Increased Complement-Associated Inflammation in Cytomegalovirus-Positive Hypertensive Anterior Uveitis Patients Based on the Aqueous Humor Proteomics Analysis
Abstract
1. Introduction
2. Methods
2.1. Human Subjects
2.2. Clinical Assessment
2.3. Collection of Human AH
2.4. Mass Spectrometry
2.4.1. Sample Preparation and LC-MS/MS Analysis
2.4.2. Protein Identification and Quantification
2.5. TM Cell Culture and CMV Virus Infection
2.6. qRT-PCR Analyses
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Proteome Functional Analyses
2.9. Statistical Analyses
3. Results
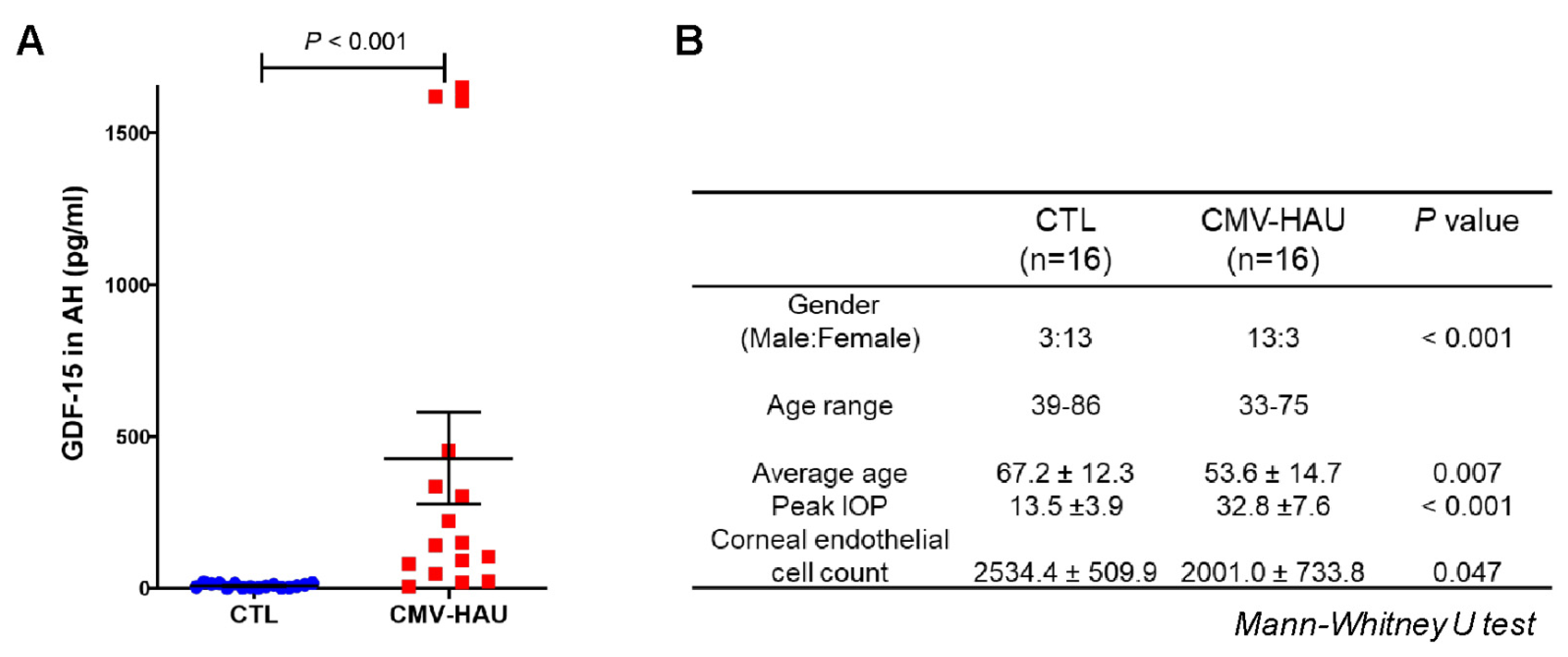
3.1. Demographic and Clinical Characteristics of Human Subjects
3.2. Evidence for Complement Activation in CMV-HAU Patients
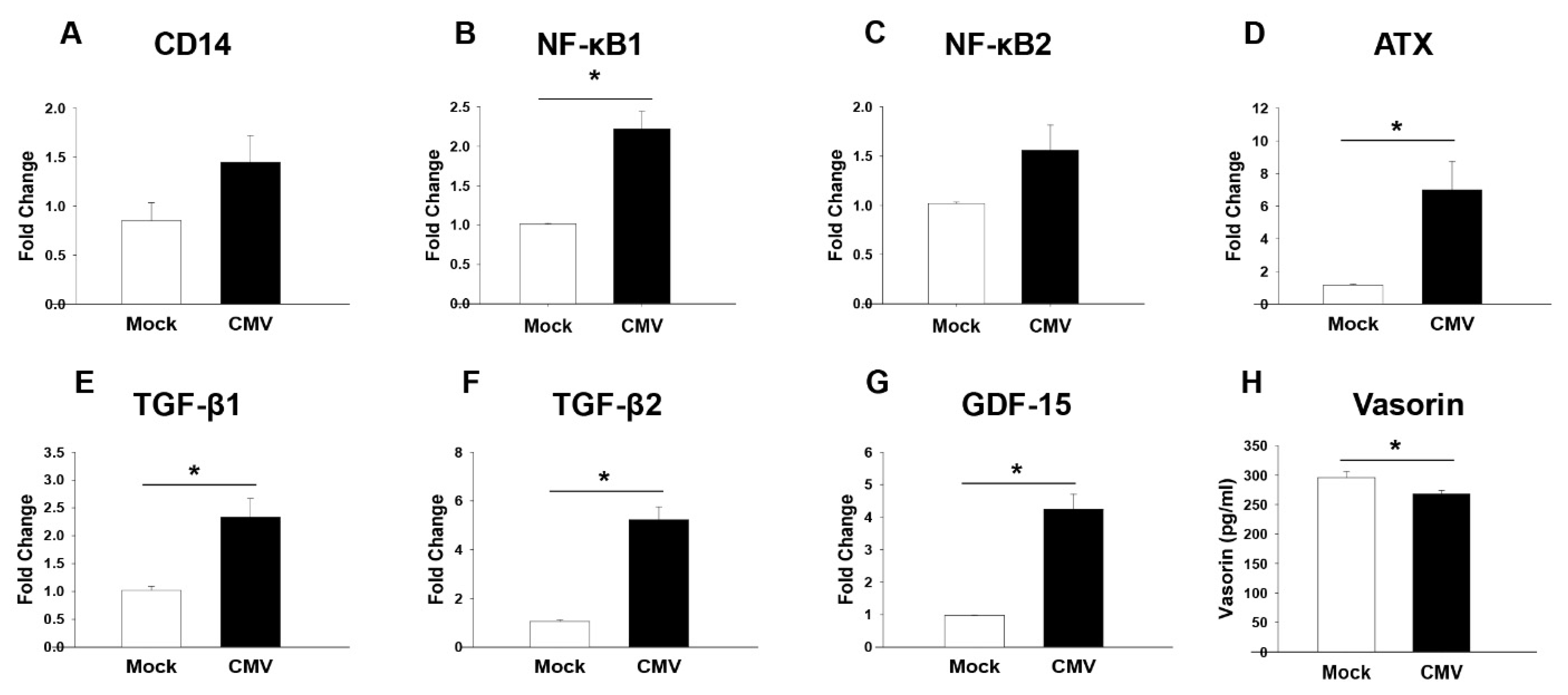
3.3. Increased Inflammation Markers in the AH of CMV-HAU Patients
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamamoto, T.; Kitazawa, Y. Vascular pathogenesis of normal-tension glaucoma: A possible pathogenetic factor, other than intraocular pressure, of glaucomatous optic neuropathy. Prog. Retin. Eye Res. 1998, 17, 127–143. [Google Scholar] [CrossRef]
- Baarsma, G.S. The epidemiology and genetics of endogenous uveitis: A review. Curr. Eye Res. 1992, 11 (Suppl. S1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.; Barton, K. Uveitic glaucoma. Ophthalmol. Clin. N. Am. 2002, 15, 375–387. [Google Scholar] [CrossRef]
- Sung, V.C.; Barton, K. Management of inflammatory glaucomas. Curr. Opin. Ophthalmol. 2004, 15, 136–140. [Google Scholar] [CrossRef]
- Jap, A.; Sivakumar, M.; Chee, S.P. Is Posner Schlossman syndrome benign? Ophthalmology 2001, 108, 913–918. [Google Scholar] [CrossRef]
- Chan, N.S.; Chee, S.P. Demystifying viral anterior uveitis: A review. Clin. Exp. Ophthalmol. 2019, 47, 320–333. [Google Scholar] [CrossRef]
- La Distia Nora, R.; Putera, I.; Mayasari, Y.D.; Hikmahwati, W.; Pertiwi, A.M.; Ridwan, A.S.; Sitompul, R.; Westcott, M.; Chee, S.P.; Pavesio, C.; et al. Clinical characteristics and treatment outcomes of cytomegalovirus anterior uveitis and endotheliitis: A systematic review and meta-analysis. Surv. Ophthalmol. 2021, S0039-6257, 00225-00223. [Google Scholar] [CrossRef]
- Joye, A.; Gonzales, J.A. Ocular manifestations of cytomegalovirus in immunocompetent hosts. Curr. Opin. Ophthalmol. 2018, 29, 535–542. [Google Scholar] [CrossRef]
- Choi, J.A.; Ju, H.H.; Kim, J.E.; Lee, J.; Jee, D.; Park, C.K.; Paik, S.Y. Cytokine profile and cytoskeletal changes after herpes simplex virus type 1 infection in human trabecular meshwork cells. J. Cell. Mol. Med. 2021, 25, 9295–9305. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.A.; Ju, H.H.; Kim, J.E.; Paik, S.Y.; Rao, P.V. Role of MCP-1 and IL-8 in viral anterior uveitis, and contractility and fibrogenic activity of trabecular meshwork cells. Sci. Rep. 2021, 11, 14950. [Google Scholar] [CrossRef]
- Classification Criteria for Cytomegalovirus Anterior Uveitis. Am. J. Ophthalmol. 2021, 228, 89–95. [CrossRef] [PubMed]
- Hughes, C.S.; Foehr, S.; Garfield, D.A.; Furlong, E.E.; Steinmetz, L.M.; Krijgsveld, J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Reidel, B.; Thompson, J.W.; Farsiu, S.; Moseley, M.A.; Skiba, N.P.; Arshavsky, V.Y. Proteomic profiling of a layered tissue reveals unique glycolytic specializations of photoreceptor cells. Mol. Cell. Proteom. MCP 2011, 10, M110.002469. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Choi, J.A.; Kim, J.E.; Noh, S.J.; Kyoung Kim, E.; Park, C.K.; Paik, S.Y. Enhanced cytomegalovirus infection in human trabecular meshwork cells and its implication in glaucoma pathogenesis. Sci. Rep. 2017, 7, 43349. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Per Cent Endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Breit, S.N.; Brown, D.A.; Tsai, V.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Mullican, S.E.; Rangwala, S.M. Uniting GDF15 and GFRAL: Therapeutic Opportunities in Obesity and Beyond. Trends Endocrinol. Metab. TEM 2018, 29, 560–570. [Google Scholar] [CrossRef]
- Ban, N.; Siegfried, C.J.; Lin, J.B.; Shui, Y.B.; Sein, J.; Pita-Thomas, W.; Sene, A.; Santeford, A.; Gordon, M.; Lamb, R.; et al. GDF15 is elevated in mice following retinal ganglion cell death and in glaucoma patients. JCI Insight 2017, 2, e91455. [Google Scholar] [CrossRef]
- Brandt, J.D.; O’Donnell, M.E. How does the trabecular meshwork regulate outflow? Clues from the vascular endothelium. J. Glaucoma 1999, 8, 328–339. [Google Scholar] [CrossRef]
- Llobet, A.; Gasull, X.; Gual, A. Understanding trabecular meshwork physiology: A key to the control of intraocular pressure? News Physiol. Sci. Int. J. Physiol. Prod. Jt. Int. Union Physiol. Sci. Am. Physiol. Soc. 2003, 18, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Imai, Y.; Kumagai, H.; Nosaka, T.; Morikawa, Y.; Hisaoka, T.; Manabe, I.; Maemura, K.; Nakaoka, T.; Imamura, T.; et al. Vasorin, a transforming growth factor beta-binding protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 10732–10737. [Google Scholar] [CrossRef] [PubMed]
- Malapeira, J.; Esselens, C.; Bech-Serra, J.J.; Canals, F.; Arribas, J. ADAM17 (TACE) regulates TGFβ signaling through the cleavage of vasorin. Oncogene 2011, 30, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Niederkorn, J.Y. Mechanisms of immune privilege in the eye and hair follicle. J. Investig. Dermatol. Symp. Proc. 2003, 8, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Streilein, J.W.; Stein-Streilein, J. Does innate immune privilege exist? J. Leukoc. Biol. 2000, 67, 479–487. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. The Eye Sees Eye to Eye with the Immune System: The 2019 Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4489–4495. [Google Scholar] [CrossRef]
- Reis, E.S.; Mastellos, D.C.; Hajishengallis, G.; Lambris, J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019, 19, 503–516. [Google Scholar] [CrossRef]
- Mohlin, C.; Sandholm, K.; Ekdahl, K.N.; Nilsson, B. The link between morphology and complement in ocular disease. Mol. Immunol. 2017, 89, 84–99. [Google Scholar] [CrossRef]
- Adav, S.S.; Wei, J.; Terence, Y.; Ang, B.C.H.; Yip, L.W.L.; Sze, S.K. Proteomic Analysis of Aqueous Humor from Primary Open Angle Glaucoma Patients on Drug Treatment Revealed Altered Complement Activation Cascade. J. Proteome Res. 2018, 17, 2499–2510. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Kaplan, H.J. Oxidative stress and the regulation of complement activation in human glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5071–5082. [Google Scholar] [CrossRef]
- Mondino, B.J.; Glovsky, M.M.; Ghekiere, L. Activated complement in inflamed aqueous humor. Investig. Ophthalmol. Vis. Sci. 1984, 25, 871–873. [Google Scholar]
- Vergani, S.; Di Mauro, E.; Davies, E.T.; Spinelli, D.; Mieli-Vergani, G.; Vergani, D. Complement activation in uveitis. Br. J. Ophthalmol. 1986, 70, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Maddala, R.; Ho, L.T.Y.; Karnam, S.; Navarro, I.; Osterwald, A.; Stinnett, S.S.; Ullmer, C.; Vann, R.R.; Challa, P.; Rao, P.V. Elevated Levels of Growth/Differentiation Factor-15 in the Aqueous Humor and Serum of Glaucoma Patients. J. Clin. Med. 2022, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, M.; Nasu, N.; Yoshida, S.; Higuchi, Y.; Akizuki, S.; Yamamoto, S. Mouse and human CD14 (myeloid cell-specific leucine-rich glycoprotein) primary structure deduced from cDNA clones. Biochim. Biophys. Acta 1989, 1008, 213–222. [Google Scholar] [CrossRef]
- Streilein, J.W.; Niederkorn, J.Y. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J. Exp. Med. 1981, 153, 1058–1067. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef]
- Yeung, V.; Sriram, S.; Tran, J.A.; Guo, X.; Hutcheon, A.E.K.; Zieske, J.D.; Karamichos, D.; Ciolino, J.B. FAK Inhibition Attenuates Corneal Fibroblast Differentiation In Vitro. Biomolecules 2021, 11, 1682. [Google Scholar] [CrossRef]
- Conedera, F.M.; Quintela Pousa, A.M.; Presby, D.M.; Mercader, N.; Enzmann, V.; Tschopp, M. Diverse Signaling by TGFβ Isoforms in Response to Focal Injury is Associated with Either Retinal Regeneration or Reactive Gliosis. Cell. Mol. Neurobiol. 2021, 41, 43–62. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.K.; Zieske, J.D. Molecular insights on the effect of TGF-β1/-β3 in human corneal fibroblasts. Exp. Eye Res. 2016, 146, 233–241. [Google Scholar] [CrossRef]
- Michelson, S.; Alcami, J.; Kim, S.J.; Danielpour, D.; Bachelerie, F.; Picard, L.; Bessia, C.; Paya, C.; Virelizier, J.L. Human cytomegalovirus infection induces transcription and secretion of transforming growth factor beta 1. J. Virol. 1994, 68, 5730–5737. [Google Scholar] [CrossRef]
- Faralli, J.A.; Clark, R.W.; Filla, M.S.; Peters, D.M. NFATc1 activity regulates the expression of myocilin induced by dexamethasone. Exp. Eye Res. 2015, 130, 9–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prat, C.; Belville, C.; Comptour, A.; Marceau, G.; Clairefond, G.; Chiambaretta, F.; Sapin, V.; Blanchon, L. Myocilin expression is regulated by retinoic acid in the trabecular meshwork-derived cellular environment. Exp. Eye Res. 2017, 155, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Guo, M.; He, Q.; Hu, Y. Effects of transforming growth factor-β2 on myocilin expression and secretion in human primary cultured trabecular meshwork cells. Int. J. Clin. Exp. Pathol. 2014, 7, 4827–4836. [Google Scholar] [PubMed]
- Li, G.; Cui, G.; Dismuke, W.M.; Navarro, I.; Perkumas, K.; Woodward, D.F.; Stamer, W.D. Differential response and withdrawal profile of glucocorticoid-treated human trabecular meshwork cells. Exp. Eye Res. 2017, 155, 38–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, G.C.; Liu, Y.; Millar, J.C.; Clark, A.F. Glucocorticoid receptor GRβ regulates glucocorticoid-induced ocular hypertension in mice. Sci. Rep. 2018, 8, 862. [Google Scholar] [CrossRef]
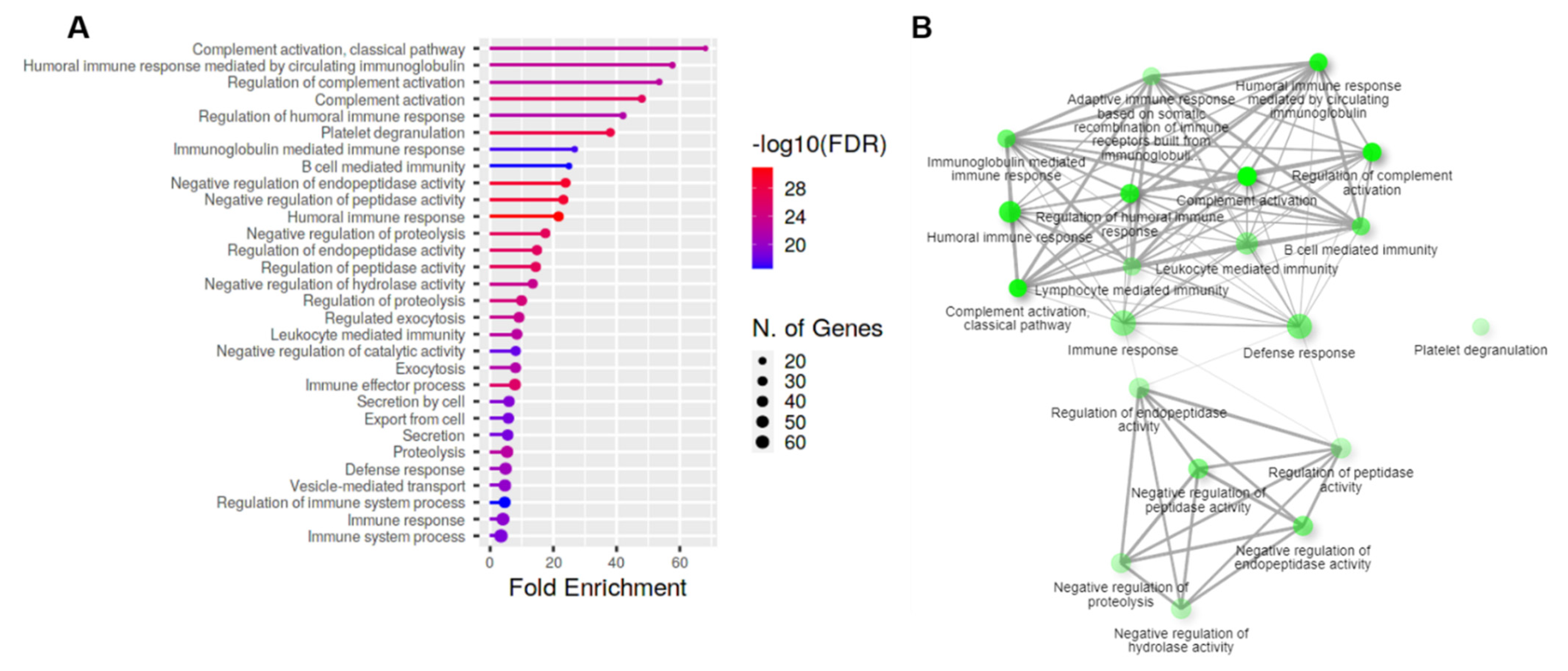
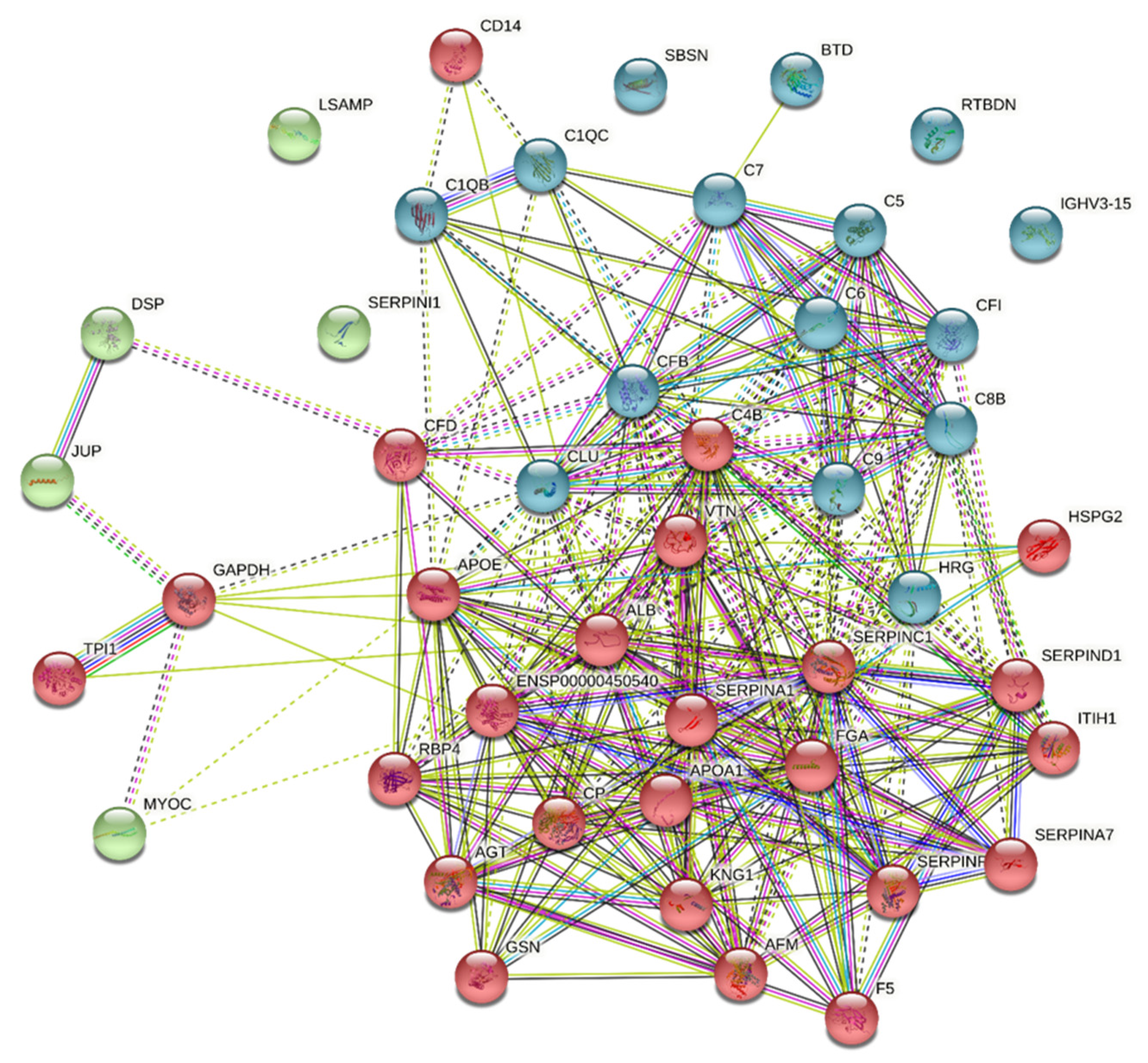

| SwissProt Accession | Protein Name | Gene Name | Confidence Score | Mass | Fold Change | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Q99574 | Neuroserpin | SERPINI1 | 36.52 | 46,397 | 107.418 | 35.202 | 0.034 |
| P08571 | Monocyte differentiation antigen CD14 | CD14 | 221.71 | 40,678 | 49.448 | 19.642 | 0.014 |
| P02747 | Complement C1q subcomponent subunit C | C1QC | 96.28 | 25,985 | 31.305 | 7.597 | 0.001 |
| P06310 | Immunoglobulin kappa variable 2–30 | IGKV2-30 | 171.55 | 13,291 | 18.993 | 11.785 | 0.047 |
| P02671 | Fibrinogen alpha chain | FGA | 882.32 | 95,656 | 15.914 | 3.252 | 0.049 |
| P00746 | Complement factor D | CFD | 371.47 | 27,529 | 12.955 | 2.630 | 0.021 |
| P08697 | Alpha-2-antiplasmin | SERPINF2 | 780.89 | 54,873 | 10.648 | 3.051 | 0.029 |
| P98160 | Basement membrane-specific heparan sulfate proteoglycan core protein | HSPG2 | 463.75 | 479,253 | 9.577 | 1.577 | 0.003 |
| Q99972 | Myocilin | MYOC | 545.94 | 57,450 | 9.492 | 1.951 | 0.002 |
| P13671 | Complement component C6 | C6 | 426.5 | 108,367 | 8.149 | 1.789 | 0.027 |
| P07358 | Complement component C8 beta chain | C8B | 181.35 | 68,714 | 8.097 | 2.285 | 0.048 |
| P0C0L5 | Complement C4-B | C4B | 836.96 | 194,170 | 8.028 | 2.454 | 0.044 |
| P05546 | Heparin cofactor 2 | SERPIND1 | 734.9 | 57,205 | 7.671 | 1.750 | 0.044 |
| P04004 | Vitronectin | VTN | 690.61 | 55,069 | 7.582 | 1.678 | 0.044 |
| Q9BSG5 | Retbindin | RTBDN | 40.8 | 25,397 | 7.302 | 2.058 | 0.050 |
| P01042 | Kininogen-1 | KNG1 | 764.45 | 72,996 | 7.016 | 1.639 | 0.035 |
| P06396 | Gelsolin | GSN | 2330.63 | 86,043 | 6.891 | 2.099 | 0.022 |
| P01031 | Complement C5 | C5 | 312.35 | 189,897 | 6.741 | 1.119 | 0.012 |
| Q9Y6R7 | IgGFc-binding protein | FCGBP | 399.89 | 596,443 | 6.624 | 2.003 | 0.034 |
| P02746 | Complement C1q subcomponent subunit B | C1QB | 135.42 | 26,933 | 6.401 | 1.021 | 0.007 |
| P02748 | Complement component C9 | C9 | 789.9 | 64,615 | 6.118 | 1.195 | 0.034 |
| P43251 | Biotinidase | BTD | 223.98 | 62,006 | 5.655 | 2.312 | 0.046 |
| P19827 | Inter-alpha-trypsin inhibitor heavy chain H1 | ITIH1 | 743.03 | 101,782 | 5.215 | 0.933 | 0.011 |
| P01009 | Alpha-1-antitrypsin | SERPINA1 | 3504.62 | 46,878 | 5.167 | 0.940 | 0.006 |
| P01700 | Immunoglobulin lambda variable 1–47 | IGLV1-47 | 181.44 | 12,447 | 4.759 | 0.767 | 0.001 |
| P10643 | Complement component C7 | C7 | 548.4 | 96,650 | 4.472 | 0.752 | 0.036 |
| P43652 | Afamin | AFM | 724.79 | 70,963 | 4.327 | 0.651 | 0.013 |
| P02647 | Apolipoprotein A-I | APOA1 | 2280.23 | 30,759 | 4.140 | 0.719 | 0.050 |
| P01011 | Alpha-1-antichymotrypsin | SERPINA3 | 2881.01 | 47,792 | 4.126 | 0.676 | 0.016 |
| P02649 | Apolipoprotein E | APOE | 1047.38 | 36,246 | 3.775 | 0.562 | 0.041 |
| P02768 | Albumin | ALB | 13,704.76 | 71,317 | 3.640 | 0.640 | 0.030 |
| Q13449 | Limbic system-associated membrane protein | LSAMP | 40.52 | 37,883 | 3.584 | 0.559 | 0.006 |
| P02753 | Retinol-binding protein 4 | RBP4 | 516.31 | 23,337 | 3.501 | 0.651 | 0.048 |
| P05543 | Thyroxine-binding globulin | SERPINA7 | 537.84 | 46,637 | 3.490 | 0.435 | 0.009 |
| P00751 | Complement factor B | CFB | 1454.73 | 86,847 | 3.411 | 0.631 | 0.047 |
| P04196 | Histidine-rich glycoprotein | HRG | 1350.42 | 60,510 | 3.381 | 0.500 | 0.000 |
| P01008 | Antithrombin-III | SERPINC1 | 2746.51 | 53,025 | 3.057 | 0.424 | 0.011 |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 40.47 | 36,201 | 2.760 | 0.831 | 0.045 |
| A0A075B6Q5 | Immunoglobulin heavy variable 3–64 | IGHV3-64 | 34.36 | 13,053 | 2.714 | 0.366 | 0.018 |
| P05156 | Complement factor I | CFI | 548.08 | 68,102 | 2.708 | 0.343 | 0.000 |
| P48740 | Complement C1s subcomponent | MASP1 | 845.36 | 78,174 | 2.529 | 0.300 | 0.016 |
| P15924 | Desmoplakin | DSP | 282.15 | 334,021 | 2.518 | 0.915 | 0.038 |
| P12259 | Coagulation factor V | F5 | 249.16 | 252,686 | 2.377 | 0.721 | 0.037 |
| Q6UWP8 | Suprabasin | SBSN | 145.13 | 60,562 | 2.275 | 0.843 | 0.003 |
| P60174 | Triosephosphate isomerase | TPI1 | 60.32 | 31,057 | 2.241 | 0.813 | 0.037 |
| A0A0B4J1V0 | Immunoglobulin heavy variable 3–15 | IGHV3-15 | 345.55 | 13,089 | 2.209 | 0.251 | 0.037 |
| P10909 | Clusterin | CLU | 1756.16 | 53,031 | 2.203 | 0.372 | 0.040 |
| P01019 | Angiotensinogen | AGT | 955.58 | 53,406 | 2.115 | 0.473 | 0.035 |
| P14923 | Junction plakoglobin | JUP | 138.53 | 82,434 | 2.110 | 0.783 | 0.016 |
| P00450 | Ceruloplasmin | CP | 4829.27 | 122,983 | 2.076 | 0.208 | 0.008 |
| SwissProt Accession | Protein Name | Gene Name | Confidence Score | Mass | Fold Change | SEM | p Value |
|---|---|---|---|---|---|---|---|
| P51884 | Lumican | LUM | 172.21 | 38,747 | 0.262 | 0.031 | 0.002 |
| P98164 | Low-density lipoprotein receptor-related protein 2 | LRP2 | 90.94 | 540,376 | 0.343 | 0.042 | 0.001 |
| Q92823 | Neuronal cell adhesion molecule | NRCAM | 143.1 | 144,655 | 0.356 | 0.058 | 0.001 |
| O00533 | Neural cell adhesion molecule L1-like protein | CHL1 | 43.02 | 136,070 | 0.370 | 0.053 | 0.000 |
| P07451 | Carbonic anhydrase 3 | CA3 | 41.84 | 29,824 | 0.375 | 0.069 | 0.003 |
| P78509 | Reelin | RELN | 27.64 | 394,980 | 0.392 | 0.079 | 0.023 |
| Q16610 | Extracellular matrix protein 1 | ECM1 | 123.22 | 62,232 | 0.393 | 0.055 | 0.002 |
| O60938 | Keratocan | KERA | 63.67 | 40,882 | 0.402 | 0.185 | 0.030 |
| Q99435 | Protein kinase C-binding protein NELL2 | NELL2 | 38.93 | 96,359 | 0.405 | 0.113 | 0.016 |
| Q12841 | Follistatin-related protein 1 | FSTL1 | 34.61 | 36,103 | 0.446 | 0.081 | 0.012 |
| Q6EMK4 | Vasorin | VASN | 211.36 | 72,751 | 0.448 | 0.063 | 0.009 |
| Q5D862 | Filaggrin-2 | FLG2 | 193.17 | 249,296 | 0.482 | 0.050 | 0.019 |
| Q14515 | SPARC-like protein 1 | SPARCL1 | 363.95 | 76,017 | 0.486 | 0.053 | 0.004 |
| P24592 | Insulin-like growth factor-binding protein 6 | IGFBP6 | 55.43 | 26,219 | 0.492 | 0.116 | 0.027 |
| Q9BY67 | Cell adhesion molecule 1 | CADM1 | 90.24 | 48,935 | 0.492 | 0.066 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.A.; Ju, H.-h.; Lee, J.; Kim, J.-E.; Paik, S.-Y.; Skiba, N.P.; Rao, P.V. Increased Complement-Associated Inflammation in Cytomegalovirus-Positive Hypertensive Anterior Uveitis Patients Based on the Aqueous Humor Proteomics Analysis. J. Clin. Med. 2022, 11, 2337. https://doi.org/10.3390/jcm11092337
Choi JA, Ju H-h, Lee J, Kim J-E, Paik S-Y, Skiba NP, Rao PV. Increased Complement-Associated Inflammation in Cytomegalovirus-Positive Hypertensive Anterior Uveitis Patients Based on the Aqueous Humor Proteomics Analysis. Journal of Clinical Medicine. 2022; 11(9):2337. https://doi.org/10.3390/jcm11092337
Chicago/Turabian StyleChoi, Jin A, Hyun-hee Ju, Jiyoung Lee, Ju-Eun Kim, Soon-Young Paik, Nikolai P. Skiba, and Ponugoti Vasantha Rao. 2022. "Increased Complement-Associated Inflammation in Cytomegalovirus-Positive Hypertensive Anterior Uveitis Patients Based on the Aqueous Humor Proteomics Analysis" Journal of Clinical Medicine 11, no. 9: 2337. https://doi.org/10.3390/jcm11092337
APA StyleChoi, J. A., Ju, H.-h., Lee, J., Kim, J.-E., Paik, S.-Y., Skiba, N. P., & Rao, P. V. (2022). Increased Complement-Associated Inflammation in Cytomegalovirus-Positive Hypertensive Anterior Uveitis Patients Based on the Aqueous Humor Proteomics Analysis. Journal of Clinical Medicine, 11(9), 2337. https://doi.org/10.3390/jcm11092337







