Accuracy of DICOM–DICOM vs. DICOM–STL Protocols in Computer-Guided Surgery: A Human Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- patients of both binary genders and all races, aged between 18 and 99 years;
- patients for whom full supportive implant rehabilitation with multiple implants was already established;
- patients physically able to tolerate surgical and prosthetic procedures;
- patients who agreed to going back to the dental clinic for a follow-up visit.
- patients with active infection or severe inflammation in the areas identified for implant placement;
- patients with a smoking habit exceeding 10 cigarettes per day;
- patients with uncontrolled diabetes or metabolic bone disease or other uncontrolled systemic diseases;
- patients with a history of radiotherapy treatment of the craniofacial area;
- patients with a known gestational state;
- patients with evidence of severe bruxism or grinding;
- patients requiring augmentation procedures for dental implant placement and/or with buccal–lingual bone ridge dimensions of less than 5 mm.
- Group A:
- Five patients treated with mucosa-supported surgical guides made using the DICOM–DICOM protocol (or double scan protocol) (Nobel Biocare Services AG P.O. Box CH-8058 Zurich-Flughafen Switzerland).
- Group B:
- Five patients treated with mucosa-supported surgical guides made using the DICOM–STL protocol (or extraoral stent protocol) (GEASS srl Via Madonna della Salute, 23 33050 Pozzuolo del Friuli, UD).
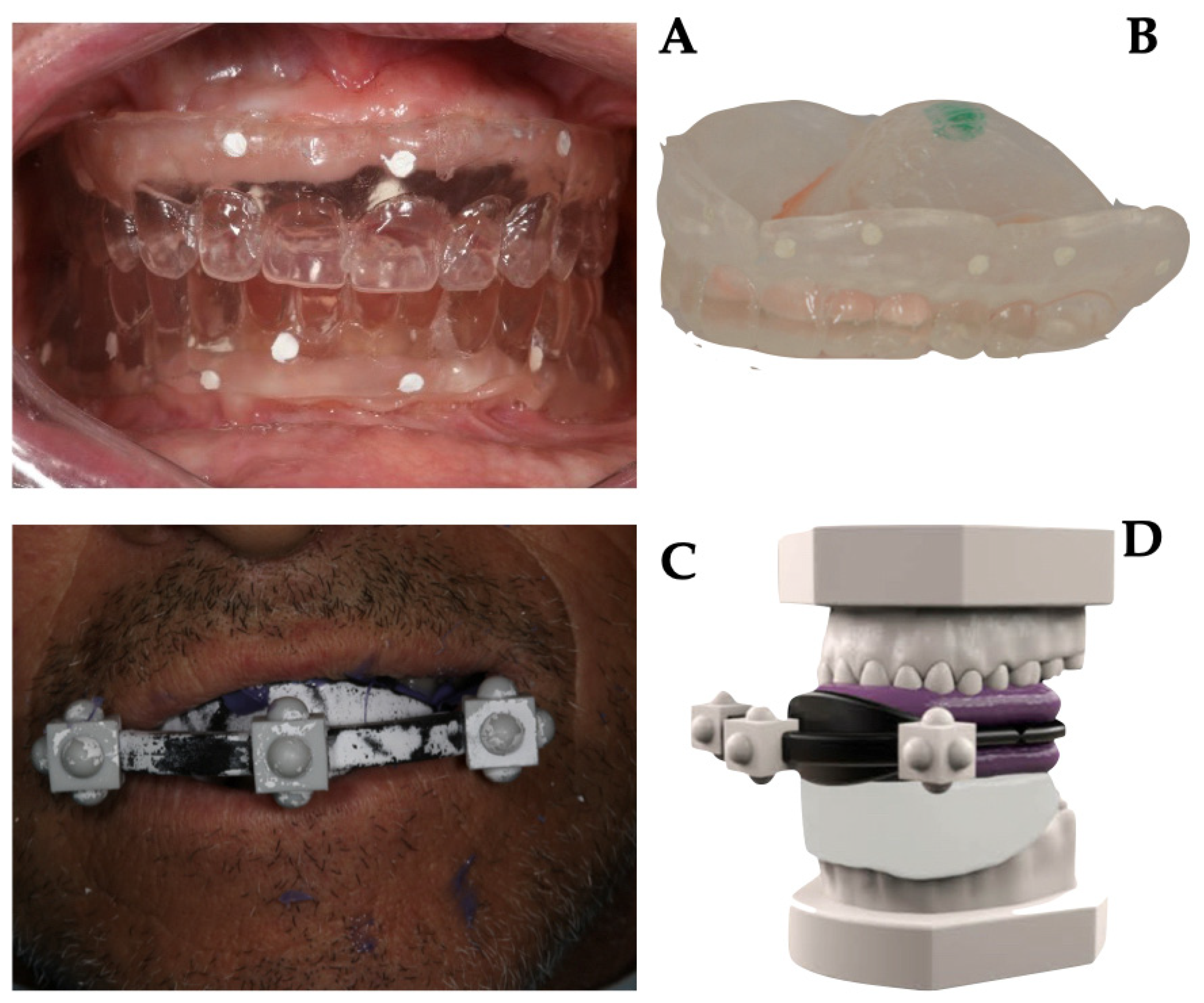
2.2. Data Acquisition and Templates Realization
- master model with radiological template and stent;
- master model at the mucosal level;
- master model without stent with only radiological template in place;
- opposite arch model.
2.3. Surgical Phase
2.4. Prosthetic Phase
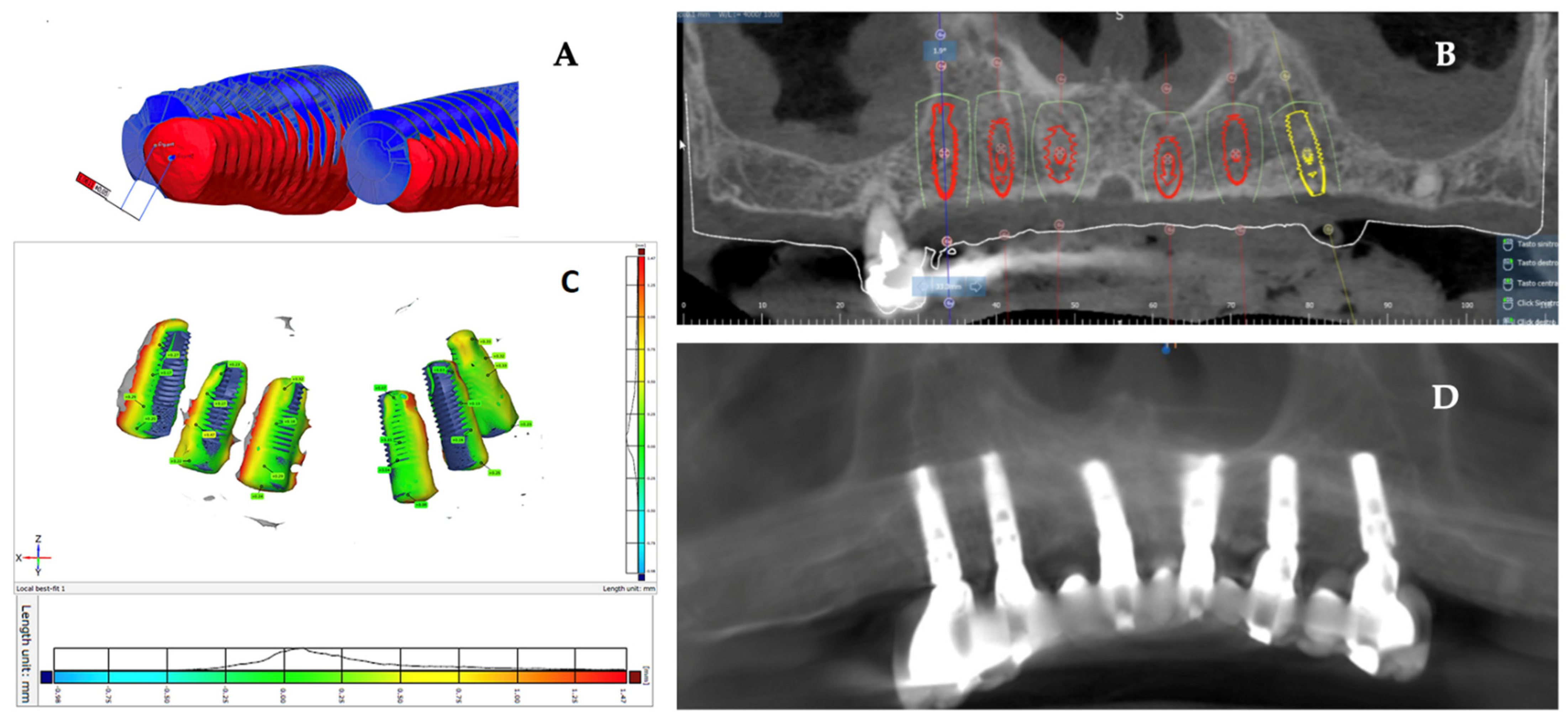
2.5. Data Analysis
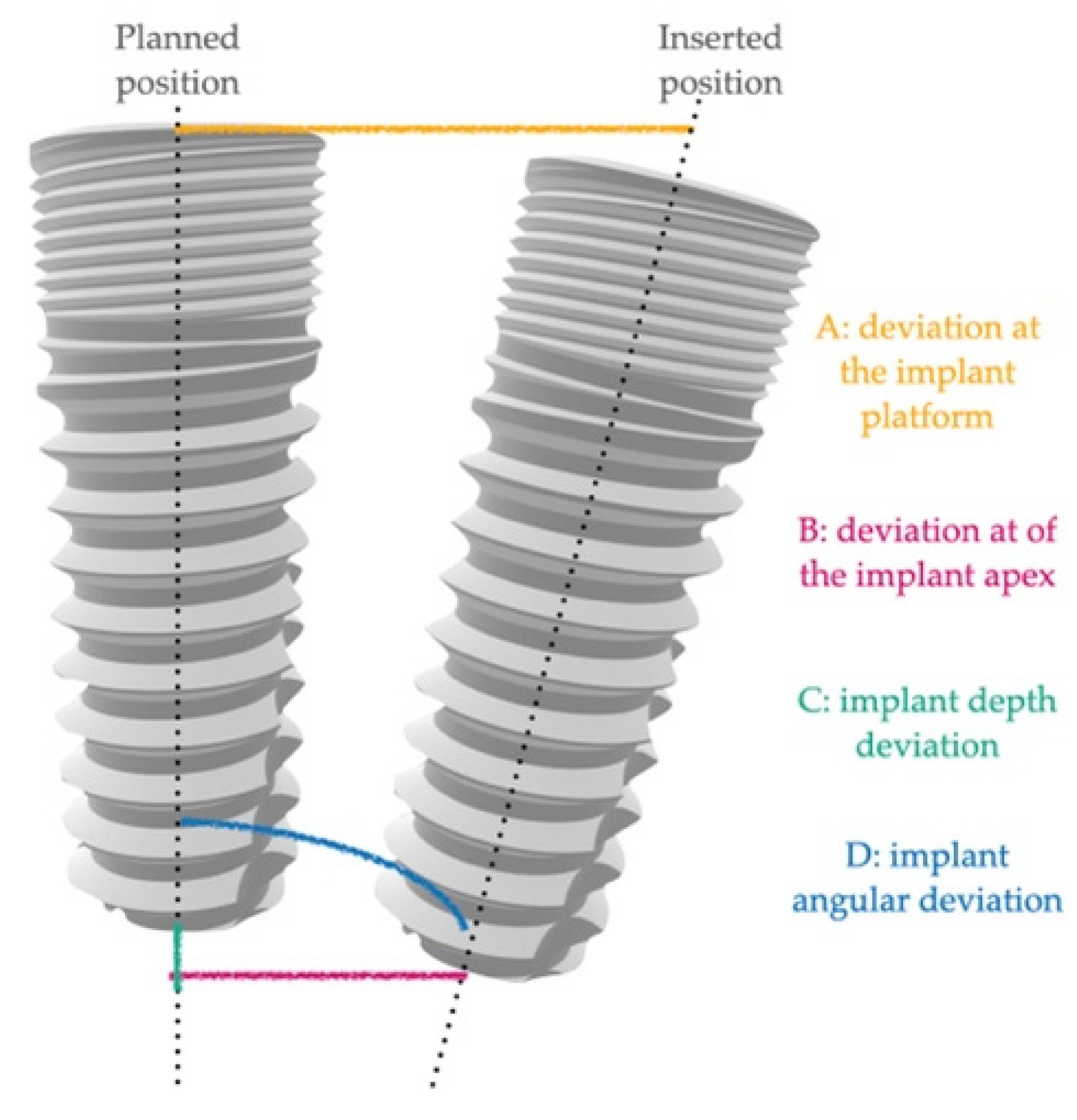
- -
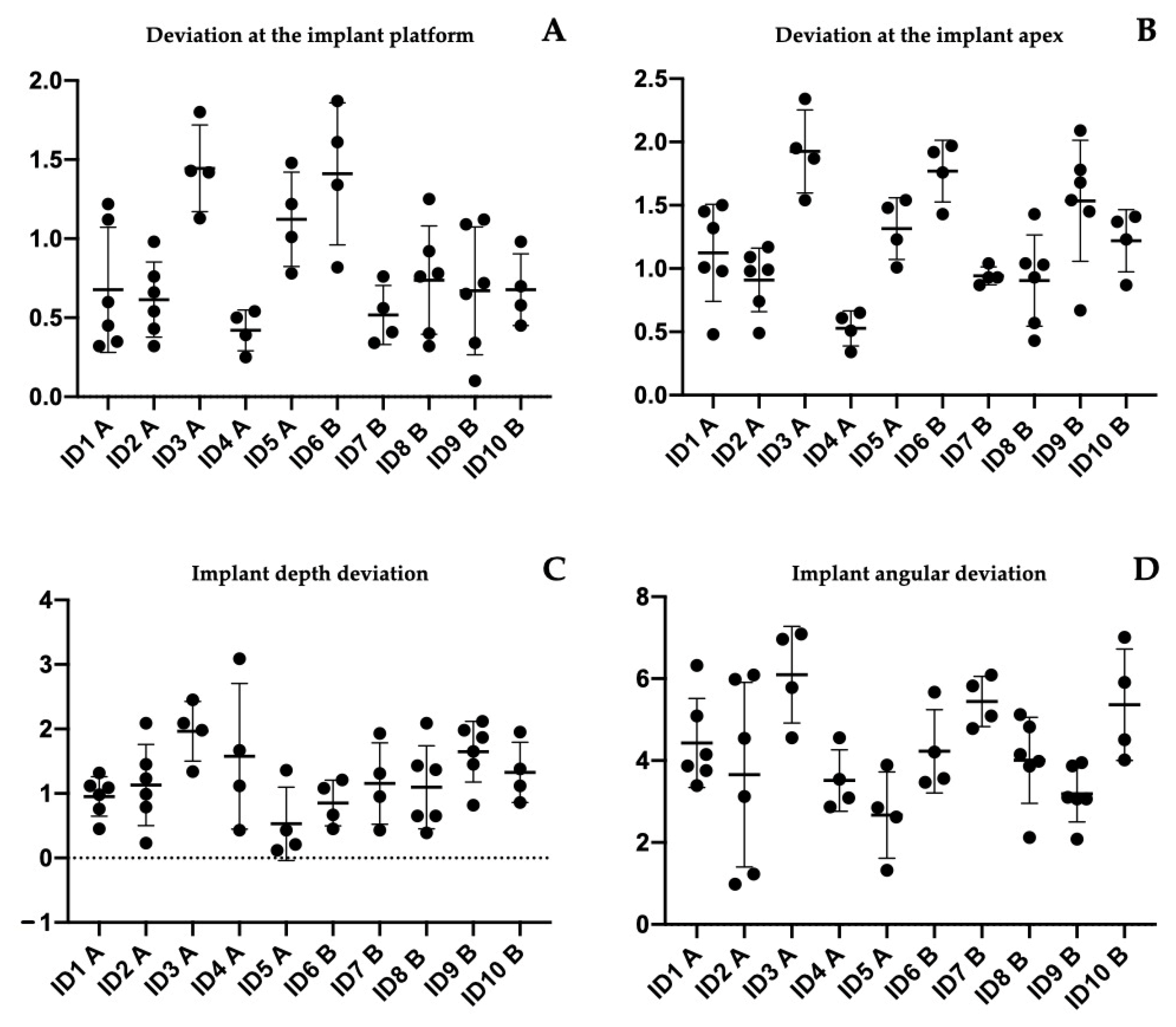
- A: deviation at the implant platform as the spatial distance between the center of the platform of the planned and positioned implants;
- -
- B: deviation of the implant apex as the deviation at the apex level of the planned implant;
- -
- C: implant depth deviation as the distance of the planned and positioned implants on the vertical axis;
- -
- D: implant angular deviation the spatial angle between the planned and the positioned.
2.6. Statistical Analysis
2.7. Ethical Consideration
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinjari, B.; D’Addazio, G.; Traini, T.; Varvara, G.; Scarano, A.; Murmura, G.; Caputi, S. A 10-year retrospective comparative human study on screw-retained versus cemented dental implant abutments. J. Biol. Regul. Homeost. Agents 2019, 33, 787–797. [Google Scholar] [PubMed]
- Trullenque-Eriksson, A.; Guisado-Moya, B. Retrospective long-term evaluation of dental implants in totally and partially edentulous patients. Part I: Survival and marginal bone loss. Implant Dent. 2014, 23, 732–737. [Google Scholar] [CrossRef]
- Merli, M.; Moscatelli, M.; Pagliaro, U.; Mariotti, G.; Merli, I.; Nieri, M. Implant prosthetic rehabilitation in partially edentulous patients with bone atrophy. An umbrella review based on systematic reviews of randomised controlled trials. Eur. J. Oral Implantol. 2018, 11, 261–280. [Google Scholar] [PubMed]
- Merlone, A.; Tetè, G.; Cantile, N.; Manacorda, M.; Cattoni, F. Minimally invasive digital implant-prosthetic procedure in "all on 4" rehabilitation in patients with special needs: A three-year follow-up. J. Biol. Regul. Homeost. Agents 2021, 35, 71–85. [Google Scholar] [PubMed]
- Urban, I.A.; Monje, A.; Lozada, J.L.; Wang, H.L. Long-term Evaluation of Peri-implant Bone Level after Reconstruction of Severely Atrophic Edentulous Maxilla via Vertical and Horizontal Guided Bone Regeneration in Combination with Sinus Augmentation: A Case Series with 1 to 15 Years of Loading. Clin. Implant Dent. Relat. Res. 2017, 19, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Papi, P.; Di Murro, B.; Pranno, N.; Bisogni, V.; Saracino, V.; Letizia, C.; Polimeni, A.; Pompa, G. Prevalence of peri-implant diseases among an Italian population of patients with metabolic syndrome: A cross-sectional study. J. Periodontol. 2019, 90, 1374–1382. [Google Scholar] [CrossRef]
- Valente, F.; Scarano, A.; Murmura, G.; Varvara, G.; Sinjari, B.; Mandelli, F.; Piattelli, M.; Caputi, S.; Traini, T. Collagen Fibres Orientation in the Bone Matrix around Dental Implants: Does the Implant’s Thread Design Play a Role? Int. J. Mol. Sci. 2021, 22, 7860. [Google Scholar] [CrossRef]
- Testori, T.; Weinstein, T.; Scutellà, F.; Wang, H.L.; Zucchelli, G. Implant placement in the esthetic area: Criteria for positioning single and multiple implants. Periodontol. 2000 2018, 77, 176–196. [Google Scholar] [CrossRef]
- Malchiodi, L.; Giacomazzi, E.; Cucchi, A.; Ricciotti, G.; Caricasulo, R.; Bertossi, D.; Gherlone, E. Relationship Between Crestal Bone Levels and Crown-to-Implant Ratio of Ultra-Short Implants with a Micro-rough Surface: A Prospective Study With 48 Months of Follow-Up. J. Oral Implantol. 2019, 45, 18–28. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-Implant Bone Resorption during Healing Abutment Placement: The Effect of a 0.20% Chlorhexidine Gel vs. Placebo-A Randomized Double Blind Controlled Human Study. BioMed Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef] [Green Version]
- Mangano, C.; Mangano, F.G.; Shibli, J.A.; Roth, L.A.; d’Addazio, G.; Piattelli, A.; Iezzi, G. Immunohistochemical Evaluation of Peri-Implant Soft Tissues around Machined and Direct Metal Laser Sintered (DMLS) Healing Abutments in Humans. Int. J. Environ. Res. Public Health 2018, 15, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zukauskas, S.; Puisys, A.; Andrijauskas, P.; Zaleckas, L.; Vindasiute-Narbute, E.; Linkevičius, T. Influence of Implant Placement Depth and Soft Tissue Thickness on Crestal Bone Stability Around Implants With and Without Platform Switching: A Comparative Clinical Trial. Int. J. Periodontics Restor. Dent. 2021, 41, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Venezia, P.; Torsello, F.; Santomauro, V.; Dibello, V.; Cavalcanti, R. Full Digital Workflow for the Treatment of an Edentulous Patient with Guided Surgery, Immediate Loading, and 3D-Printed Hybrid Prosthesis: The BARI Technique 2.0. A Case Report. Int. J. Environ. Res. Public Health 2019, 16, 5160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattoni, F.; Chirico, L.; Cantile, N.; Merlone, A. Traditional prosthetic workflow for implant rehabilitations with a reduced number of fixtures: Proposal of a protocol. J. Biol. Regul. Homeost. Agents 2021, 35, 31–40. [Google Scholar] [PubMed]
- Makarov, N.; Pompa, G.; Papi, P. Computer-assisted implant placement and full-arch immediate loading with digitally prefabricated provisional prostheses without cast: A prospective pilot cohort study. Int. J. Implant Dent. 2021, 7, 80. [Google Scholar] [CrossRef]
- Vieira, D.M.; Sotto-Maior, B.S.; Barros, C.A.; Reis, E.S.; Francischone, C.E. Clinical accuracy of flapless computer-guided surgery for implant placement in edentulous arches. Int. J. Oral Maxillofac. Implant. 2013, 28, 1347–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wismeijer, D.; Joda, T.; Flügge, T.; Fokas, G.; Tahmaseb, A.; Bechelli, D.; Bohner, L.; Bornstein, M.; Burgoyne, A.; Caram, S.; et al. Group 5 ITI Consensus Report: Digital technologies. Clin. Oral Implant. Res. 2018, 29, 436–442. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 416–435. [Google Scholar] [CrossRef] [Green Version]
- Wegmüller, L.; Halbeisen, F.; Sharma, N.; Kühl, S.; Thieringer, F.M. Consumer vs. High-End 3D Printers for Guided Implant Surgery-An In Vitro Accuracy Assessment Study of Different 3D Printing Technologies. J. Clin. Med. 2021, 10, 4894. [Google Scholar] [CrossRef]
- Mistry, A.; Ucer, C.; Thompson, J.D.; Khan, R.S.; Karahmet, E.; Sher, F. 3D Guided Dental Implant Placement: Impact on Surgical Accuracy and Collateral Damage to the Inferior Alveolar Nerve. Dent. J. 2021, 9, 99. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, C.Z.; Huang, M.S.; Huang, C.F.; Cheng, H.C.; Wang, D.P. Fully Digital Workflow for Planning Static Guided Implant Surgery: A Prospective Accuracy Study. J. Clin. Med. 2020, 9, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattoni, F.; Chirico, L.; Merlone, A.; Manacorda, M.; Vinci, R.; Gherlone, E.F. Digital Smile Designed Computer-Aided Surgery versus Traditional Workflow in “All on Four” Rehabilitations: A Randomized Clinical Trial with 4-Years Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 3449. [Google Scholar] [CrossRef] [PubMed]
- D’haese, R.; Vrombaut, T.; Hommez, G.; De Bruyn, H.; Vandeweghe, S. Accuracy of Guided Implant Surgery in the Edentulous Jaw Using Desktop 3D-Printed Mucosal Supported Guides. J. Clin. Med. 2021, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Mozer, P.S. Accuracy and Deviation Analysis of Static and Robotic Guided Implant Surgery: A Case Study. Int. J. Oral Maxillofac. Implant. 2020, 35, e86–e90. [Google Scholar] [CrossRef]
- Bover-Ramos, F.; Viña-Almunia, J.; Cervera-Ballester, J.; Peñarrocha-Diago, M.; García-Mira, B. Accuracy of Implant Placement with Computer-Guided Surgery: A Systematic Review and Meta-Analysis Comparing Cadaver, Clinical, and In Vitro Studies. Int. J. Oral Maxillofac. Implant. 2018, 33, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Guentsch, A.; Sukhtankar, L.; An, H.; Luepke, P.G. Precision, and trueness of implant placement with and without static surgical guides: An in vitro study. J. Prosthet. Dent. 2021, 126, 398–404. [Google Scholar] [CrossRef]
- Guerrero, M.E.; Jacobs, R.; Loubele, M.; Schutyser, F.; Suetens, P.; Van Steenberghe, D. State-of-the-art on cone beam CT imaging for preoperative planning of implant placement. Clin. Oral Investig. 2006, 10, 1–7. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Barootchi, S.; Salomó-Coll, O.; Wang, H.-L. Advantages, and disadvantages of implant navigation surgery. A systematic review. Ann. Anat. Anat. Anz. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, J.; Wang, C.; Huang, Y.; Kang, L. Modular Preoperative Planning Software for Computer- Aided Oral Im-plantology and the Application of a Novel Stereolithographic Template: A Pilot Study. Clin. Implant. Dent. Relat. Res. 2009, 12, 181–193. [Google Scholar]
- Vercruyssen, M.; Jacobs, R.; Van Assche, N.; van Steenberghe, D. The use of CT scan-based planning for oral rehabilitation by means of implants and its transfer to the surgical field: A critical review on accuracy. J. Oral Rehabil. 2008, 35, 454–474. [Google Scholar] [CrossRef]
- Modica, F.; Fava, C.; Benech, A.; Preti, G. Radiologic-prosthetic planning of the surgical phase of the treatment of edentulism by osseointegrated implants: An in vitro study. J. Prosthet. Dent. 1991, 65, 541–546. [Google Scholar] [CrossRef]
- Kernen, F.; Kramer, J.; Wanner, L.; Wismeijer, D.; Nelson, K.; Flügge, T. A review of virtual planning software for guided implant surgery—Data import and visualization, drill guide design and manufacturing. BMC Oral Health 2020, 20, 251. [Google Scholar] [CrossRef] [PubMed]
- Vinci, R.; Manacorda, M.; Abundo, R.; Lucchina, A.G.; Scarano, A.; Crocetta, C.; Muzio, L.L.; Gherlone, E.F.; Mastrangelo, F. Accuracy of Edentulous Computer-Aided Implant Surgery as Compared to Virtual Planning: A Retrospective Multicenter Study. J. Clin. Med. 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felice, P.; Soardi, E.; Piattelli, M.; Pistilli, R.; Jacotti, M.; Esposito, M. Immediate non-occlusal loading of immediate post-extractive versus delayed placement of single implants in preserved sockets of the anterior maxilla: 4-month post-loading results from a pragmatic multicentre randomised controlled trial. Eur. J. Oral Implantol. 2011, 4, 329–344. [Google Scholar]
- Sample Size Calculator. Available online: https://clincalc.com/stats/samplesize.aspx (accessed on 3 July 2021).
- Zhou, W.; Liu, Z.; Song, L.; Kuo, C.L.; Shafer, D.M. Clinical Factors Affecting the Accuracy of Guided Implant Surgery-A Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2018, 18, 28–40. [Google Scholar] [CrossRef]
- Rivara, F.; Lumetti, S.; Calciolari, E.; Toffoli, A.; Forlani, G.; Manfredi, E. Photogrammetric method to measure the discrepancy between clinical and software-designed positions of implants. J. Prosthet. Dent. 2016, 115, 703–711. [Google Scholar] [CrossRef]
- Cattoni, F.; Merlone, A.; Broggi, R.; Manacorda, M.; Vinci, R. Computer-assisted prosthetic planning, and implant design with integrated digital bite registration: A treatment protocol. J. Biol. Regul. Homeost. Agents 2021, 35, 11–29. [Google Scholar]
- D’Addazio, G.; Sinjari, B.; Arcuri, L.; Femminella, B.; Murmura, G.; Santilli, M.; Caputi, S. Mechanical Pull-Out Test of a New Hybrid Fixture-Abutment Connection: An In Vitro Study. Materials 2021, 14, 1555. [Google Scholar] [CrossRef]
- Winkler, S.; Ring, K.; Ring, J.D.; Boberick, K.G. Implant screw mechanics and the settling effect: An overview. J. Oral Implantol. 2003, 29, 242–245. [Google Scholar] [CrossRef]
- Varvara, G.; Sinjari, B.; Caputi, S.; Scarano, A.; Piattelli, M. The Relationship Between Time of Retightening and Preload Loss of Abutment Screws for Two Different Implant Designs: An In Vitro Study. J. Oral Implantol. 2020, 46, 13–17. [Google Scholar]
- Cucchi, A.; Chierico, A.; Fontana, F.; Mazzocco, F.; Cinquegrana, C.; Belleggia, F.; Rossetti, P.; Soardi, C.M.; Todisco, M.; Luongo, R.; et al. Statements and Recommendations for Guided Bone Regeneration: Consensus Report of the Guided Bone Regeneration Symposium Held in Bologna, October 15 to 16, 2016. Implant Dent. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Raabe, C.; Janner, S.F.M.; Abou-Ayash, S. Comprehensive Digital Workflow and Computer-Assisted Implant Surgery in a Patient with Reduced Crest Width. Case Report of a Split-Mouth Approach. Swiss Dent. J. 2021, 131, 437–441. [Google Scholar] [PubMed]
- Poli, P.P.; Muktadar, A.K.; Souza, F.Á.; Maiorana, C.; Beretta, M. Computer-guided implant placement associated with computer-aided bone regeneration in the treatment of atrophied partially edentulous alveolar ridges: A proof-of-concept study. J. Dent. Sci. 2021, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
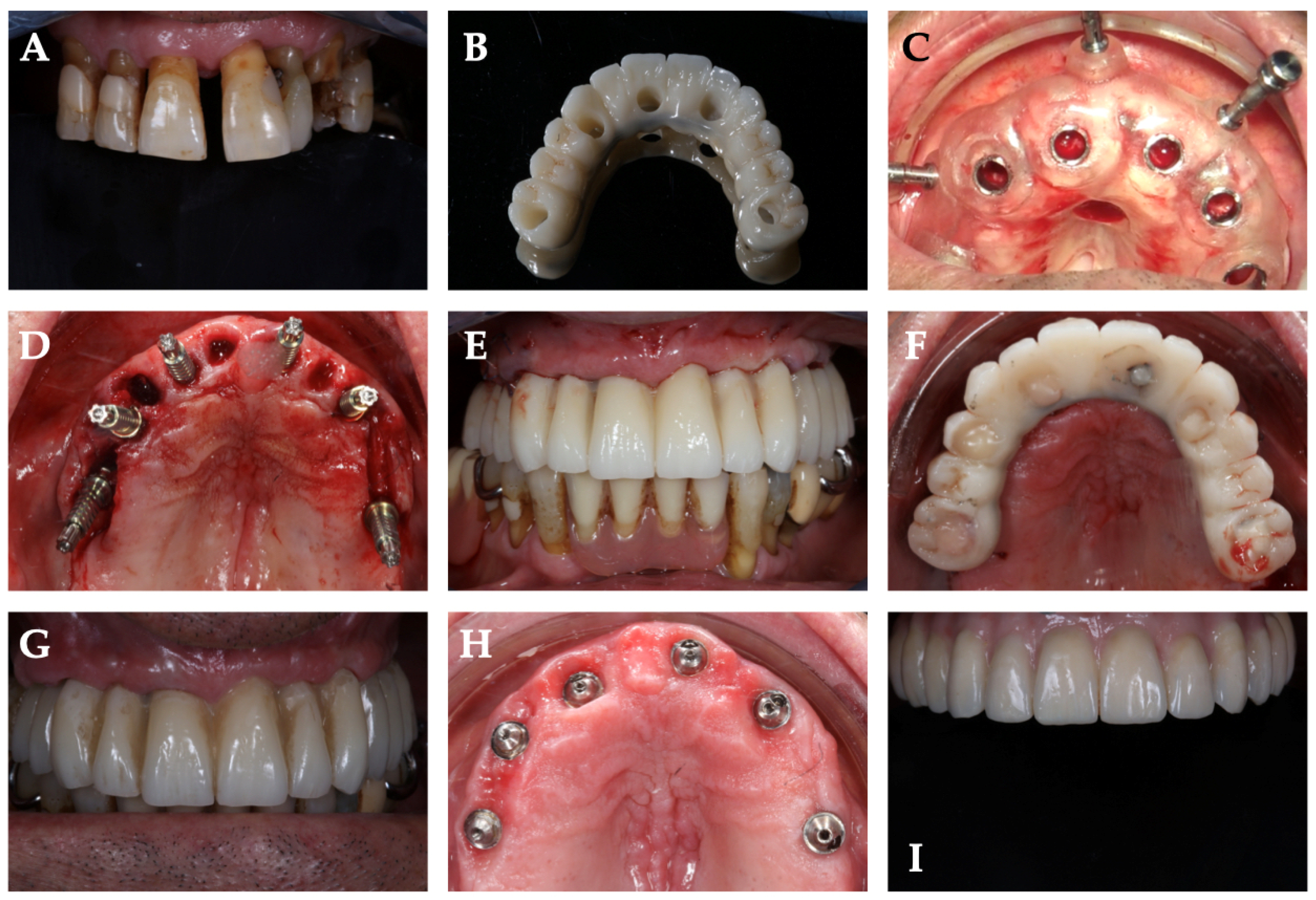
| Patient ID | Sex | Age | Group | Implant Site | Final Torque | Immediate Loading | Six Month Complication | One Year Complication |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | A | 47 | 45 | Yes | No complication recorded | Screw loosening on one abutment. It was retightened at 15 Ncm |
| 46 | 55 | Yes | ||||||
| 44 | 60 | Yes | ||||||
| 34 | 50 | Yes | ||||||
| 36 | 35 | No | ||||||
| 37 | 55 | Yes | ||||||
| 2 | M | 64 | A | 46 | 40 | Yes | Screw loosening on one abutment. It was retightened at 15 Ncm | Bleeding on implants 46 and 36. Patient needed oral hygiene and instructions |
| 44 | 45 | Yes | ||||||
| 43 | 30 | No | ||||||
| 32 | 45 | Yes | ||||||
| 34 | 40 | Yes | ||||||
| 36 | 60 | Yes | ||||||
| 3 | M | 64 | A | 14 | 40 | Yes | No complication recorded | Occlusal adjustment |
| 12 | 50 | Yes | ||||||
| 22 | 55 | Yes | ||||||
| 24 | 40 | Yes | ||||||
| 4 | M | 68 | A | 46 | 65 | Yes | No complication recorded | No complication recorded |
| 32 | 55 | Yes | ||||||
| 34 | 50 | Yes | ||||||
| 36 | 55 | Yes | ||||||
| 5 | F | 57 | A | 14 | 40 | Yes | No complication recorded | No complication recorded |
| 12 | 30 | No | ||||||
| 22 | 55 | Yes | ||||||
| 24 | 45 | Yes | ||||||
| 6 | F | 83 | B | 12 | 45 | Yes | No complication recorded | Screw loosening on one abutment. It was retightened at 15 Ncm |
| 22 | 50 | Yes | ||||||
| 24 | 45 | Yes | ||||||
| 25 | 45 | Yes | ||||||
| 7 | M | 92 | B | 44 | 45 | Yes | No complication recorded | No complication recorded |
| 42 | 40 | Yes | ||||||
| 32 | 40 | Yes | ||||||
| 34 | 50 | Yes | ||||||
| 8 | M | 69 | B | 16 | 50 | Yes | Fracture of the prosthesis with immediate loading in the portion where there was a tooth in extension [26] | No complication recorded |
| 14 | 45 | Yes | ||||||
| 12 | 30 | No | ||||||
| 22 | 30 | No | ||||||
| 24 | 45 | Yes | ||||||
| 25 | 45 | Yes | ||||||
| 9 | M | 69 | B | 46 | 60 | Yes | Screw loosening on two abutments. They were retightened at 15 Ncm | Relining was necessary to improve the fit between the prosthesis and the gingiva |
| 44 | 55 | Yes | ||||||
| 43 | 30 | No | ||||||
| 32 | 45 | Yes | ||||||
| 34 | 50 | Yes | ||||||
| 36 | 50 | Yes | ||||||
| 10 | F | 73 | B | 44 | 55 | Yes | No complication recorded | No complication recorded |
| 42 | 45 | Yes | ||||||
| 34 | 50 | Yes | ||||||
| 32 | 45 | Yes |
| Patient ID | Implant Position | Deviation at the Implant Platform (A) | Deviation at the Implant Apex (B) | Implant Depth Deviation (C) | Implant Angular Deviation (D) |
|---|---|---|---|---|---|
| 1 (A) | 47 | 1.22 | 1.5 | 1.09 | 3.39 |
| 46 | 0.45 | 0.98 | 1.12 | 4.15 | |
| 44 | 0.32 | 0.48 | 0.98 | 3.87 | |
| 34 | 0.6 | 1.01 | 1.32 | 6.32 | |
| 36 | 0.35 | 1.45 | 0.45 | 5.09 | |
| 37 | 1.12 | 1.32 | 0.76 | 3.76 | |
| 2 (A) | 46 | 0.98 | 1.17 | 0.23 | 3.12 |
| 44 | 0.43 | 0.49 | 1.23 | 4.54 | |
| 43 | 0.66 | 0.98 | 2.09 | 5.98 | |
| 32 | 0.54 | 0.74 | 1.45 | 6.09 | |
| 34 | 0.76 | 1.09 | 0.79 | 0.98 | |
| 36 | 0.32 | 0.99 | 0.99 | 1.23 | |
| 3 (A) | 14 | 1.43 | 2.34 | 1.34 | 6.96 |
| 12 | 1.13 | 1.54 | 1.98 | 4.56 | |
| 22 | 1.42 | 1.87 | 2.09 | 5.78 | |
| 24 | 1.8 | 1.95 | 2.45 | 7.09 | |
| 4 (A) | 46 | 0.25 | 0.34 | 3.09 | 2.87 |
| 32 | 0.54 | 0.65 | 1.12 | 4.56 | |
| 34 | 0.39 | 0.51 | 0.43 | 3.09 | |
| 36 | 0.5 | 0.61 | 1.67 | 3.54 | |
| 5 (A) | 14 | 1.01 | 1.23 | 0.21 | 3.89 |
| 12 | 1.22 | 1.48 | 0.12 | 2.62 | |
| 22 | 1.48 | 1.54 | 0.43 | 1.32 | |
| 24 | 0.78 | 1.01 | 1.36 | 2.85 | |
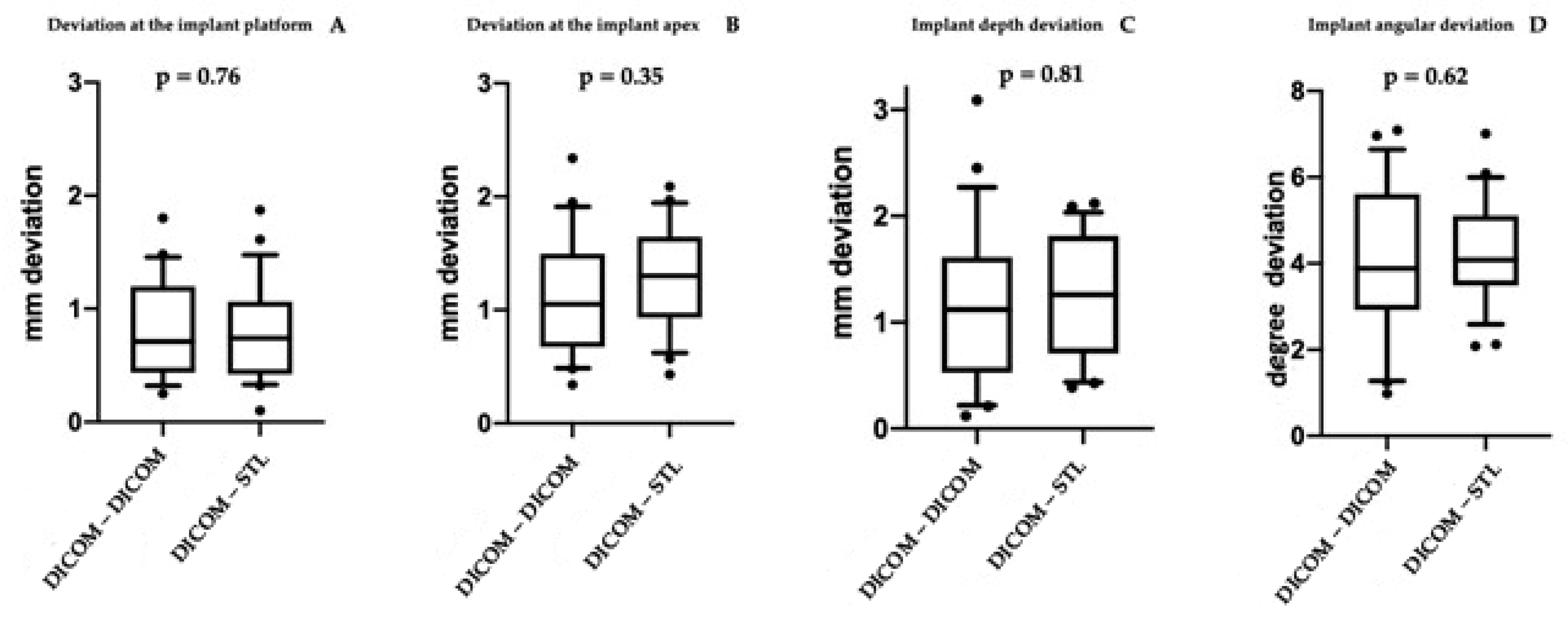
| Mean | 0.8208 mm | 1.1362 mm | 1.1995 mm | 4.0687° | |
| St. Dev | 0.4449 mm | 0.5114 mm | 0.7514 mm | 1.7184° |
| Patient ID | Implant Position | Deviation at the Implant Platform | Deviation at the Implant Apex | Implant Depth Deviation | Implant Angular Deviation |
|---|---|---|---|---|---|
| 6 (B) | 12 | 1.34 | 1.43 | 1.21 | 3.56 |
| 22 | 1.61 | 1.92 | 1.08 | 3.47 | |
| 24 | 1.87 | 1.97 | 0.45 | 5.67 | |
| 25 | 0.82 | 1.76 | 0.67 | 4.21 | |
| 7 (B) | 44 | 0.34 | 0.87 | 0.95 | 4.78 |
| 42 | 0.41 | 0.93 | 1.31 | 6.09 | |
| 32 | 0.76 | 1.04 | 0.43 | 5.82 | |
| 34 | 0.56 | 0.93 | 1.93 | 5.09 | |
| 8 (B) | 16 | 0.76 | 0.93 | 0.65 | 3.86 |
| 14 | 0.92 | 1.04 | 1.43 | 4.15 | |
| 12 | 0.32 | 0.43 | 1.37 | 3.98 | |
| 22 | 0.4 | 0.57 | 0.65 | 5.12 | |
| 24 | 0.78 | 1.03 | 0.39 | 4.82 | |
| 25 | 1.25 | 1.43 | 2.09 | 2.12 | |
| 9 (B) | 46 | 1.12 | 1.78 | 2.12 | 3.06 |
| 44 | 1.09 | 2.09 | 1.87 | 3.87 | |
| 43 | 0.72 | 1.68 | 0.82 | 3.1 | |
| 32 | 0.1 | 0.67 | 1.45 | 3.06 | |
| 34 | 0.34 | 1.54 | 1.98 | 2.08 | |
| 36 | 0.65 | 1.45 | 1.65 | 3.95 | |
| 10 (B) | 44 | 0.45 | 0.87 | 1.38 | 4.02 |
| 42 | 0.98 | 1.41 | 1.95 | 4.51 | |
| 34 | 0.7 | 1.23 | 1.12 | 5.91 | |
| 32 | 0.58 | 1.37 | 0.86 | 7.01 | |
| Mean | 0.7862 mm | 1.2654 mm | 1.2420 mm | 4.3045° | |
| St. Dev | 0.4299 mm | 0.4580 mm | 0.5587 mm | 1.2390° |
| Tukey’s Multiple Comparisons Test | Mean Diff. | 95.00% CI of Diff. | Sig. | Adjusted p Value |
|---|---|---|---|---|
| deviation at the implant platform (A) | ||||
| ID1 A vs. ID3 A | −0.7683 | −1.460 to −0.07632 | Yes | 0.0195 |
| ID1 A vs. ID6 B | −0.7333 | −1.425 to −0.04132 | Yes | 0.0303 |
| ID2 A vs. ID3 A | −0.83 | −1.522 to −0.1380 | Yes | 0.0087 |
| ID2 A vs. ID6 B | −0.795 | −1.487 to −0.1030 | Yes | 0.0138 |
| ID3 A vs. ID4 A | 1.025 | 0.2669 to 1.783 | Yes | 0.002 |
| ID3 A vs. ID7 B | 0.9275 | 0.1694 to 1.686 | Yes | 0.0069 |
| ID3 A vs. ID9 B | 0.775 | 0.08299 to 1.467 | Yes | 0.0179 |
| ID3 A vs. ID10 B | 0.7675 | 0.009440 to 1.526 | Yes | 0.0451 |
| ID4 A vs. ID6 B | −0.99 | −1.748 to −0.2319 | Yes | 0.0032 |
| ID6 B vs. ID7 B | 0.8925 | 0.1344 to 1.651 | Yes | 0.0107 |
| ID6 B vs. ID9 B | 0.74 | 0.04799 to 1.432 | Yes | 0.0279 |
| deviation at the implant apex (B) | ||||
| ID1 A vs. ID3 A | −0.8017 | −1.485 to −0.1183 | Yes | 0.0111 |
| ID2 A vs. ID3 A | −1.015 | −1.698 to −0.3316 | Yes | 0.0005 |
| ID2 A vs. ID6 B | −0.86 | −1.543 to −0.1766 | Yes | 0.005 |
| ID3 A vs. ID4 A | 1.398 | 0.6489 to 2.146 | Yes | <0.0001 |
| ID3 A vs. ID7 B | 0.9825 | 0.2339 to 1.731 | Yes | 0.003 |
| ID4 A vs. ID5 A | −0.7875 | −1.536 to −0.03890 | Yes | 0.0324 |
| ID4 A vs. ID6 B | −1.243 | −1.991 to −0.4939 | Yes | <0.0001 |
| ID6 B vs. ID7 B | 0.8275 | 0.07890 to 1.576 | Yes | 0.0204 |
| ID8 B vs. ID9 B | −0.63 | −1.241 to −0.01877 | Yes | 0.0388 |
| Implant angular deviation (D) | ||||
| ID3 A vs. ID9 B | 2.911 | 0.2288 to 5.593 | Yes | 0.0243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Addazio, G.; Xhajanka, E.; Traini, T.; Santilli, M.; Rexhepi, I.; Murmura, G.; Caputi, S.; Sinjari, B. Accuracy of DICOM–DICOM vs. DICOM–STL Protocols in Computer-Guided Surgery: A Human Clinical Study. J. Clin. Med. 2022, 11, 2336. https://doi.org/10.3390/jcm11092336
D’Addazio G, Xhajanka E, Traini T, Santilli M, Rexhepi I, Murmura G, Caputi S, Sinjari B. Accuracy of DICOM–DICOM vs. DICOM–STL Protocols in Computer-Guided Surgery: A Human Clinical Study. Journal of Clinical Medicine. 2022; 11(9):2336. https://doi.org/10.3390/jcm11092336
Chicago/Turabian StyleD’Addazio, Gianmaria, Edit Xhajanka, Tonino Traini, Manlio Santilli, Imena Rexhepi, Giovanna Murmura, Sergio Caputi, and Bruna Sinjari. 2022. "Accuracy of DICOM–DICOM vs. DICOM–STL Protocols in Computer-Guided Surgery: A Human Clinical Study" Journal of Clinical Medicine 11, no. 9: 2336. https://doi.org/10.3390/jcm11092336
APA StyleD’Addazio, G., Xhajanka, E., Traini, T., Santilli, M., Rexhepi, I., Murmura, G., Caputi, S., & Sinjari, B. (2022). Accuracy of DICOM–DICOM vs. DICOM–STL Protocols in Computer-Guided Surgery: A Human Clinical Study. Journal of Clinical Medicine, 11(9), 2336. https://doi.org/10.3390/jcm11092336













