Relationship between Rate of Force Development of Tongue Pressure and Physical Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Tongue Pressure
2.3. Oral and Swallowing Function
2.4. Physical Condition and Cognitive Function Assessment
2.5. Physical Performance
2.6. Statistical Analyses
3. Results
Summary of Subjects
4. Discussion
4.1. Factors Influencing RFD-TP
4.2. Relationship between Tongue Pressure and Oral and Physical Performance
4.3. Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolford, S.J.; Sohan, O.; Dennison, E.M.; Cooper, C.; Patel, H.P. Approaches to the diagnosis and prevention of frailty. Aging Clin. Exp. Res. 2020, 32, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E. Editorial: Oral Frailty. J. Nutr. Health Aging 2020, 24, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tsuji, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Robbins, J.; Levine, R.; Wood, J.; Roecker, E.B.; Luschei, E. Age effects on lingual pressure generation as a risk factor for dysphagia. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M257–M262. [Google Scholar] [CrossRef]
- Kobuchi, R.; Okuno, K.; Kusunoki, T.; Inoue, T.; Takahashi, K. The relationship between sarcopenia and oral sarcopenia in elderly people. J. Oral. Rehabil. 2020, 47, 636–642. [Google Scholar] [CrossRef]
- Butler, S.G.; Stuart, A.; Leng, X.; Wilhelm, E.; Rees, C.; Williamson, J.; Kritchevsky, S.B. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of force development: Physiological and methodological considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef] [Green Version]
- de Ruiter, C.J.; Van Leeuwen, D.; Heijblom, A.; Bobbert, M.F.; de Haan, A. Fast unilateral isometric knee extension torque development and bilateral jump height. Med. Sci. Sports Exerc. 2006, 38, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H.; Mercer, V.S.; Giuliani, C.A.; Sloane, P.D. Relationship between hip abductor rate of force development and mediolateral stability in older adults. Arch. Phys. Med. Rehabil. 2005, 86, 1843–1850. [Google Scholar] [CrossRef]
- Winters, J.D.; Christiansen, C.L.; Stevens-Lapsley, J.E. Preliminary investigation of rate of torque development deficits following total knee arthroplasty. Knee 2014, 21, 382–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klass, M.; Baudry, S.; Duchateau, J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J. Appl. Physiol. 2008, 104, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Schettino, L.; Luz, C.P.; de Oliveira, L.E.; de Assuncao, P.L.; da Silva Coqueiro, R.; Fernandes, M.H.; Brown, L.E.; Machado, M.; Pereira, R. Comparison of explosive force between young and elderly women: Evidence of an earlier decline from explosive force. Age 2014, 36, 893–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, T.; Ono, T.; Hori, K.; Wada, Y.; Uchiyama, Y.; Kasama, S.; Yoshikawa, H.; Domen, K. Tongue Pressure Measurement and Videofluoroscopic Study of Swallowing in Patients with Parkinson’s Disease. Dysphagia 2019, 34, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Kumakura, I.; Hori, K.; Tamine, K.I.; Ono, T. Differences in biomechanical features of tongue pressure production between articulation and swallow. J. Oral Rehabil. 2012, 39, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Belafsky, P.C.; Mouadeb, D.A.; Rees, C.J.; Pryor, J.C.; Postma, G.N.; Allen, J.; Leonard, R.J. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann. Otol. Rhinol. Laryngol. 2008, 117, 919–924. [Google Scholar] [CrossRef]
- Sakayori, T.; Maki, Y.; Hirata, S.; Okada, M.; Ishii, T. Evaluation of a Japanese “Prevention of long-term care” project for the improvement in oral function in the high-risk elderly. Geriatr. Gerontol. Int. 2013, 13, 451–457. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Dick, J.P.; Guiloff, R.J.; Stewart, A.; Blackstock, J.; Bielawska, C.; Paul, E.A.; Marsden, C.D. Mini-mental state examination in neurological patients. J. Neurol. Neurosurg. Psychiatry 1984, 47, 496–499. [Google Scholar] [CrossRef] [Green Version]
- Lopopolo, R.B.; Greco, M.; Sullivan, D.; Craik, R.L.; Mangione, K.K. Effect of Therapeutic Exercise on Gait Speed in Community-Dwelling Elderly People: A Meta-analysis. Phys. Ther. 2006, 86, 520–540. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michikawa, T.; Nishiwaki, Y.; Takebayashi, T.; Toyama, Y. One-leg standing test for elderly populations. J. Orthop. Sci. 2009, 14, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Murray, S.M.; Chapman, K.; Munro, B.; Tiedemann, A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M539–M543. [Google Scholar] [CrossRef] [PubMed]
- Vianna, L.C.; Oliveira, R.B.; Araujo, C.G. Age-related decline in handgrip strength differs according to gender. J. Strength Cond. Res. 2007, 21, 1310–1314. [Google Scholar] [CrossRef]
- Suzuki, H.; Ayukawa, Y.; Ueno, Y.; Atsuta, I.; Jinnouchi, A.; Koyano, K. Relationship between Maximum Tongue Pressure Value and Age, Occlusal Status, or Body Mass Index among the Community-Dwelling Elderly. Medicina 2020, 56, 623. [Google Scholar] [CrossRef]
- Sanders, I.; Mu, L.; Amirali, A.; Su, H.; Sobotka, S. The human tongue slows down to speak: Muscle fibers of the human tongue. Anat. Rec. 2013, 296, 1615–1627. [Google Scholar] [CrossRef] [Green Version]
- Satoh, M.; Sashima, M.; Itagaki, M.; Suzuki, A. Quantitative age changes of the histological constituents of the human tongue. Jpn. J. Oral Biol. 1986, 28, 746–751. [Google Scholar] [CrossRef]
- Roubenoff, R. Sarcopenia: Effects on body composition and function. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 1012–1017. [Google Scholar] [CrossRef]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Raj, I.S.; Bird, S.R.; Shield, A.J. Aging and the force-velocity relationship of muscles. Exp. Gerontol. 2010, 45, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Akima, H.; Yoshiko, A.; Tomita, A.; Ando, R.; Saito, A.; Ogawa, M.; Kondo, S.; Tanaka, N. Relationship between quadriceps echo intensity and functional and morphological characteristics in older men and women. Arch. Gerontol. Geriatr. 2017, 70, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.; Gangnon, R.E.; Theis, S.M.; Kays, S.A.; Hewitt, A.L.; Hind, J.A. The effects of lingual exercise on swallowing in older adults. J. Am. Geriatr. Soc. 2005, 53, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Tamura, F.; Kikutani, T.; Tohara, T.; Yoshida, M.; Yaegaki, K. Tongue thickness relates to nutritional status in the elderly. Dysphagia 2012, 27, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, Y.; Yamashita, T.; Honda, K.; Katsuura, T.; Hama, Y.; Nakamura, Y.; Ando, K.; Shikukra, R.; Kodama, N.; Uchiyama, Y.; et al. Association among Age-Related Tongue Muscle Abnormality, Tongue Pressure, and Presbyphagia: A 3D MRI Study. Dysphagia 2021, 36, 483–491. [Google Scholar] [CrossRef]
- Utanohara, Y.; Hayashi, R.; Yoshikawa, M.; Yoshida, M.; Tsuga, K.; Akagawa, Y. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia 2008, 23, 286–290. [Google Scholar] [CrossRef]
- Ono, T.; Kumakura, I.; Arimoto, M.; Hori, K.; Dong, J.; Iwata, H.; Nokubi, T.; Tsuga, K.; Akagawa, Y. Influence of bite force and tongue pressure on oro-pharyngeal residue in the elderly. Gerodontology 2007, 24, 143–150. [Google Scholar] [CrossRef]
- Ogawa, N.; Mori, T.; Fujishima, I.; Wakabayashi, H.; Itoda, M.; Kunieda, K.; Shigematsu, T.; Nishioka, S.; Tohara, H.; Yamada, M.; et al. Ultrasonography to Measure Swallowing Muscle Mass and Quality in Older Patients With Sarcopenic Dysphagia. J. Am. Med. Dir. Assoc. 2018, 19, 516–522. [Google Scholar] [CrossRef]
- Kajisa, E.; Tohara, H.; Nakane, A.; Wakasugi, Y.; Hara, K.; Yamaguchi, K.; Yoshimi, K.; Minakuchi, S. The relationship between jaw-opening force and the cross-sectional area of the suprahyoid muscles in healthy elderly. J. Oral. Rehabil. 2018, 45, 222–227. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepulveda, D.; Baumgartner, R.N.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar] [CrossRef]
- Mizuno, T.; Matsui, Y.; Tomida, M.; Suzuki, Y.; Nishita, Y.; Tange, C.; Shimokata, H.; Imagama, S.; Otsuka, R.; Arai, H. Differences in the mass and quality of the quadriceps with age and sex and their relationships with knee extension strength. J. Cachexia Sarcopenia Muscle 2021, 12, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, T.; Guralnik, J.M.; Ferrucci, L.; Penninx, B.W.; Leveille, S.; Sipila, S.; Fried, L.P. Coimpairments as predictors of severe walking disability in older women. J. Am. Geriatr. Soc. 2001, 49, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-leg balance is an important predictor of injurious falls in older persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef] [PubMed]
- di Vico, R.; Ardego, L.P.; Salernitano, G.; Chamari, K.; Padulo, J. The acute effect of the tongue position in the mouth on knee isokinetic test performance: A highly surprising pilot study. Muscles Ligaments Tendons J. 2013, 3, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosio-Lima, L.M.; Reynolds, K.L.; Winter, C.; Paolone, V.; Jones, M.T. Effects of Physioball and Conventional Floor Exercises on Early Phase Adaptations in Back and Abdominal Core Stability and Balance in Women. J. Strength Cond. Res. 2003, 17, 721–725. [Google Scholar] [CrossRef]
- Yoshimi, K.; Hara, K.; Tohara, H.; Nakane, A.; Nakagawa, K.; Yamaguchi, K.; Kurosawa, Y.; Yoshida, S.; Ariya, C.; Minakuchi, S. Relationship between swallowing muscles and trunk muscle mass in healthy elderly individuals: A cross-sectional study. Arch. Gerontol. Geriatr. 2018, 79, 21–26. [Google Scholar] [CrossRef]
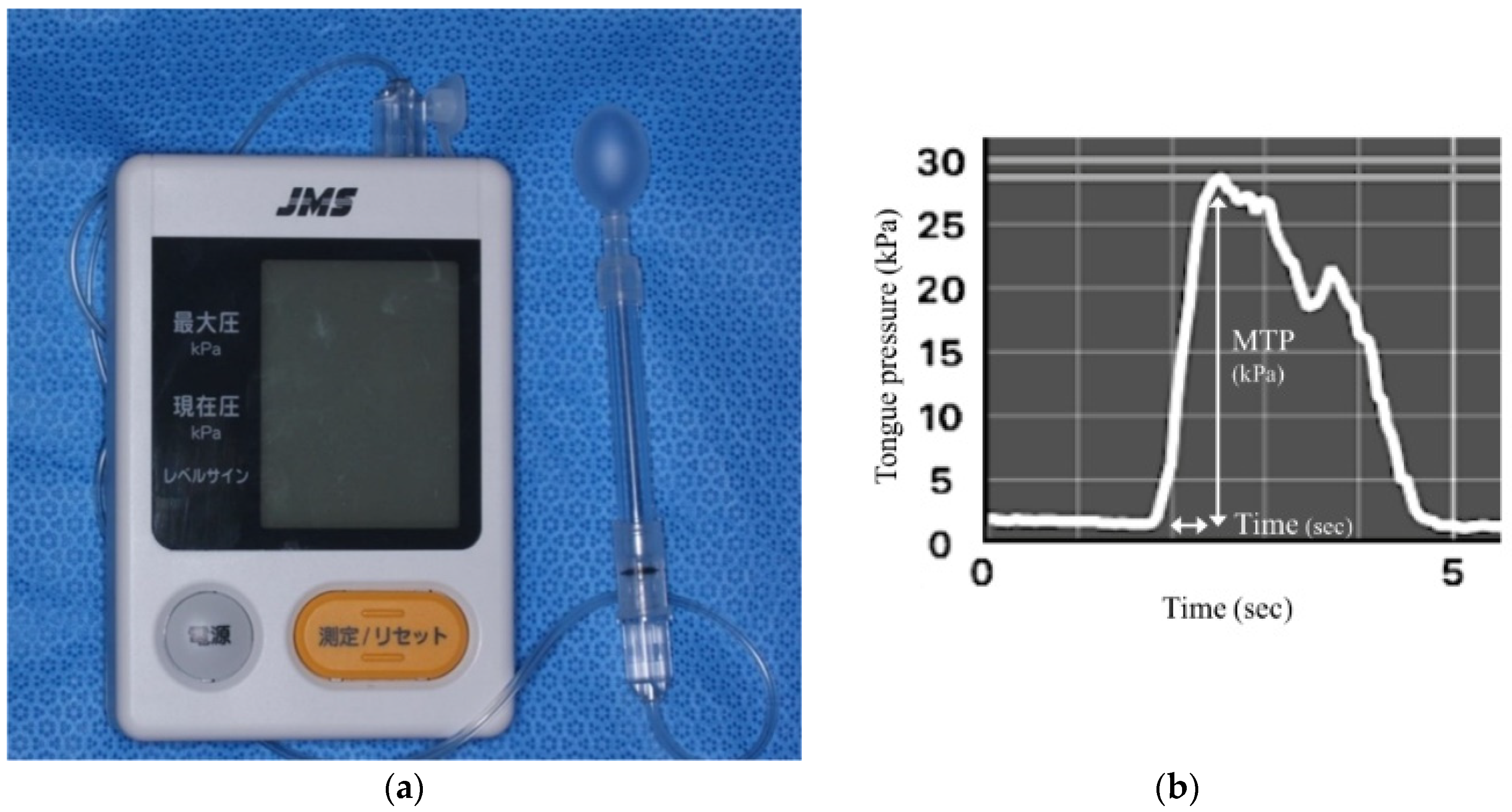
| Measurement Variables | Overall (n = 87) | Male (n = 29) | Female (n = 58) | p-Value | Rage | p-Value |
|---|---|---|---|---|---|---|
| Physical/Cognitive condition | ||||||
| Age (years) | 74.3 (69.0, 80.0) | 75.2 (69.0, 81.0) | 73.8 (69.0, 78.0) | 0.358 | - | - |
| BMI (kg/m2) * | 22.6 (20.5, 24.4) | 23.8 (22.1, 25.5) | 22.0 (20.2, 24.2) | 0.001 | 0.003 | 0.977 |
| SMI (kg/m2) * | 6.4 (5.7, 7.2) | 7.5 (7.1, 7.9) | 5.9 (5.5, 6.2) | <0.001 | −0.113 | 0.299 |
| MMSE (score) # | 28.1 (27.0, 30.0) | 27.7 (26.0, 30.0) | 28.2 (27.0, 30.0) | 0.298 | −0.295 | 0.006 |
| Oral function | ||||||
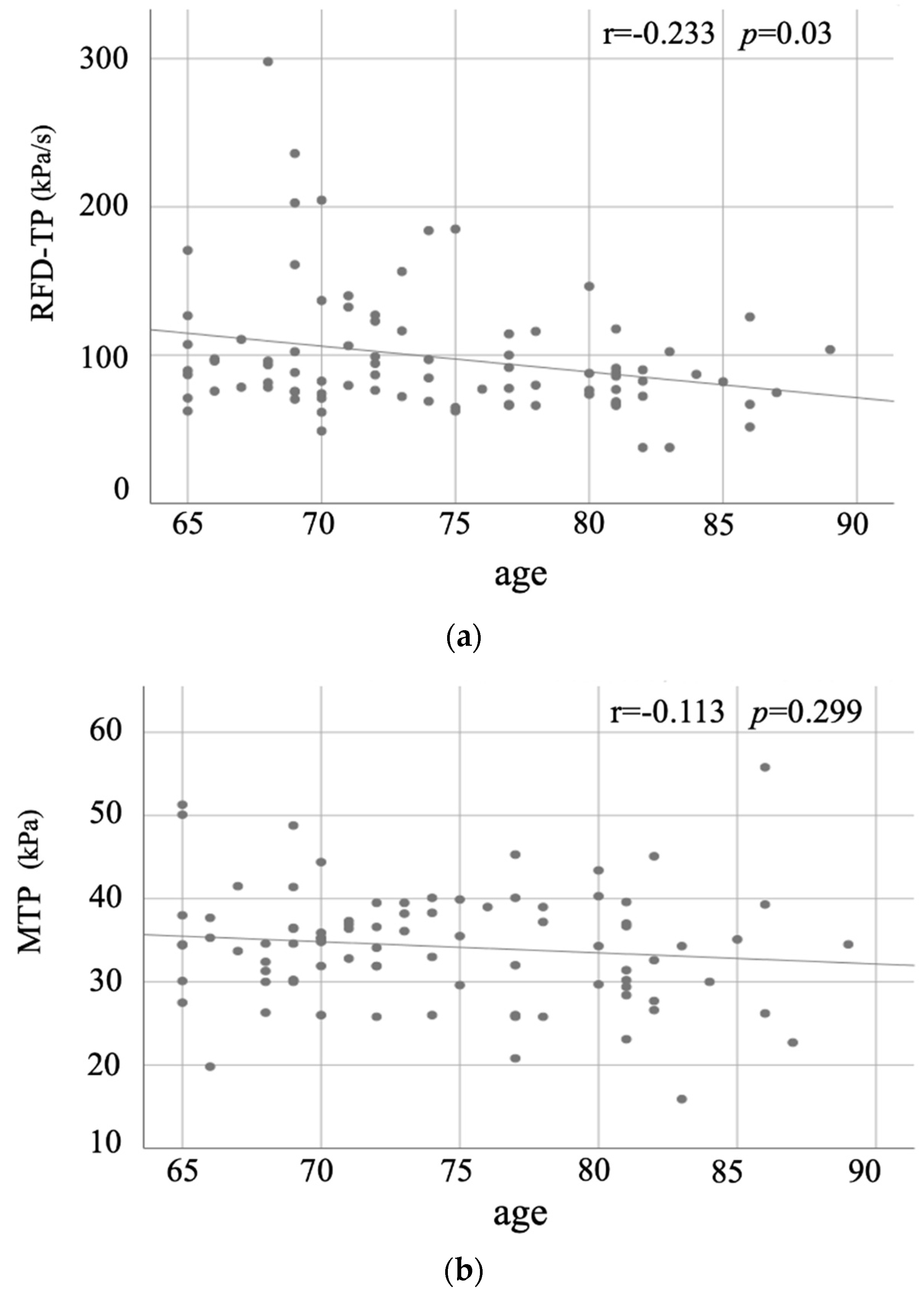
| RFD-TP (kPa/s) # | 98.6 (73.5, 110.6) | 109.0(71.6, 151.4) | 93.4 (73.9, 102.3) | 0.331 | −0.233 | 0.03 |
| MTP (kPa) | 34.3 (30.0, 38.2) | 33.9 (27.6, 39.4) | 34.5 (30.2, 37.8) | 0.709 | −0.113 | 0.299 |
| ODK /ta/ (times/5 s) # | 31.1 (29.0, 34.0) | 30.1 (27.5, 34.5) | 31.5 (29.0, 34.0) | 0.307 | −0.219 | 0.041 |
| RSST (times/30 s) * | 5.1 (3.0, 7.0) | 6.0 (4.0, 7.5) | 4.7 (3.0, 6.0) | 0.012 | −0.133 | 0.221 |
| EAT-10 (score) | 0.2 (0, 0) | 0.2 (0, 0) | 0.2 (0, 0) | 0.363 | 0.126 | 0.245 |
| Physical performance | ||||||
| Walking speed (m/s) | 1.5 (1.3, 1.7) | 1.4 (1.2, 1.5) | 1.5 (1.3, 1.7) | 0.11 | −0.085 | 0.435 |
| TUG (s) # | 6.0 (5.1, 6.5) | 5.9 (5.0, 6.3) | 6.1 (5.3, 6.5) | 0.163 | 0.581 | <0.001 |
| One-leg standing (s) # | 32.7 (11.8, 60.0) | 34.5 (12.6, 60.0) | 31.8 (11.4, 60.0) | 0.559 | −0.575 | <0.001 |
| 5CS (s) # | 6.87 (5.36, 7.40) | 7.61 (5.57, 8.34) | 6.49 (5.28, 7.12) | 0.192 | 0.345 | 0.001 |
| Grip strength (kg) *# | 27.6 (22.0, 32.0) | 36.4 (30.3, 41.3) | 23.2 (20.4, 26.5) | <0.001 | −0.295 | 0.006 |
| Knee extension force (N) *# | 358.6 (276.0, 418.0) | 467.1 (350.0, 540.5) | 304.3 (255.8, 343.0) | <0.001 | −0.368 | <0.001 |
| RFD-TP | MTP | |||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| Physical/Cognitive condition | ||||
| Age | −0.233 | 0.03 * | −0.113 | 0.299 |
| BMI | 0.079 | 0.472 | 0.184 | 0.09 |
| SMI | 0.173 | 0.112 | 0.092 | 0.401 |
| MMSE | 0.21 | 0.053 | 0.009 | 0.937 |
| Oral function | ||||
| ODK/ta/ | −0.022 | 0.839 | −0.054 | 0.619 |
| RSST | 0.048 | 0.66 | −0.083 | 0.445 |
| EAT-10 | 0.021 | 0.847 | −0.035 | 0.744 |
| Physical performance | ||||
| Walking speed | −0.187 | 0.083 | −0.215 | 0.046 * |
| TUG | −0.172 | 0.111 | −0.104 | 0.338 |
| One-leg standing | 0.284 | 0.008 * | 0.099 | 0.361 |
| 5CS | −0.177 | 0.101 | −0.175 | 0.106 |
| Grip strength | 0.12 | 0.268 | −0.118 | 0.277 |
| Knee extension force | 0.316 | 0.003 * | 0.029 | 0.793 |
| RFD-TP | B | β | p-Value | βAge | p-Value | βSex | p-Value |
|---|---|---|---|---|---|---|---|
| Physical/Cognitive condition | |||||||
| BMI | 0.025 | 0.032 | 0.782 | −0.154 | 0.187 | ||
| SMI | 2.84 | 0.266 | 0.772 | 0.224 | 0.772 | ||
| MMSE | 0.171 | 0.168 | 0.127 | −0.228 | 0.04 | ||
| Oral function | |||||||
| ODK | 0.106 | 0.011 | 0.917 | −0.271 | 0.013 | ||
| RSST | 0.098 | 0.025 | 0.823 | −0.164 | 0.145 | ||
| Physical performance | |||||||
| TUG | 0.34 | 0.054 | 0.662 | −0.298 | 0.018 | ||
| One-leg standing * | 0.249 | 0.273 | 0.034 | −0.108 | 0.397 | ||
| 5CS | −0.047 | −0.003 | 0.956 | −0.258 | 0.023 | ||
| Grip strength | 0.062 | 0.012 | 0.95 | −0.275 | 0.029 | −0.19 | 0.302 |
| Knee extension force * | 0.19 | 0.308 | 0.048 | −0.153 | 0.214 | 0.017 | 0.907 |
| MTP | B | β | p-value | βAge | p-value | βsex | p-value |
| Physical/Cognitive condition | |||||||
| BMI | 0.037 | 0.156 | 0.181 | 0.079 | 0.498 | ||
| SMI | 1.014 | 0.315 | 0.105 | 0.284 | 0.143 | ||
| MMSE | −0.01 | −0.034 | 0.764 | −0.201 | 0.079 | ||
| Oral function | |||||||
| ODK | 0.381 | 0.128 | 0.24 | −0.153 | 0.162 | ||
| RSST | 1.317 | 0.093 | 0.408 | 0.017 | 0.877 | ||
| Physical performance | |||||||
| TUG | 0.271 | 0.14 | 0.273 | −0.21 | 0.101 | ||
| One-leg standing | 0.015 | 0.051 | 0.702 | −0.106 | 0.432 | ||
| 5CS | −0.135 | −0.103 | 0.378 | −0.097 | 0.404 | ||
| Grip strength | −0.161 | −0.215 | 0.274 | −0.221 | 0.09 | −0.142 | 0.453 |
| Knee extension force | 0.013 | 0.068 | 0.683 | −0.104 | 0.431 | 0.073 | 0.637 |
| Confidence Interval | |||||||
|---|---|---|---|---|---|---|---|
| Variables | B | S.E. | β | t-Value | p-Value | Lower | Upper |
| One-leg standing | 0.100 | 0.037 | 0.283 | 2.714 | 0.008 | 0.027 | 0.173 |
| Knee extension force | 0.508 | 0.206 | 0.257 | 2.470 | 0.016 | 0.099 | 0.917 |
| intercept | 46.944 | 13.431 | 3.495 | 0.001 | 20.226 | 73.662 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Nakao, Y.; Hasegawa, Y.; Nagai, K.; Sano, K.; Uchiyama, Y.; Kishimoto, H.; Shinmura, K.; Domen, K. Relationship between Rate of Force Development of Tongue Pressure and Physical Performance. J. Clin. Med. 2022, 11, 2347. https://doi.org/10.3390/jcm11092347
Saito S, Nakao Y, Hasegawa Y, Nagai K, Sano K, Uchiyama Y, Kishimoto H, Shinmura K, Domen K. Relationship between Rate of Force Development of Tongue Pressure and Physical Performance. Journal of Clinical Medicine. 2022; 11(9):2347. https://doi.org/10.3390/jcm11092347
Chicago/Turabian StyleSaito, Syota, Yuta Nakao, Yoko Hasegawa, Koutatsu Nagai, Kyoko Sano, Yuki Uchiyama, Hiromitsu Kishimoto, Ken Shinmura, and Kazuhisa Domen. 2022. "Relationship between Rate of Force Development of Tongue Pressure and Physical Performance" Journal of Clinical Medicine 11, no. 9: 2347. https://doi.org/10.3390/jcm11092347
APA StyleSaito, S., Nakao, Y., Hasegawa, Y., Nagai, K., Sano, K., Uchiyama, Y., Kishimoto, H., Shinmura, K., & Domen, K. (2022). Relationship between Rate of Force Development of Tongue Pressure and Physical Performance. Journal of Clinical Medicine, 11(9), 2347. https://doi.org/10.3390/jcm11092347








