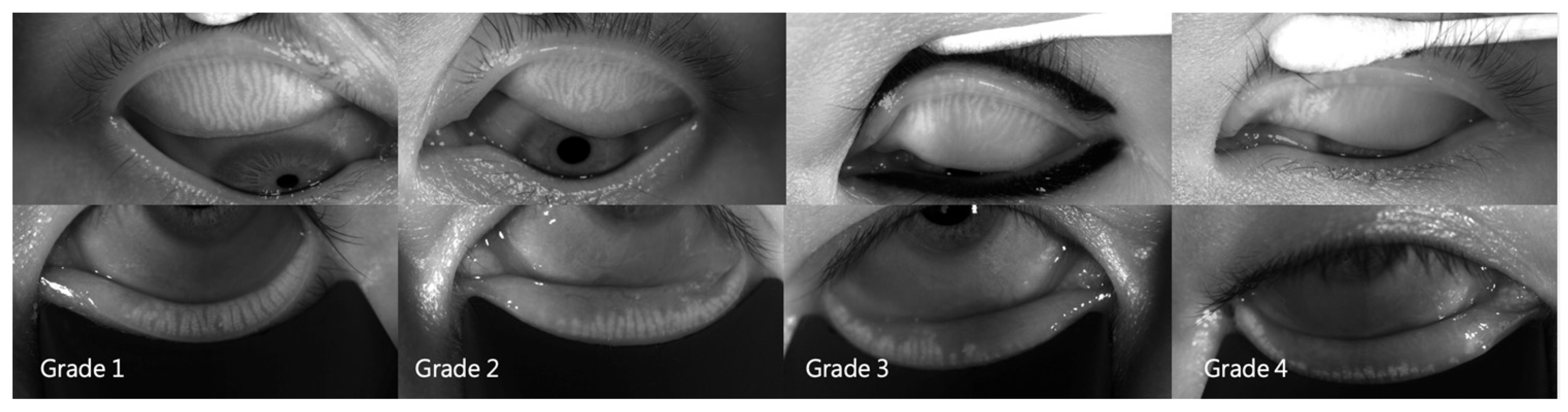
3.3. Dermatochalasis Severity and Meibomian Parameters
3.4. Dermatochalasis Severity and Tear-Film/Blink Parameters
3.5. Differences in High LLT Distribution between Females and Males
3.6. Effects of Lid Hygiene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karnaz, A.; Katircioglu, Y.A.; Ozdemir, E.S.; Celebli, P.; Hucumenoglu, S.; Ornek, F. The Histopathological Findings of Patients Who Underwent Blepharoplasty Due to Dermatochalasis. Semin Ophthalmol. 2018, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Nagi, K.S.; Carlson, J.A.; Wladis, E.J. Histologic assessment of dermatochalasis: Elastolysis and lymphostasis are fundamental and interrelated findings. Ophthalmology 2011, 118, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Kashkouli, M.B.; Karimi, N.; Johari-Moghadam, M.M.; Shayanfar, N.; Aghamirsalim, M.; Abdolalizadeh, P. Dermatochalasis Through Decades: A Histopathologic Study. Ann. Plast. Surg. 2021, 86, 340–344. [Google Scholar] [CrossRef]
- Boros, K.; Freemont, T. Physiology of ageing of the musculoskeletal system. Best Pract. Res. Clin. Rheumatol. 2017, 31, 203–217. [Google Scholar] [CrossRef]
- Fukada, K.; Kajiya, K. Age-related structural alterations of skeletal muscles and associated capillaries. Angiogenesis 2020, 23, 79–82. [Google Scholar] [CrossRef]
- DeAngelis, K.D.; Rider, A.; Potter, W.; Jensen, J.; Fowler, B.T.; Fleming, J.C. Eyelid Spontaneous Blink Analysis and Age-Related Changes Through High-Speed Imaging. Ophthalmic Plast. Reconstr. Surg. 2019, 35, 487–490. [Google Scholar] [CrossRef]
- Tomlinson, A.; Bron, A.J.; Korb, D.R.; Amano, S.; Paugh, J.R.; Pearce, E.I.; Yee, R.; Yokoi, N.; Arita, R.; Dogru, M. The international workshop on meibomian gland dysfunction: Report of the diagnosis subcommittee. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2006–2049. [Google Scholar] [CrossRef]
- Knop, E.; Knop, N.; Millar, T.; Obata, H.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest. Ophthalmol. Vis. Sci. 2011, 52, 1938–1978. [Google Scholar] [CrossRef] [Green Version]
- Ngo, W.; Situ, P.; Keir, N.; Korb, D.; Blackie, C.; Simpson, T. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea 2013, 32, 1204–1210. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. (Chicago, Ill.: 1960) 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Weng, H.Y.; Ho, W.T.; Chiu, C.Y.; Tsai, T.Y.; Chang, S.W. Characteristics of tear film lipid layer in young dry eye patients. J. Formos Med. Assoc. 2021, 120, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Finis, D.; Pischel, N.; König, C.; Hayajneh, J.; Borrelli, M.; Schrader, S.; Geerling, G. Comparison of the OSDI and SPEED questionnaires for the evaluation of dry eye disease in clinical routine. Ophthalmologe 2014, 111, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Sillevis Smitt-Kamminga, N.; Nibourg, S.A.; Hammond, C.J. Predictors of Discordance between Symptoms and Signs in Dry Eye Disease. Ophthalmology 2017, 124, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Lee, J.; Lee, H.; Seo, K.Y.; Kim, E.K.; Kim, T.I. Automated Measurement of Tear Film Dynamics and Lipid Layer Thickness for Assessment of Non-Sjögren Dry Eye Syndrome With Meibomian Gland Dysfunction. Cornea 2017, 36, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Bunya, V.; Maguire, M.; Asbell, P.; Ying, G.S. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology 2021, 128, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Butovich, I.A.; Suzuki, T. Effects of Aging on Human Meibum. Investig. Ophthalmol. Vis. Sci. 2021, 62, 23. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Adil, M.Y.; Chen, X.; Utheim Ø, A.; Ræder, S.; Tønseth, K.A.; Lagali, N.S.; Dartt, D.A.; Utheim, T.P. Functional and Morphological Evaluation of Meibomian Glands in the Assessment of Meibomian Gland Dysfunction Subtype and Severity. Am. J. Ophthalmol. 2020, 209, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ma, J.; Hu, M.; Yu, J.; Zhao, Y. Assessment of tear film lipid layer thickness in patients with Meibomian gland dysfunction at different ages. BMC Ophthalmol. 2020, 20, 394. [Google Scholar] [CrossRef]
- Eom, Y.; Lee, J.S.; Kang, S.Y.; Kim, H.M.; Song, J.S. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am. J. Ophthalmol. 2013, 155, 1104–1110e1102. [Google Scholar] [CrossRef]
- Adil, M.Y.; Xiao, J.; Olafsson, J.; Chen, X.; Lagali, N.S.; Ræder, S.; Utheim Ø, A.; Dartt, D.A.; Utheim, T.P. Meibomian Gland Morphology Is a Sensitive Early Indicator of Meibomian Gland Dysfunction. Am. J. Ophthalmol. 2019, 200, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.W.; Park, S.Y.; Kim, J.S.; Kim, E.K.; Seo, K.Y.; Kim, T.I. Analysis of Factors Associated With the Tear Film Lipid Layer Thickness in Normal Eyes and Patients With Dry Eye Syndrome. Invest. Ophthalmol. Vis. Sci. 2016, 57, 4076–4083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finis, D.; König, C.; Hayajneh, J.; Borrelli, M.; Schrader, S.; Geerling, G. Six-month effects of a thermodynamic treatment for MGD and implications of meibomian gland atrophy. Cornea 2014, 33, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Kasetsuwan, N.; Suwajanakorn, D.; Tantipat, C.; Reinprayoon, U. The Efficacy Between Conventional Lid Hygiene and Additional Thermal Pulsatile System in Meibomian Gland Dysfunction Patients Treated with Long-Term Anti-Glaucoma Medications in a Randomized Controlled Trial. Clin. Ophthalmol. 2020, 14, 2891–2902. [Google Scholar] [CrossRef] [PubMed]
- Finis, D.; Hayajneh, J.; König, C.; Borrelli, M.; Schrader, S.; Geerling, G. Evaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: A prospective, randomized, observer-masked trial. Ocul. Surf. 2014, 12, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Giannaccare, G.; Mencucci, R.; Rubino, P.; Cantera, E.; Rolando, M. Modern approach to the treatment of dry eye, a complex multifactorial disease: A P. I.C.A.S.S.O. board review. Br. J. Ophthalmol. 2021, 105, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.C.; Liu, F.; Bleyen, I.; Gunn, D.A.; Hofman, A.; Klaver, C.C.; Uitterlinden, A.G.; Neumann, H.A.; Bataille, V.; Spector, T.D.; et al. Intrinsic and extrinsic risk factors for sagging eyelids. JAMA dermatology 2014, 150, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pult, H.; Riede-Pult, B. Comparison of subjective grading and objective assessment in meibography. Contact lens & anterior eye: The journal of the British Contact Lens Association 2013, 36, 22–27. [Google Scholar] [CrossRef]
- Su, T.Y.; Ho, W.T.; Lu, C.Y.; Chang, S.W.; Chiang, H.K. Correlations among ocular surface temperature difference value, the tear meniscus height, Schirmer’s test and fluorescein tear film break up time. Br. J. Ophthalmol. 2015, 99, 482–487. [Google Scholar] [CrossRef]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef]
- Mudgil, P.; Borchman, D.; Gerlach, D.; Yappert, M.C. Sebum/Meibum Surface Film Interactions and Phase Transitional Differences. Invest. Ophthalmol. Vis. Sci. 2016, 57, 2401–2411. [Google Scholar] [CrossRef] [Green Version]
- Finis, D.; Pischel, N.; Schrader, S.; Geerling, G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea 2013, 32, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Spierer, O.; Felix, E.R.; McClellan, A.L.; Parel, J.M.; Gonzalez, A.; Feuer, W.J.; Sarantopoulos, C.D.; Levitt, R.C.; Ehrmann, K.; Galor, A. Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Investig. Ophthalmol. Vis. Sci. 2016, 57, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Mitchell, T.; Murri, M.; Pflugfelder, S.C. Video Viewing Blink Rate in Normal and Dry Eyes. Eye Contact Lens. 2021, 47, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Muntz, A.; Turnbull, P.R.; Kim, A.D.; Gokul, A.; Wong, D.; Tsay, T.S.; Zhao, K.; Zhang, S.; Kingsnorth, A.; Wolffsohn, J.S.; et al. Extended screen time and dry eye in youth. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2021, 101541. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Han, K.E.; Yoon, S.C.; Ahn, J.M.; Nam, S.M.; Stulting, R.D.; Kim, E.K.; Seo, K.Y. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am. J. Ophthalmol. 2014, 157, 1144–1150e1141. [Google Scholar] [CrossRef]
- Park, J.; Yoo, Y.S.; Shin, K.; Han, G.; Arita, R.; Lim, D.H.; Chung, T.Y. Effects of Lipiflow Treatment Prior to Cataract Surgery: A Prospective, Randomized, Controlled Study. Am. J. Ophthalmol. 2021, 230, 264–275. [Google Scholar] [CrossRef]
- Kim, K.Y.; Chung, B.; Kim, E.K.; Seo, K.Y.; Jun, I.; Kim, T.I. Changes in ocular surface and Meibomian gland after penetrating Keratoplasty. BMC Ophthalmol. 2021, 21, 85. [Google Scholar] [CrossRef]
| (A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age (Years) | ||||||||
| N | Median | Q1 | Q3 | Minimum | Maximum | p | ||
| DM-1 | 917 | 50.9 | 41.0 | 62.0 | 20.0 | 90.0 | <0.001 | |
| DM-2 | 880 | 53.9 | 44.0 | 64.8 | 20.0 | 90.0 | ||
| DM-3 | 304 | 63.5 | 57.0 | 70.0 | 29.0 | 93.0 | ||
| DM-4 | 227 | 66.8 | 61.0 | 74.0 | 36.0 | 91.0 | ||
| Total | 2328 | 55.3 | 46.0 | 66.0 | 20.0 | 93.0 | ||
| (B) | ||||||||
| Sex | Female | Male | Total | |||||
| Group | N | % within Female | N | % within Male | N | % | p | |
| DM-1 | 712 | 40.3% | 205 | 36.7% | 917 | 39.4% | 0.012 | |
| DM-2 | 679 | 38.3% | 201 | 36.0% | 880 | 37.8% | ||
| DM-3 | 209 | 11.8% | 95 | 17.0% | 304 | 13.1% | ||
| DM-4 | 170 | 9.6% | 57 | 10.2% | 227 | 9.7% | ||
| Total | 1770 | 100.0% | 558 | 100.0% | 2328 | 100.0% | ||
| (A) | ||||||
|---|---|---|---|---|---|---|
| DM-1 | DM-2 | DM-3 | DM-4 | Total | p | |
| N | 917 | 880 | 304 | 227 | 2328 | |
| SPEED | 12.0 [8.0–16.0] | 12.0 [8.0–16.0] | 11.0 [7.0–15.0] | 10.0 [6.0–15.0] | 12.0 [8.0–16.0] | 0.001 |
| Frequency (SPEED) | 5.0 [4.0–7.0] | 5.0 [4.0–7.0] | 5.0 [3.0–7.0] | 5.0 [3.0–7.0] | 5.0 [4.0–7.0] | 0.001 |
| Severity (SPEED) | 6.0 [4.0–9.0] | 6.0 [4.0–8.0] | 6.0 [3.0–8.0] | 5.0 [3.0–8.0] | 6.0 [4.0–8.0] | 0.003 |
| OSDI | 37.5 [25.0–54.9] | 37.0 [22.5–56.3] | 33.3 [20.6–54.8] | 36.8 [18.8–55.4] | 36.4 [22.7–55.6] | 0.036 |
| OSDI (A) | 8.0 [5.0–11.0] | 8.0 [5.0–12.0] | 8.0 [4.0–12.0] | 8.0 [4.0–12.0] | 8.0 [5.0–12.0] | 0.984 |
| OSDI (B) | 4.0 [2.0–8.0] | 4.0 [2.0–8.0] | 4.0 [2.0–7.0] | 4.0 [1.0–7.0] | 4.0 [2.0–8.0] | 0.007 |
| OSDI (C) | 3.0 [2.0–7.0] | 3.0 [1.0–6.0] | 3.0 [1.0–6.0] | 3.0 [0.0–5.0] | 3.0 [1.0–7.0] | <0.001 |
| (B) | ||||||
| DM | Age | Age Adjusted DM | ||||
| rs | ρ | rs | ρ | rs | ρ | |
| Age | 0.351 | <0.001 | 1 | |||
| SPEED | −0.085 | <0.001 | −0.149 | <0.001 | 0.053 | 0.005 |
| Frequency (SPEED) | −0.076 | 0.001 | −0.133 | <0.001 | 0.064 | 0.001 |
| Severity (SPEED) | −0.085 | <0.001 | −0.147 | <0.001 | 0.041 | 0.029 |
| OSDI | −0.041 | 0.076 | 0.016 | 0.487 | 0.040 | 0.036 |
| OSDI (A) | −0.026 | 0.273 | 0.077 | 0.001 | 0.054 | 0.004 |
| OSDI (B) | −0.063 | 0.007 | −0.111 | <0.001 | 0.033 | 0.077 |
| OSDI (C) | −0.110 | <0.001 | −0.153 | <0.001 | −0.005 | 0.810 |
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| DM-1 | DM-2 | DM-3 | DM-4 | Total | p | ||||
| N | 917 | 880 | 304 | 227 | 2328 | ||||
| LLT (nm) | 66.5 [56.0–86.0] | 69.0 [58.0–88.0] | 76.0 [58.0–97.0] | 81.0 [63.0–110.0] | 69.0 [57.0–91.0] | <0.001 | |||
| MGE | 8.0 [5.0–11.0] | 8.0 [5.0–11.0] | 8.0 [5.0–10.0] | 7.0 [5.0–10.0] | 8.0 [5.0–10.0] | 0.022 | |||
| MGE (upper) | 5.0 [3.0–6.0] | 5.0 [3.0–6.0] | 4.0 [2.0–6.0] | 4.0 [2.0–6.0] | 5.0 [3.0–6.0] | <0.001 | |||
| MGE (lower) | 3.0 [2.0–5.0] | 3.0 [2.0–5.0] | 3.0 [2.0–5.0] | 3.0 [2.0–5.0] | 3.0 [2.0–5.0] | 0.657 | |||
| Meiboscale (grade) | 1.0 [1.0–1.5] | 1.0 [1.0–1.5] | 1.5 [1.0–2.0] | 1.5 [1.0–2.0] | 1.5 [1.0–2.0] | <0.001 | |||
| Meiboscale (upper) | 1.0 [1.0–2.0] | 1.0 [1.0–2.0] | 2.0 [1.0–2.0] | 2.0 [1.0–3.0] | 1.0 [1.0–2.0] | <0.001 | |||
| Meiboscale (lower) | 1.0 [1.0–1.0] | 1.0 [1.0–1.0] | 1.0 [1.0–2.0] | 1.0 [1.0–2.0] | 1.0 [1.0–1.0] | 0.003 | |||
| (B) | |||||||||
| DM (Grade) | Age (Years) | Age Adjusted DM | LLT (nm) | ||||||
| rs | ρ | rs | rs | rs | ρ | rs | ρ | ||
| LLT | 0.122 | <0.001 | 0.178 | <0.001 | 0.043 | 0.022 | 1 | ||
| MGE | Total | −0.040 | 0.051 | −0.074 | <0.001 | −0.005 | 0.781 | 0.117 | <0.001 |
| upper | −0.064 | 0.002 | −0.052 * | 0.012 | −0.014 | 0.462 | 0.075 | <0.001 | |
| lower | 0.000 | 0.991 | −0.066 | 0.001 | 0.000 | 0.988 | 0.117 | <0.001 | |
| Meiboscale | Total | 0.096 | <0.001 | 0.278 | <0.001 | 0.040 | 0.034 | −0.01 | 0.626 |
| upper | 0.100 | <0.001 | 0.297 | <0.001 | 0.057 | 0.003 | 0.003 | 0.896 | |
| lower | 0.033 | 0.111 | 0.122 | <0.001 | 0.011 | 0.560 | −0.052 | 0.012 | |
| LLT < 100 | LLT ≥ 100 | Total | p | ||
|---|---|---|---|---|---|
| DM-1 | N | 774 | 144 | 918 | <0.001 |
| % within DM-1 | 84.3% | 15.7% | 100.0% | ||
| DM-2 | N | 744 | 135 | 879 | |
| % within DM-2 | 84.6% | 15.4% | 100.0% | ||
| DM-3 | N | 235 | 69 | 304 | |
| % within DM-3 | 77.3% | 22.7% | 100.0% | ||
| DM-4 | N | 150 | 77 | 227 | |
| % within DM-4 | 66.1% | 33.9% | 100.0% | ||
| Total | N | 1903 | 425 | 2328 | |
| % within DM | 81.7% | 18.3% | 100.0% |
| (A) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DM-1 | DM-2 | DM-3 | DM-4 | Total | p | |||||
| N | 917 | 880 | 304 | 227 | 2328 | |||||
| PB | 3.0 [1.0–6.0] | 3.0 [1.0–7.0] | 3.0 [1.0–5.0] | 2.0 [1.0–5.0] | 3.0 [1.0–6.0] | <0.001 | ||||
| PB (%) | 62.5 [27.3–92.9] | 66.7 [33.3–90.0] | 63.6 [26.8–91.3] | 50.0 [20.0–84.2] | 62.5 [28.6–90.9] | 0.106 | ||||
| TB | 7.0 [4.0–11.0] | 7.0 [4.0–10.0] | 6.0 [3.0–8.0] | 6.0 [3.0–9.0] | 6.0 [4.0–10.0] | <0.001 | ||||
| Schirmer (mm) | 4.0 [2.0–7.0] | 4.0 [2.0–6.0] | 4.0 [2.0–7.0] | 4.0 [2.0–7.0] | 4.0 [2.0–7.0] | 0.776 | ||||
| TBUT (s) | 3.0 [2.0–3.0] | 3.0 [2.0–3.0] | 3.0 [2.0–3.0] | 2.5 [2.0–3.0] | 3.0 [2.0–3.0] | 0.284 | ||||
| SPK (grade) | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.417 | ||||
| (B) | ||||||||||
| DM (Grade) | Age (Year) | Age Adjusted DM | Schirmer (mm) | SPK (Grade) | ||||||
| rs | ρ | rs | ρ | rs | ρ | rs | ρ | rs | ρ | |
| TB | −0.115 | <0.001 | −0.218 | <0.001 | −0.087 | <0.001 | −0.019 | 0.416 | 0.017 | 0.471 |
| PB | −0.085 | <0.001 | −0.194 | <0.001 | −0.054 | 0.004 | −0.044 | 0.059 | 0.040 | 0.091 |
| PB (%) | −0.011 | 0.628 | −0.076 | 0.001 | −0.015 | 0.438 | −0.030 | 0.213 | 0.026 | 0.270 |
| Schirmer (mm) | 0.008 | 0.743 | −0.065 | 0.005 | −0.017 | 0.362 | 1 | −0.073 | 0.002 | |
| FTBUT (s) | −0.027 | 0.251 | −0.094 | <0.001 | −0.020 | 0.290 | 0.151 | <0.001 | −0.151 | <0.001 |
| SPK (grade) | −0.005 | 0.828 | 0.038 | 0.100 | 0.022 | 0.257 | −0.073 | 0.002 | 1 | |
| N (% within Group) | Female | Male | Total | p |
|---|---|---|---|---|
| LLT < 100 | 1410 (79.70%) | 493 (88.40%) | 1903 (81.70%) | <0.001 |
| LLT ≥ 100 | 360 (20.30%) | 65 (11.60%) | 425 (18.30%) | |
| Total | 1770 | 558 | 2328 |
| (A) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM 1–4 (N = 644) | DM-1 (N = 223) | DM-2 (N = 279) | DM-3 (N = 95) | DM4 (N = 47) | ||||||||||||
| Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | ||
| SPEED (total) | Before | 12.0 | 8.0 | <0.001 | 12.0 | 8.0 | <0.001 | 12.0 | 8.0 | <0.001 | 11.80 | 7.0 | <0.001 | 12.0 | 7.00 | 0.007 |
| After | 8.0 | 7.0 | 9.0 | 8.0 | 8.0 | 6.0 | 9.0 | 7.0 | 9.0 | 8.00 | ||||||
| Frequency (SPEED) | Before | 5.0 | 3.0 | <0.001 | 6.0 | 4.0 | <0.001 | 6.0 | 3.0 | <0.001 | 5.57 | 3.0 | 0.002 | 6.0 | 4.00 | 0.031 |
| After | 4.0 | 3.0 | 4.0 | 4.0 | 4.0 | 2.0 | 4.0 | 3.0 | 4.0 | 3.00 | ||||||
| Severity (SPEED) | Before | 6.0 | 4.0 | <0.001 | 6.0 | 5.0 | <0.001 | 6.0 | 4.0 | <0.001 | 6.23 | 5.0 | <0.001 | 6.0 | 5.00 | 0.008 |
| After | 4.0 | 5.0 | 5.0 | 5.0 | 4.0 | 3.0 | 5.0 | 4.0 | 5.0 | 5.00 | ||||||
| OSDI (total) | Before | 36.4 | 33.5 | <0.001 | 35.4 | 33.3 | <0.001 | 37.5 | 31.8 | <0.001 | 41.70 | 35.8 | 0.002 | 45.5 | 46.53 | 0.018 |
| After | 25.0 | 26.7 | 25.0 | 25.0 | 27.1 | 25.0 | 29.4 | 33.5 | 31.3 | 26.45 | ||||||
| OSDI (A) | Before | 7.0 | 6.0 | <0.001 | 8.0 | 6.0 | <0.001 | 8.0 | 6.0 | <0.001 | 8.54 | 8.0 | 0.122 | 10.0 | 9.00 | <0.001 |
| After | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 6.0 | 8.0 | 6.0 | 6.00 | ||||||
| OSDI (B) | Before | 4.0 | 5.8 | <0.001 | 4.0 | 4.0 | <0.001 | 4.0 | 5.0 | <0.001 | 5.38 | 6.0 | 0.004 | 4.0 | 7.00 | 0.139 |
| After | 3.0 | 4.0 | 3.0 | 4.0 | 3.0 | 4.0 | 3.0 | 5.0 | 4.0 | 6.00 | ||||||
| OSDI (C) | Before | 3.0 | 4.8 | <0.001 | 3.0 | 5.0 | <0.001 | 3.0 | 5.0 | 0.006 | 4.06 | 5.0 | 0.001 | 3.0 | 6.00 | 0.194 |
| After | 3.0 | 4.0 | 3.0 | 5.0 | 3.0 | 4.0 | 3.0 | 5.0 | 3.0 | 5.00 | ||||||
| (B) | ||||||||||||||||
| DM 1–4 (N = 644) | DM-1 (N = 223) | DM-2 (N = 279) | DM-3 (N = 95) | DM-4 (N = 47) | ||||||||||||
| Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | ||
| LLT (nm) | Before | 63.0 | 35.0 | 0.004 | 64.0 | 32.0 | 0.003 | 67.0 | 37.0 | 0.004 | 69.0 | 38.0 | 0.159 | 73.0 | 53.0 | 0.105 |
| After | 63.0 | 29.0 | 62.0 | 27.3 | 65.0 | 28.0 | 68.0 | 34.0 | 61.5 | 32.0 | ||||||
| MGE | Before | 7.0 | 5.0 | 0.127 | 7.0 | 6.0 | 0.260 | 8.0 | 4.0 | 0.901 | 8.0 | 5.0 | 0.453 | 8.0 | 6.0 | 0.647 |
| After | 8.0 | 5.0 | 8.0 | 6.0 | 8.0 | 5.0 | 8.0 | 4.0 | 8.0 | 5.0 | ||||||
| Meiboscale(grade) | Before | 1.0 | 1.0 | 0.740 | 1.5 | 1.0 | 1.000 | 1.5 | 0.5 | 0.414 | 1.5 | 1.0 | 0.782 | 2.0 | 1.0 | 0.180 |
| After | 1.0 | 1.0 | 1.5 | 1.0 | 1.5 | 0.5 | 1.5 | 1.0 | 1.5 | 1.0 | ||||||
| PB | Before | 3.0 | 5.0 | <0.001 | 3.0 | 5.0 | <0.001 | 4.0 | 6.0 | <0.001 | 3.0 | 5.0 | 0.022 | 2.0 | 5.0 | 0.355 |
| After | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 4.0 | 2.0 | 5.3 | ||||||
| PB (%) | Before | 64.3 | 67.9 | <0.001 | 50.0 | 65.9 | 0.008 | 66.7 | 59.0 | <0.001 | 71.4 | 70.8 | 0.002 | 50.0 | 76.8 | 0.128 |
| After | 40.0 | 65.0 | 40.0 | 65.0 | 40.0 | 68.6 | 40.0 | 75.8 | 33.3 | 67.1 | ||||||
| TB | Before | 7.0 | 7.0 | 0.627 | 7.0 | 8.0 | 0.532 | 7.0 | 7.0 | 0.754 | 6.0 | 6.0 | 1.000 | 6.0 | 4.0 | 0.435 |
| After | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 6.0 | 7.0 | 8.0 | 7.0 | 6.3 | ||||||
| Schirmer test (mm) | Before | 4.0 | 5.0 | <0.001 | 4.0 | 6.0 | 0.028 | 4.0 | 5.0 | 0.002 | 4.0 | 5.0 | 0.138 | 4.0 | 5.0 | 0.262 |
| After | 4.0 | 4.0 | 3.0 | 5.0 | 3.0 | 4.3 | 4.0 | 4.0 | 4.0 | 6.0 | ||||||
| FTBUT (sec) | Before | 3.0 | 1.0 | 0.007 | 3.0 | 1.0 | 0.152 | 3.0 | 1.0 | 0.204 | 3.0 | 1.0 | 0.072 | 2.0 | 1.0 | 0.405 |
| After | 3.0 | 1.0 | 3.0 | 2.0 | 3.0 | 2.0 | 3.0 | 1.0 | 3.0 | 1.0 | ||||||
| SPK (grade) | Before | 0.0 | 0.0 | 0.103 | 0.0 | 0.0 | 0.206 | 0.0 | 0.0 | 0.895 | 0.0 | 0.0 | 0.197 | 0.0 | 0.0 | 0.102 |
| After | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| (A) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM 1–4 (N = 238) | DM-1 (N =103) | DM-2 (N = 83) | DM-3 (N = 31) | DM-4 (N = 21) | ||||||||||||
| Exam | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | |
| SPEED (total) | 1st | 11.0 | 8.0 | 0.087 | 12.0 | 8.0 | 0.179 | 11.0 | 7.0 | 0.265 | 10.0 | 7.0 | 0.883 | 10.0 | 8.0 | 0.458 |
| 2nd | 10.0 | 8.0 | 10.0 | 8.0 | 11.0 | 8.0 | 10.0 | 9.0 | 7.0 | 7.0 | ||||||
| Frequency (SPEED) | 1st | 5.0 | 3.0 | 0.062 | 5.0 | 3.0 | 0.312 | 5.0 | 3.0 | 0.154 | 4.0 | 3.0 | 0.726 | 5.0 | 3.0 | 0.308 |
| 2nd | 4.5 | 4.0 | 5.0 | 4.0 | 5.0 | 2.0 | 4.0 | 4.0 | 4.0 | 3.5 | ||||||
| Severity (SPEED) | 1st | 6.0 | 4.0 | 0.149 | 6.0 | 4.5 | 0.155 | 6.0 | 4.0 | 0.437 | 5.0 | 5.0 | 0.980 | 5.0 | 5.0 | 0.726 |
| 2nd | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 7.0 | 4.0 | 4.5 | ||||||
| OSDI(total) | 1st | 36.4 | 33.1 | 0.091 | 37.5 | 30.0 | 0.305 | 36.0 | 34.3 | 0.305 | 31.8 | 32.7 | 0.595 | 32.5 | 34.0 | 0.498 |
| 2nd | 31.3 | 31.5 | 31.3 | 38.9 | 31.8 | 27.5 | 32.5 | 22.2 | 29.2 | 24.9 | ||||||
| OSDI (A) | 1st | 8.0 | 6.3 | 0.093 | 8.0 | 6.5 | 0.180 | 8.0 | 8.0 | 0.506 | 7.0 | 7.0 | 0.498 | 7.0 | 8.0 | 0.539 |
| 2nd | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 6.0 | 5.0 | 5.0 | 8.0 | ||||||
| OSDI (B) | 1st | 4.0 | 5.0 | <0.001 | 4.0 | 6.0 | 0.049 | 4.0 | 5.0 | 0.024 | 3.0 | 4.0 | 0.070 | 3.0 | 5.0 | 0.378 |
| 2nd | 3.0 | 5.0 | 4.0 | 5.0 | 3.0 | 3.0 | 3.0 | 4.0 | 2.0 | 3.5 | ||||||
| OSDI (C) | 1st | 3.0 | 5.0 | 0.727 | 3.0 | 5.5 | 0.881 | 3.0 | 5.0 | 0.841 | 3.0 | 5.0 | 0.381 | 3.0 | 5.0 | 0.324 |
| 2nd | 3.0 | 4.3 | 3.0 | 4.0 | 3.0 | 5.0 | 3.0 | 7.0 | 3.0 | 2.5 | ||||||
| (B) | ||||||||||||||||
| DM 1–4 (N = 238) | DM-1 (N =103) | DM-2 (N = 83) | DM-3 (N = 31) | DM-4 (N = 21) | ||||||||||||
| Exam | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | Median | IQR | p | |
| LLT (nm) | 1st | 69.0 | 33.0 | 0.368 | 66.0 | 28.0 | 0.797 | 69.0 | 29.0 | 0.244 | 77.0 | 38.0 | 0.748 | 86.0 | 48.0 | 0.370 |
| 2nd | 68.5 | 36.0 | 64.0 | 35.0 | 67.0 | 29.0 | 74.0 | 34.0 | 83.0 | 42.5 | ||||||
| MGE | 1st | 8.0 | 6.0 | 0.720 | 8.0 | 5.0 | 0.208 | 8.0 | 7.0 | 0.757 | 8.0 | 5.0 | 0.146 | 8.0 | 6.0 | 0.479 |
| 2nd | 8.0 | 6.0 | 8.0 | 6.0 | 8.0 | 6.0 | 8.0 | 6.0 | 5.0 | 6.0 | ||||||
| Meiboscale (grade) | 1st | 1.0 | 0.5 | 0.020 | 1.0 | 0.5 | 0.126 | 1.0 | 0.5 | 0.185 | 1.5 | 1.0 | 0.261 | 1.5 | 1.0 | 0.596 |
| 2nd | 1.5 | 1.0 | 1.5 | 1.0 | 1.5 | 1.0 | 1.5 | 1.5 | 1.5 | 1.0 | ||||||
| PB | 1st | 3.0 | 5.0 | 0.004 | 4.0 | 5.0 | 0.331 | 3.0 | 5.0 | 0.051 | 3.0 | 4.0 | 0.176 | 3.0 | 4.0 | 0.029 |
| 2nd | 2.0 | 4.0 | 3.0 | 5.0 | 2.0 | 3.0 | 2.0 | 3.0 | 1.0 | 3.0 | ||||||
| PB (%) | 1st | 62.5 | 60.8 | 0.002 | 62.5 | 61.6 | 0.048 | 60.0 | 62.6 | 0.028 | 63.1 | 58.5 | 0.890 | 50.0 | 60.1 | 0.110 |
| 2nd | 50.0 | 63.3 | 50.0 | 57.5 | 48.3 | 62.3 | 66.7 | 63.3 | 33.3 | 77.2 | ||||||
| TB | 1st | 6.0 | 6.0 | 0.901 | 7.0 | 7.0 | 0.389 | 6.0 | 6.0 | 0.905 | 6.0 | 5.0 | 0.459 | 6.0 | 6.0 | 0.506 |
| 2nd | 6.0 | 7.0 | 7.0 | 7.0 | 6.0 | 7.0 | 5.0 | 6.0 | 4.0 | 6.0 | ||||||
| Schirmer test (mm) | 1st | 4.0 | 5.0 | 0.003 | 4.0 | 5.0 | 0.021 | 4.0 | 5.0 | 0.296 | 4.0 | 4.0 | 0.651 | 4.0 | 5.0 | 0.015 |
| 2nd | 3.0 | 5.0 | 3.0 | 5.0 | 4.0 | 5.0 | 4.0 | 4.3 | 2.0 | 4.0 | ||||||
| FTBUT (sec) | 1st | 3.0 | 1.0 | 0.555 | 3.0 | 1.0 | 0.575 | 3.0 | 1.0 | 0.846 | 3.0 | 1.0 | 0.881 | 3.0 | 1.0 | 0.678 |
| 2nd | 3.0 | 1.0 | 3.0 | 2.0 | 3.0 | 1.0 | 3.0 | 1.0 | 2.0 | 1.0 | ||||||
| SPK (grade) | 1st | 0.0 | 0.0 | 0.187 | 0.0 | 0.0 | 0.030 | 0.0 | 0.0 | 0.815 | 0.0 | 1.0 | 0.647 | 0.0 | 0.0 | 0.672 |
| 2nd | 0.0 | 1.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-L.; Chang, S.-W. Dermatochalasis Aggravates Meibomian Gland Dysfunction Related Dry Eyes. J. Clin. Med. 2022, 11, 2379. https://doi.org/10.3390/jcm11092379
Wu W-L, Chang S-W. Dermatochalasis Aggravates Meibomian Gland Dysfunction Related Dry Eyes. Journal of Clinical Medicine. 2022; 11(9):2379. https://doi.org/10.3390/jcm11092379
Chicago/Turabian StyleWu, Wan-Lin, and Shu-Wen Chang. 2022. "Dermatochalasis Aggravates Meibomian Gland Dysfunction Related Dry Eyes" Journal of Clinical Medicine 11, no. 9: 2379. https://doi.org/10.3390/jcm11092379
APA StyleWu, W.-L., & Chang, S.-W. (2022). Dermatochalasis Aggravates Meibomian Gland Dysfunction Related Dry Eyes. Journal of Clinical Medicine, 11(9), 2379. https://doi.org/10.3390/jcm11092379






