Olfactory Neuroblastomas: What Actually Happens in the Long-Term?
Abstract
1. Introduction
2. Materials and Methods
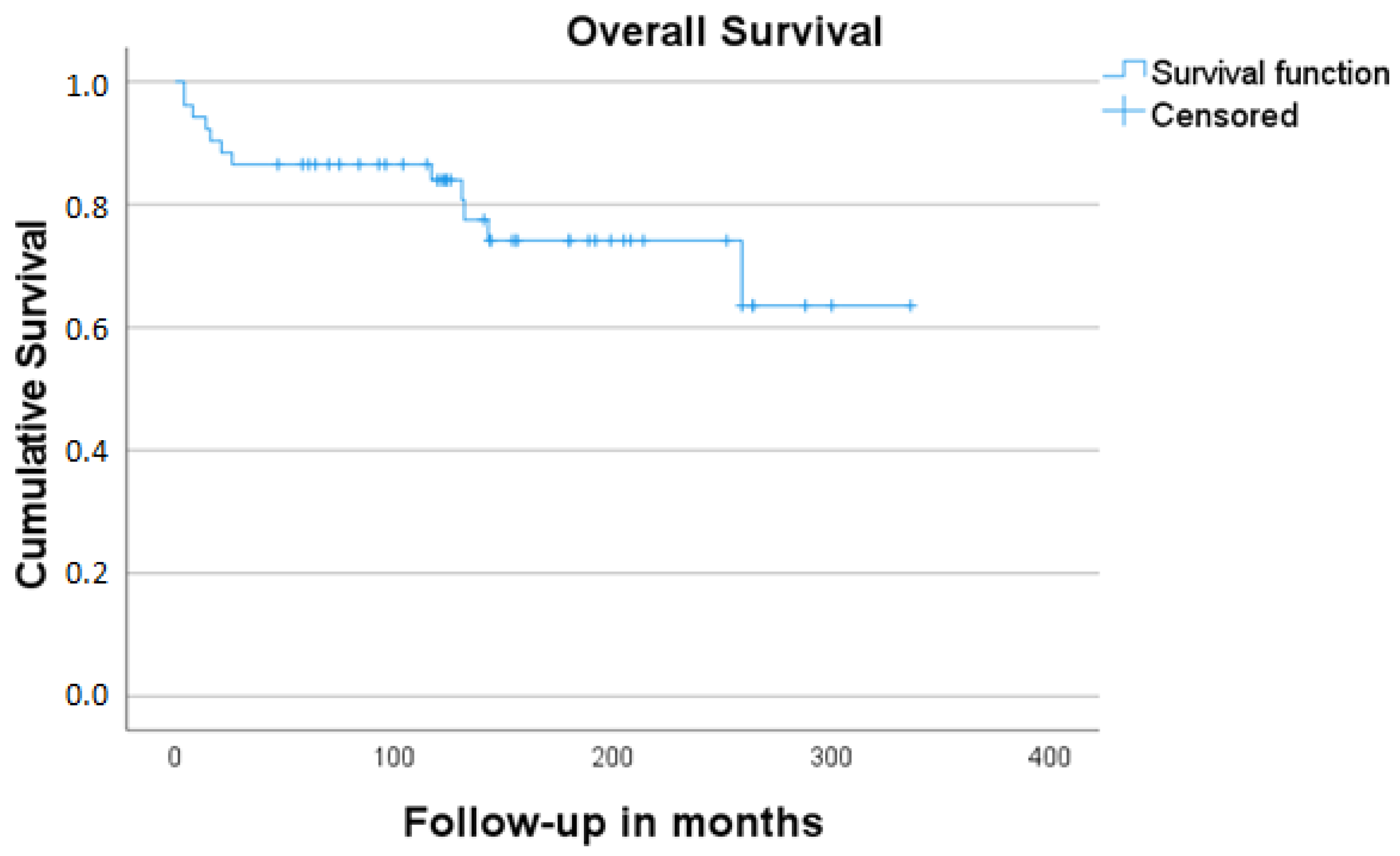
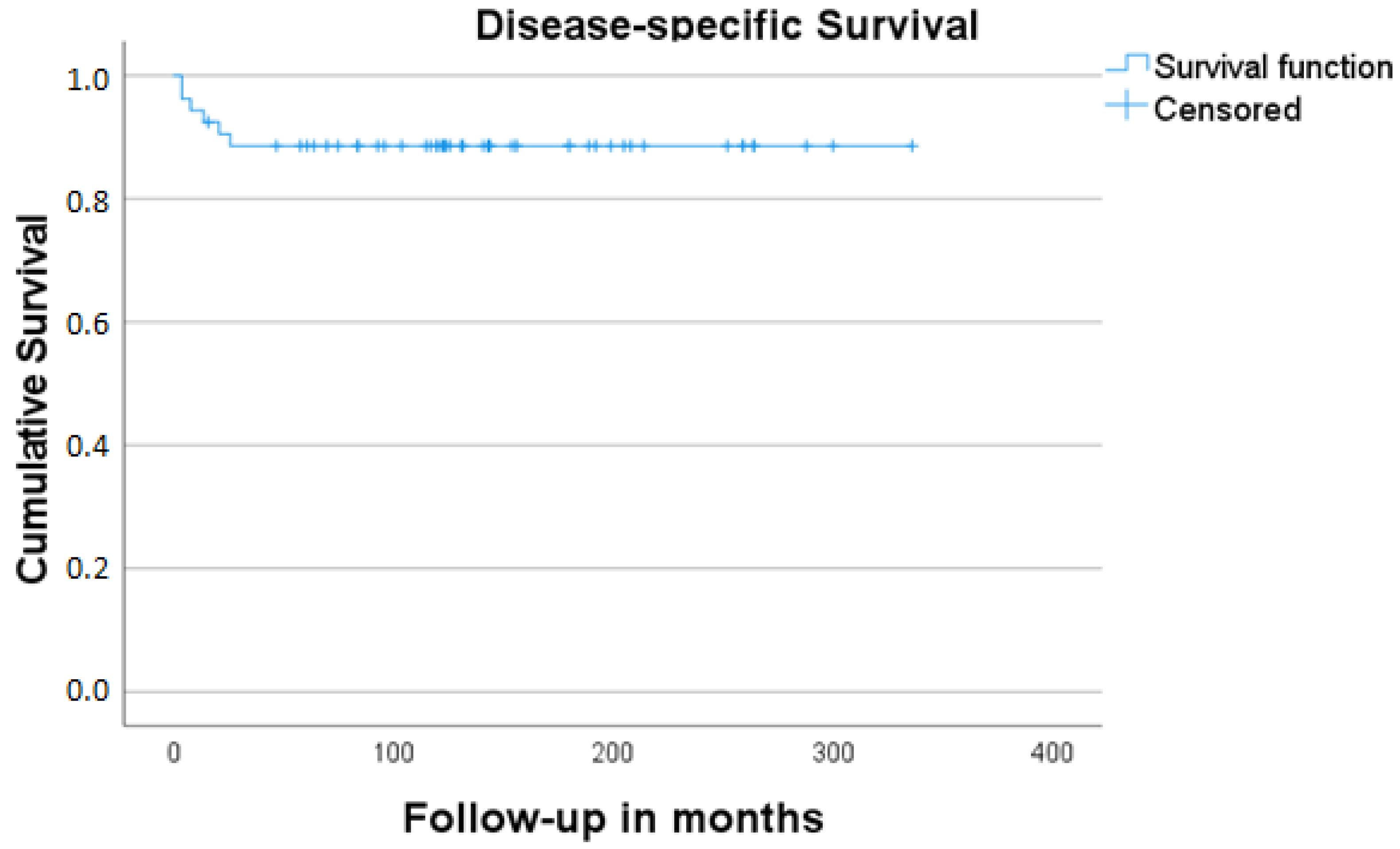
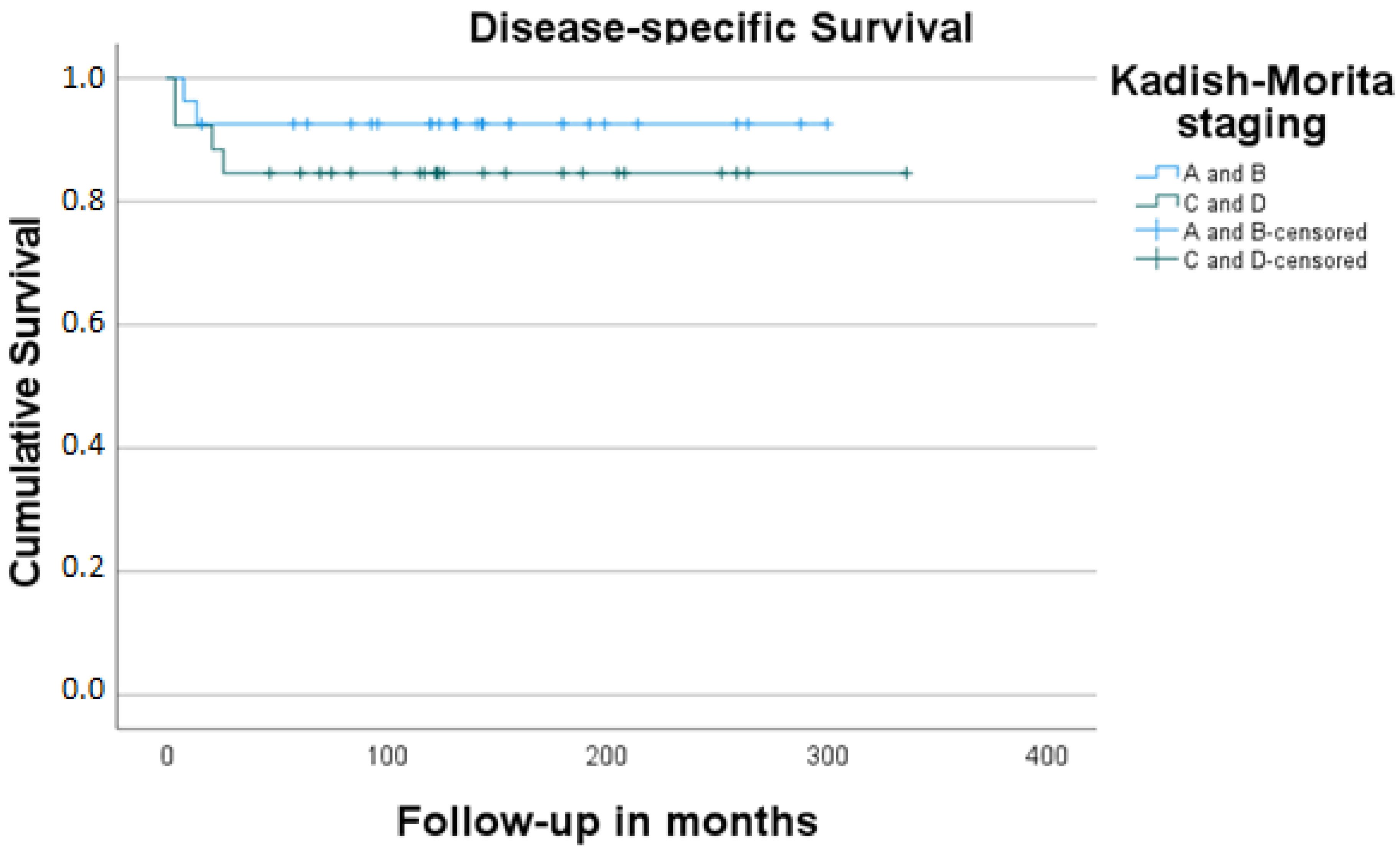
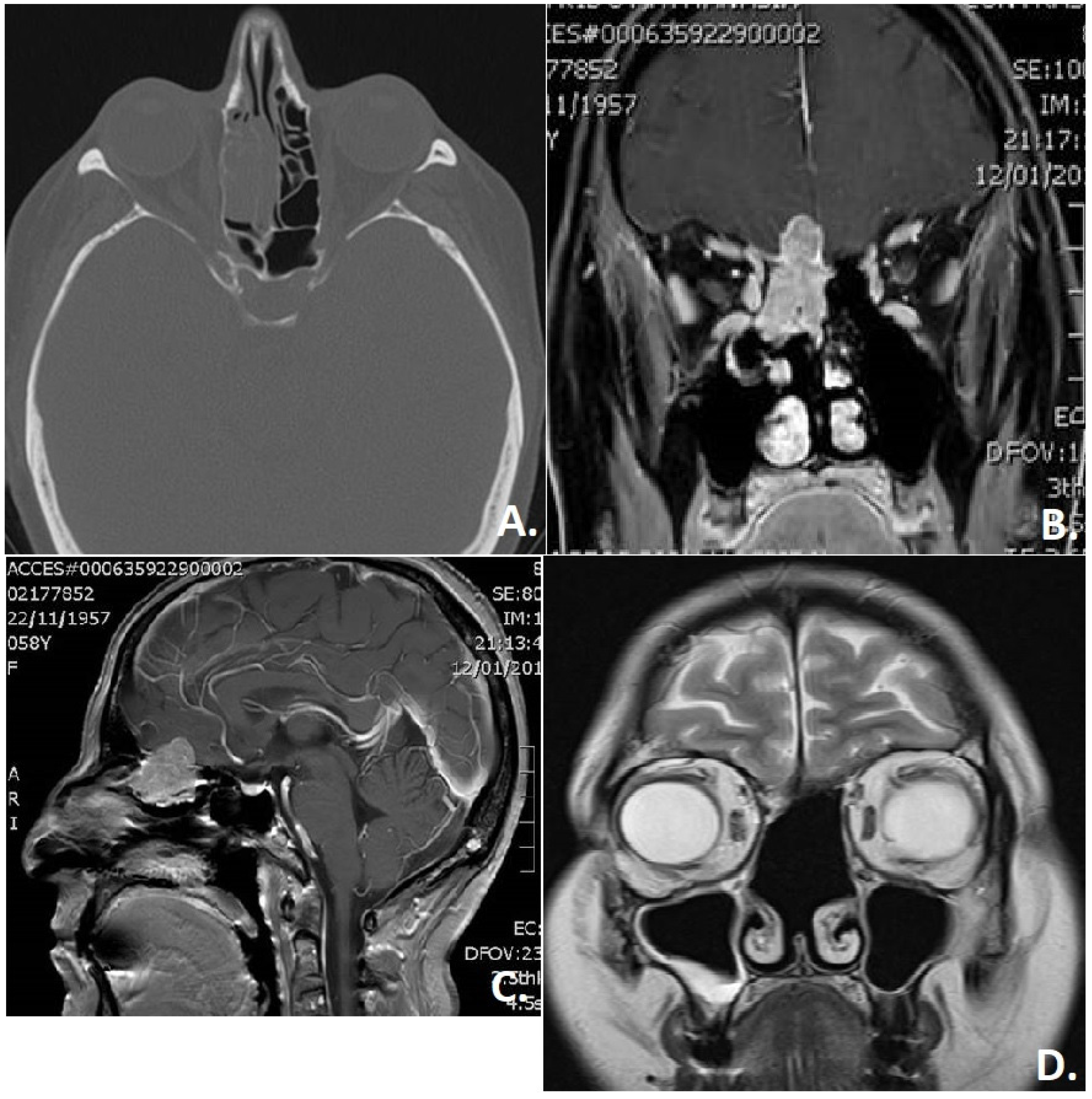
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, L.L.R.; Richard, D. L’esthesioneuroepithe liome olfactif. Bull. Assoc. Fr. Etud. Cancer 1924, 13, 410–421. [Google Scholar]
- Ferlito, A.; Rinaldo, A.; Rhys-Evans, P.H. Contemporary clinical commentary: Esthesioneuroblastoma: An update on management of the neck. Laryngoscope 2003, 113, 1935–1938. [Google Scholar] [CrossRef]
- Koch, M.; Constantinidis, J.; Dimmler, A.; Strauss, C.; Iro, H. Long-term experiences in the therapy of esthesioneuroblastoma. Laryngorhinootologie 2006, 85, 723–730. [Google Scholar] [CrossRef]
- Dulguerov, P.; Calcaterra, T. Esthesioneuroblastoma: The UCLA experience 1970–1990. Laryngoscope 1992, 102, 843–849. [Google Scholar] [CrossRef]
- Rimmer, J.; Lund, V.J.; Beale, T.; Wei, W.I.; Howard, D. Olfactory neuroblastoma: A 35-year experience and suggested follow-up protocol. Laryngoscope 2014, 124, 1542–1549. [Google Scholar] [CrossRef]
- Dumont, B.; Lemelle, L.; Cordero, C.; Couloigner, V.; Bernard, S.; Cardoen, L.; Brisse, H.J.; Jehanno, N.; Fréneaux, P.; Helfre, S.; et al. Esthesioneuroblastoma in children, adolescents and young adults. Bull. Cancer 2020, 107, 934–945. [Google Scholar] [CrossRef]
- Demiroz, C.; Gutfeld, O.; Aboziada, M.; Brown, D.; Marentette, L.J.; Eisbruch, A. Esthesioneuroblastoma: Is there a need for elective neck treatment? Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e255–e261. [Google Scholar] [CrossRef]
- Kadish, S.; Goodman, M.; Wang, C.C. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer 1976, 37, 1571–1576. [Google Scholar] [CrossRef]
- Biller, H.F.; Lawson, W.; Sachdev, V.P.; Som, P. Esthesioneuroblastoma: Surgical treatment without radiation. Laryngoscope 1990, 100, 1199–1201. [Google Scholar] [CrossRef]
- Foote, R.L.; Morita, A.; Ebersold, M.J.; Olsen, K.D.; Lewis, J.E.; Quast, L.M.; Ferguson, J.A.; O’Fallon, W.M. Esthesioneuroblastoma: The role of adjuvant radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 835–842. [Google Scholar] [CrossRef]
- Sun, M.; Wang, K.; Qu, Y.; Zhang, J.; Zhang, S.; Chen, X.; Wang, J.; Wu, R.; Zhang, Y.; Yi, J.; et al. Proposal of a TNM classification-based staging system for esthesioneuroblastoma: More precise prediction of prognosis. Head Neck 2021, 43, 1097–1104. [Google Scholar] [CrossRef]
- Naples, J.G.; Spiro, J.; Tessema, B.; Kuwada, C.; Kuo, C.L.; Brown, S.M. Neck recurrence and mortality in esthesioneuroblastoma: Implications for management of the N0 neck. Laryngoscope 2016, 126, 1373–1379. [Google Scholar] [CrossRef]
- Hyams, V.J. Olfactory neuroblastoma. In Tumors of the Upper Respiratory Tract and Ear; Hyams, V.J.B.J., Michaels, L., Eds.; Armed Forces Institute of Pathology: Washington, DC, USA, 1988; pp. 240–248. [Google Scholar]
- Koka, V.N.; Julieron, M.; Bourhis, J.; Janot, F.; Le Ridant, A.M.; Marandas, P.; Luboinski, B.; Schwaab, G. Aesthesioneuroblastoma. J. Laryngol. Otol. 1998, 112, 628–633. [Google Scholar] [CrossRef]
- Lund, V.J.; Howard, D.; Wei, W.; Spittle, M. Olfactory neuroblastoma: Past, present, and future? Laryngoscope 2003, 113, 502–507. [Google Scholar] [CrossRef]
- Zafereo, M.E.; Fakhri, S.; Prayson, R.; Batra, P.S.; Lee, J.; Lanza, D.C.; Citardi, M.J. Esthesioneuroblastoma: 25-year experience at a single institution. Otolaryngol. Head Neck Surg. 2008, 138, 452–458. [Google Scholar] [CrossRef]
- Diaz, E.M., Jr.; Johnigan, R.H., III; Pero, C.; El-Naggar, A.K.; Roberts, D.B.; Barker, J.L.; DeMonte, F. Olfactory neuroblastoma: The 22-year experience at one comprehensive cancer center. Head Neck 2005, 27, 138–149. [Google Scholar] [CrossRef]
- Platek, M.E.; Merzianu, M.; Mashtare, T.L.; Popat, S.R.; Rigual, N.R.; Warren, G.W.; Singh, A.K. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: Analysis of the SEER database. Radiat. Oncol. 2011, 6, 41. [Google Scholar] [CrossRef]
- Meerwein, C.M.; Nikolaou, G.; Binz, G.H.A.; Soyka, M.B.; Holzmann, D. Surgery as Single-Modality Treatment for Early-Stage Olfactory Neuroblastoma: An Institutional Experience, Systematic Review and Meta-analysis. Am. J. Rhinol. Allergy 2021, 35, 525–534. [Google Scholar] [CrossRef]
- Jiang, W.; Mohamed, A.S.R.; Fuller, C.D.; Kim, B.Y.S.; Tang, C.; Gunn, G.B.; Hanna, E.Y.; Frank, S.J.; Su, S.Y.; Diaz, E.; et al. The role of elective nodal irradiation for esthesioneuroblastoma patients with clinically negative neck. Pract. Radiat. Oncol. 2016, 6, 241–247. [Google Scholar] [CrossRef][Green Version]
- Yin, Z.Z.; Luo, J.W.; Gao, L.; Yi, J.L.; Huang, X.D.; Qu, Y.; Wang, K.; Zhang, S.P.; Xiao, J.P.; Xu, G.Z.; et al. Spread patterns of lymph nodes and the value of elective neck irradiation for esthesioneuroblastoma. Radiother. Oncol. 2015, 117, 328–332. [Google Scholar] [CrossRef]
- Zlochower, A.B.; Steinklein, J.M. Doing Great with DOTATATE: Update on GA-68 DOTATATE Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Evaluation of Sinonasal Tumors. Top. Magn. Reason. Imaging 2021, 30, 151–158. [Google Scholar] [CrossRef]
- Ow, T.J.; Hanna, E.; Roberts, D.B.; Levine, N.B.; El-Naggar, A.K.; Rosenthal, D.; Demonte, F.; Kupferman, M.E. Optimization of long-term outcomes for patients with esthesioneuroblastoma. Head Neck 2014, 36, 524–530. [Google Scholar] [CrossRef]
- Casiano, R.R.; Numa, W.A.; Falquez, A.M. Endoscopic resection of esthesioneuroblastoma. Am. J. Rhinol. 2001, 15, 271–279. [Google Scholar] [CrossRef]
- Gallia, G.L.; Reh, D.D.; Salmasi, V.; Blitz, A.M.; Koch, W.; Ishii, M. Endonasal endoscopic resection of esthesioneuroblastoma: The Johns Hopkins Hospital experience and review of the literature. Neurosurg. Rev. 2011, 34, 465–475. [Google Scholar] [CrossRef]
- Morita, A.; Ebersold, M.J.; Olsen, K.D.; Foote, R.L.; Lewis, J.E.; Quast, L.M. Esthesioneuroblastoma: Prognosis and management. Neurosurgery 1993, 32, 706–714, discussion 14–15. [Google Scholar] [CrossRef]
- Liermann, J.; Syed, M.; Held, T.; Bernhardt, D.; Plinkert, P.; Jungk, C.; Unterberg, A.; Rieken, S.; Debus, J.; Herfarth, K.; et al. Advanced Radiation Techniques in the Treatment of Esthesioneuroblastoma: A 7-Year Single-Institution’s Clinical Experience. Cancers 2018, 10, 457. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Wu, Y.; Zhang, X.; Wang, F.; Wang, P.; Tao, Z.; Yuan, Z. Age distribution and age-related outcomes of olfactory neuroblastoma: A population-based analysis. Cancer Manag. Res. 2018, 10, 1359–1364. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Kim, L.J.; Bly, R.A.; Moe, K.; Ferreira, M., Jr. Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J. Clin. Neurosci. 2016, 24, 99–104. [Google Scholar] [CrossRef]
- Nichols, A.C.; Chan, A.W.; Curry, W.T.; Barker, F.G.; Deschler, D.G.; Lin, D.T. Esthesioneuroblastoma: The massachusetts eye and ear infirmary and massachusetts general hospital experience with craniofacial resection, proton beam radiation, and chemotherapy. Skull Base 2008, 18, 327–337. [Google Scholar] [CrossRef][Green Version]
- Eden, B.V.; Debo, R.F.; Larner, J.M.; Kelly, M.D.; Levine, P.A.; Stewart, F.M.; Cantrell, R.W.; Constable, W.C. Esthesioneuroblastoma. Long-term outcome and patterns of failure—The University of Virginia experience. Cancer 1994, 73, 2556–2562. [Google Scholar] [CrossRef]
- Porter, A.B.; Bernold, D.M.; Giannini, C.; Foote, R.L.; Link, M.J.; Olsen, K.D.; Moynihan, T.J.; Buckner, J.C. Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J. Neurooncol. 2008, 90, 201–204. [Google Scholar] [CrossRef]
- Constantinidis, J.; Steinhart, H.; Koch, M.; Buchfelder, M.; Schaenzer, A.; Weidenbecher, M.; Iro, H. Olfactory neuroblastoma: The University of Erlangen-Nuremberg experience 1975–2000. Otolaryngol. Head Neck Surg. 2004, 130, 567–574. [Google Scholar] [CrossRef]
- Zanation, A.M.; Ferlito, A.; Rinaldo, A.; Gore, M.R.; Lund, V.J.; McKinney, K.A.; Suárez, C.; Takes, R.P.; Devaiah, A. When, how and why to treat the neck in patients with esthesioneuroblastoma: A review. Eur. Arch. Otorhinolaryngol. 2010, 267, 1667–1671. [Google Scholar] [CrossRef]
- Banuchi, V.E.; Dooley, L.; Lee, N.Y.; Pfister, D.G.; McBride, S.; Riaz, N.; Bilsky, M.H.; Ganly, I.; Shah, J.P.; Kraus, D.H.; et al. Patterns of regional and distant metastasis in esthesioneuroblastoma. Laryngoscope 2016, 126, 1556–1561. [Google Scholar] [CrossRef]
- Weiss, M.H.; Harrison, L.B.; Isaacs, R.S. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 699–702. [Google Scholar] [CrossRef]
- Principles and Practice of Radiation Oncology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008.
- Managing Esthesioneuroblastoma—An Expert’s Guide. ERS Webinar Autumn Series. 2021. Available online: https://www.youtube.com/watch?v=6Mgl-p5-Dxo (accessed on 1 February 2022).
- Zollinger, L.V.; Wiggins, R.H., III; Cornelius, R.S.; Phillips, C.D. Retropharyngeal lymph node metastasis from esthesioneuroblastoma: A review of the therapeutic and prognostic implications. AJNR Am. J. Neuroradiol. 2008, 29, 1561–1563. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.; Yoon, J.H. Retropharyngeal lymph node metastasis from olfactory neuroblastoma: A report of two cases. Eur. Arch. Otorhinolaryngol. 2006, 263, 778–782. [Google Scholar] [CrossRef]
- Teshima, M.; Otsuki, N.; Shinomiya, H.; Morita, N.; Furukawa, T.; Morimoto, K.; Nakamura, T.; Hashikawa, K.; Kiyota, N.; Sasaki, R.; et al. Impact of retropharyngeal lymph node dissection in the surgical treatment of hypopharyngeal cancer. Head Neck 2019, 41, 1738–1744. [Google Scholar] [CrossRef]
- Sun, M.; Wang, K.; Qu, Y.; Zhang, J.; Zhang, S.; Chen, X.; Wang, J.; Wu, R.; Zhang, Y.; Yi, J.; et al. Long-term analysis of multimodality treatment outcomes and prognosis of esthesioneuroblastomas: A single center results of 138 patients. Radiat. Oncol. 2020, 15, 219. [Google Scholar] [CrossRef]

| ID | Gender | Age (y) | Stage (Kadish) | Stage (Kadish–Morita) | Histologic Grading (Hyams) | N Status | Treatment | Recurrence (After … Months) | Outcome (Follow-Up in Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 10 | B | B | IIII | N0 | Neoadjuvant RCT + BFC | Local | AND (144) |
| 2 | Male | 56 | B | B | III | N0 | Neoadjuvant RT + BFC | Local | DAD (259) |
| 3 | Female | 22 | C | D | IV | N3 | Neoadjuvant RCT + BFC | No | DOD (4) |
| 4 | Female | 38 | C | C | III | N0 | Neoadjuvant RT + TFA | Locoregional | DOD (21) |
| 5 | Male | 59 | A | A | II | N0 | ESS | No | AND (199) |
| 6 | Male | 54 | B | B | I | N0 | ESS | No | AND (156) |
| 7 | Female | 63 | B | B | II | N0 | ESS | No | AND (120) |
| 8 | Female | 77 | B | B | IV | N0 | TFA | Local | DOD (14) |
| 9 | Female | 46 | C | C | III | N0 | BFC | No | AND (122) |
| 10 | Female | 50 | B | B | III | N0 | BFC | Locoregional | DOD (8) |
| 11 | Male | 50 | C | C | III | N0 | BFC | No | DOD (4) |
| 12 | Male | 28 | B | B | II | N0 | ESS + aRT | No | AND (214) |
| 13 | Female | 67 | B | B | II | N0 | ESS + aRT | No | DAD (131) |
| 14 | Male | 16 | B | B | III | N0 | ESS + aRT | No | AND (141) |
| 15 | Male | 52 | B | D | III | N1 | ESS + aRT | No | AND (124) |
| 16 | Male | 36 | C | C | III | N0 | ESS + aRT | No | AND (208) |
| 17 | Male | 48 | C | C | III | N0 | ESS + aRT | Regional | AND (189) |
| 18 | Female | 55 | C | C | III | N0 | ESS + aRT | No | AND (154) |
| 19 | Female | 41 | C | C | III | N0 | ESS + aRT | No | AND (115) |
| 20 | Male | 71 | B | B | III | N0 | ESS + aRT | No | DAD (132) |
| 21 | Male | 27 | B | B | II | N0 | ESS + aRT | Regional | AND (180) |
| 22 | Female | 56 | B | B | III | N0 | ESS + aRT | No | AND (120) |
| 23 | Female | 64 | C | D | II | N1 | ESS + BFC+ aRT | No | AND (205) |
| 24 | Female | 62 | C | C | I | N0 | ESS + BFC+ aRT | No | AND (75) |
| 25 | Male | 80 | B | B | I | N0 | ESS + BFC + aRT | No | DAD (143) |
| 26 | Male | 57 | A | A | II | N0 | ESS + BFC + aRT | No | AND (264) |
| 27 | Female | 53 | B | B | II | N0 | ESS + BFC + aRT | No | AND (300) |
| 28 | Male | 32 | C | C | III | N0 | ESS + BFC + aRT | Local | DOD (26) |
| 29 | Male | 15 | B | B | II | N0 | TFA + aRT | No | AND (288) |
| 30 | Male | 51 | B | B | I | N0 | BFC + aRT | Local | AND (156) |
| 31 | Male | 48 | C | C | I | N0 | BFC + aRT | No | AND (264) |
| 32 | Female | 57 | C | C | I | N0 | BFC + aRT | No | AND (252) |
| 33 | Male | 62 | C | C | I | N0 | BFC + aRT | No | DAD (117) |
| 34 | Female | 38 | B | B | II | N0 | BFC + aRT | Regional | AND (192) |
| 35 | Female | 31 | B | B | II | N0 | BFC + aRT | No | AND (180) |
| 36 | Female | 84 | B | B | II | N0 | BFC + aRT | No | DAD (16) |
| 37 | Male | 36 | C | C | III | N0 | BFC + aRT | No | AND (336) |
| 38 | Female | 34 | C | D | III | N3 | BFC + aRT | Regional | AND (180) |
| 39 | Female | 45 | C | C | III | N0 | BFC + aRT | No | AND (123) |
| 40 | Female | 43 | C | C | III | N0 | BFC + aRT | No | AND (126) |
| 41 | Male | 68 | C | C | III | N0 | BFC + aRT | Distant recurrence | AND (144) |
| 42 | Female | 69 | C | C | IV | N0 | BFC + aRT | No | AND (259) |
| 43 | Female | 17 | B | B | III | N0 | ESS + aRT | No | AND (96) |
| 44 | Male | 51 | C | C | III | N0 | ESS + aRT | No | AND (84) |
| 45 | Male | 24 | C | C | IV | N0 | ESS + BFC + aRT | No | AND (70) |
| 46 | Female | 44 | B | B | III | N0 | ESS +aRT | No | AND (64) |
| 47 | Male | 62 | A | A | II | N0 | ESS + aRT | No | AND (58) |
| 48 | Male | 55 | C | C | III | N2 | Neoadjuvant RCT + ESS | No | AND (47) |
| 49 | Female | 65 | C | C | II | N0 | ESS + BFC + aRT | No | AND (61) |
| 50 | Male | 53 | B | B | II | N0 | ESS + aRT | No | AND (84) |
| 51 | Female | 67 | B | B | III | N0 | ESS + aRT | No | AND (93) |
| 52 | Female | 43 | C | C | III | N0 | ESS + BFC + aRT | Local, distant | AWD (104) |
| 53 | Male | 47 | B | B | III | N0 | ESS + aRT | Regional | AND (124) |
| Gender (n, %) | |
| Male | 26 (49.1) |
| Female | 27 (50.9) |
| Kadish [8] stage (n, %) | |
| A | 3 (5.7) |
| B | 25 (47.2) |
| C | 25 (47.2) |
| Kadish–Morita [26] grading (n, %) | |
| A | 3 (5.7) |
| B | 24 (45.3) |
| C | 22 (41.5) |
| D | 4 (7.5) |
| Hyams [13] (n, %) | |
| I | 7 (13.2) |
| II | 1 (28.3) |
| III | 26 (49.1) |
| IV | 5 (9.4) |
| Nodal stage (n, %) | |
| N0 | 48 (90.6) |
| N+ | 5 (9.4) |
| Therapeutic approach (n, %) | |
| Surgery only | 7 (13.2) |
| Surgery + adjuvant irradiation | 41 (77.3) |
| Neoadjuvant R(C)T + surgery | 5 (9.4) |
| Surgical approach (n, %) | |
| Endoscopic only | 22 (41.5) |
| Endoscopic + open (craniofacial) | 22 (41.5) |
| Open (craniofacial) | 9 (17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantsopoulos, K.; Koch, M.; Iro, H.; Constantinidis, J. Olfactory Neuroblastomas: What Actually Happens in the Long-Term? J. Clin. Med. 2022, 11, 2288. https://doi.org/10.3390/jcm11092288
Mantsopoulos K, Koch M, Iro H, Constantinidis J. Olfactory Neuroblastomas: What Actually Happens in the Long-Term? Journal of Clinical Medicine. 2022; 11(9):2288. https://doi.org/10.3390/jcm11092288
Chicago/Turabian StyleMantsopoulos, Konstantinos, Michael Koch, Heinrich Iro, and Jannis Constantinidis. 2022. "Olfactory Neuroblastomas: What Actually Happens in the Long-Term?" Journal of Clinical Medicine 11, no. 9: 2288. https://doi.org/10.3390/jcm11092288
APA StyleMantsopoulos, K., Koch, M., Iro, H., & Constantinidis, J. (2022). Olfactory Neuroblastomas: What Actually Happens in the Long-Term? Journal of Clinical Medicine, 11(9), 2288. https://doi.org/10.3390/jcm11092288







