“No-Reflow” Phenomenon: A Contemporary Review
Abstract
1. Introduction
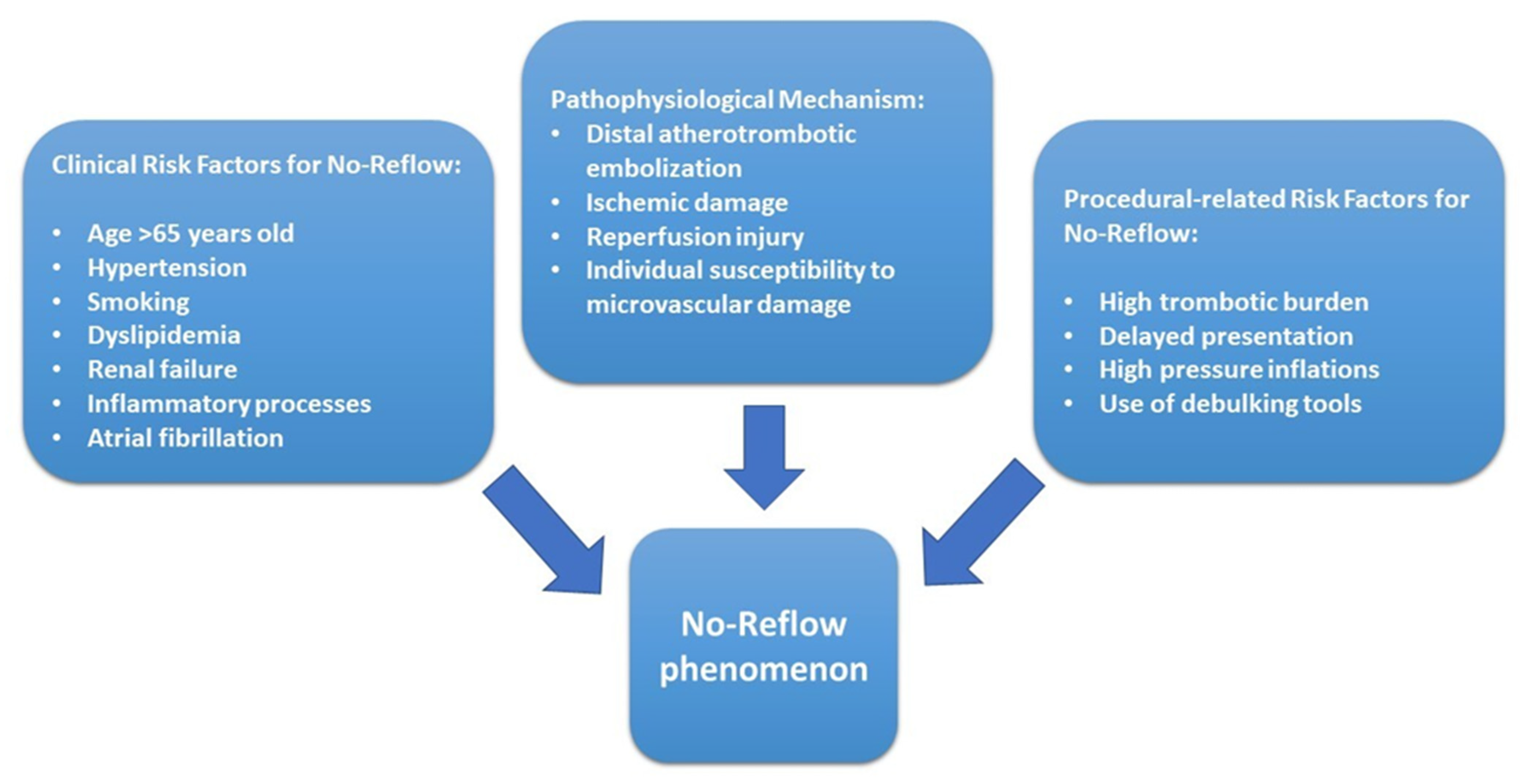
2. Pathophysiological Mechanisms
3. Diagnosis of No-Reflow
| Diagnostic Methods | Study Design | Results | Limitations |
|---|---|---|---|
| Coronary Angiography (MBG) [42] | 777 prospectively enrolled patients who underwent pPCI during a 6-year period. | MBG can be used to describe the effectiveness of myocardial reperfusion and is an independent predictor of long-term mortality. | Interobserver and intraobserver variabilities associated with subjective angiographic assessments. |
| Coronary Flow Reserve (CFR) [43] | 89 prospectively enrolled patients who underwent pPCI during a 4-year period and subsequent physiologic study. | A CFR value ≥ 2.0 is considered normal. Complimentary assessment of microcirculation by the IMR and CFR may be useful to evaluate myocardial viability and predict the long-term prognosis of STEMI patients. | Possible significant variability of tracings between different beats. Does not distinguish between epicardial and microvascular components of coronary resistances. Requires maximal hyperemia using adenosine. |
| Microvascular resistance index (IMR) [44] | 288 prospectively enrolled patients with STEMI during a 11-year period. | An IMR > 40 is a multivariable associate of left ventricular and clinical outcomes after STEMI, regardless of infarct size. IMR has superior clinical value for risk stratification. | Manual injection of saline may be a source of variability. It requires achievement of maximal hyperemia and the use of adenosine. |
| Electrocardiogram (ECG) [36] | 180 prospectively enrolled patients with a first acute STEMI. | Residual ST-segment elevation and the number of Q waves on the ECG shortly after pPCI have complementary predictive value on myocardial function, infarct size and extent, and MVO. | Discordance between resolution of ST-segment elevation and the angiographic indices of NR. |
| Myocardial Contrast Echocardiography (MCE) [40] | 110 prospectively enrolled patients who underwent pPCI in a multicenter study. | Among patients with TIMI 3 flow, MVO extension, as detected and quantified by MCE, is the most powerful independent predictor of LV remodeling after STEMI compared with persistent ST-segment elevation and degree of MBG. | Operator-dependent and limited by the possible poor acoustic window. |
| Cardiac Magnetic Resonance (CMR) [6] | Pooled analysis using individual patient data from seven randomized primary PCI trials | The presence and extent of MVO measured by CMR after primary PCI in STEMI are strongly associated with mortality and hospitalization for HF within 1 year. | Usually performed 2 to 7 days after pPCI. Not widely available locally. Not performable in all patients. |
| Positron Emission Tomography (PET) [37] | Seven porcine model with left anterior descending coronary artery occlusion/reperfusion underwent PET-CT within 3 days of infarction. | Increased regional FDG uptake in the area of acute infarction is a frequent occurrence and indicates tissue inflammation that is commonly associated with MVO. | Expensive and difficult to obtain locally. |
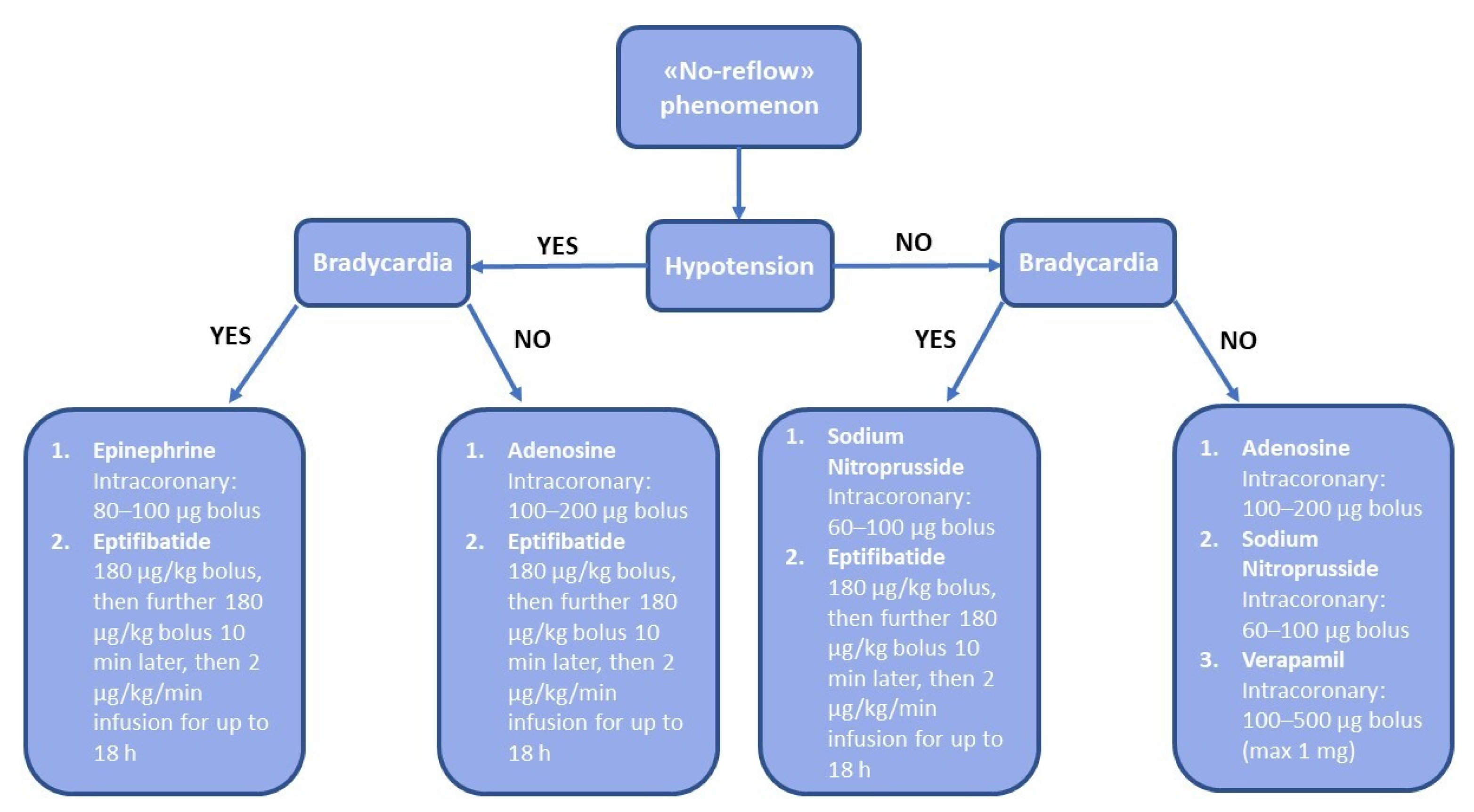
4. Management of No-Reflow
5. Pharmacological Treatment
5.1. Β-Blockers
5.2. Calcium Channel Blockers
5.3. Adenosine
5.4. Sodium Nitroprusside
5.5. Epinephrine
5.6. Nicorandil
5.7. Antiplatelet Therapy
5.8. Intracoronary Fibrinolysis
5.9. Statins
6. Non-Pharmacological Treatment
6.1. Ischemic Conditioning
6.2. Thrombus Aspiration
6.3. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N. Engl. J. Med. 1985, 312, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Kharbanda, R.K.; Crea, F.; Banning, A.P. No-reflow: Again prevention is better than treatment. Eur. Heart J. 2010, 31, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Tasar, O.; Karabay, A.K.; Oduncu, V.; Kirma, C. Predictors and outcomes of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron. Artery Dis. 2019, 30, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 2009, 54, 281–292. [Google Scholar] [CrossRef] [PubMed]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, G.; Musci, R.L.; Frediani, L.; Umińska, J.; Wanha, W.; Filipiak, K.J.; Kubica, J.; Navarese, E.P. State of the Art: No-Reflow Phenomenon. Cardiol. Clin. 2020, 38, 563–573. [Google Scholar] [CrossRef]
- Kaur, G.; Baghdasaryan, P.; Natarajan, B.; Sethi, P.; Mukherjee, A.; Varadarajan, P.; Pai, R.G. Pathophysiology, Diagnosis, and Management of Coronary No-Reflow Phenomenon. Int. J. Angiol. 2021, 30, 15–21. [Google Scholar] [CrossRef]
- Montone, R.A.; Camilli, M.; Del Buono, M.G.; Meucci, M.C.; Gurgoglione, F.; Russo, M.; Crea, F.; Niccoli, G. “No-reflow”: Update su diagnosi, fisiopatologia e strategie terapeutiche. G. Ital. Cardiol. 2020, 21, 4S–14S. [Google Scholar] [CrossRef]
- Porto, I.; Biasucci, L.M.; De Maria, G.L.; Leone, A.M.; Niccoli, G.; Burzotta, F.; Trani, C.; Tritarelli, A.; Vergallo, R.; Liuzzo, G.; et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur. Heart J. 2012, 33, 2928–2938. [Google Scholar] [CrossRef]
- Heusch, G.; Kleinbongard, P.; Böse, D.; Levkau, B.; Haude, M.; Schulz, R.; Erbel, R. Coronary microembolization: From bedside to bench and back to bedside. Circulation 2009, 120, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature 2005, 437, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bouleti, C.; Mewton, N.; Germain, S. The no-reflow phenomenon: State of the art. Arch. Cardiovasc. Dis. 2015, 108, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Tritto, I. Reperfusion injury: Experimental evidence and clinical implications. Am. Heart J. 1999, 138, S69–S75. [Google Scholar] [CrossRef]
- Fröhlich, G.M.; Meier, P.; White, S.K.; Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury: Looking beyond primary PCI. Eur. Heart J. 2013, 34, 1714–1722. [Google Scholar] [CrossRef]
- Riksen, N.P.; Franke, B.; van den Broek, P.; Smits, P.; Rongen, G.A. The 1976C>T polymorphism in the adenosine A2A receptor gene does not affect the vasodilator response to adenosine in humans in vivo. Pharmacogenet. Genom. 2007, 17, 551–554. [Google Scholar] [CrossRef]
- Kloner, R.A.; King, K.S.; Harrington, M.G. No-reflow phenomenon in the heart and brain. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H550–H562. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Daley, W.L.; Dodge, J.T.; Alexander, B.; Marble, S.J.; McCabe, C.H.; Raymond, L.; Fortin, T.; Poole, W.K.; et al. TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996, 93, 879–888. [Google Scholar] [CrossRef]
- Van ’t Hof, A.W.; Liem, A.; Suryapranata, H.; Hoorntje, J.C.; de Boer, M.J.; Zijlstra, F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef]
- Bulluck, H.; Foin, N.; Tan, J.W.; Low, A.F.; Sezer, M.; Hausenloy, D.J. Invasive Assessment of the Coronary Microcirculation in Reperfused ST-Segment-Elevation Myocardial Infarction Patients: Where Do We Stand? Circ. Cardiovasc. Interv. 2017, 10, e004373. [Google Scholar] [CrossRef]
- Knaapen, P.; Camici, P.G.; Marques, K.M.; Nijveldt, R.; Bax, J.J.; Westerhof, N.; Götte, M.J.W.; Jerosch-Herold, M.; Schelbert, H.R.; Lammertsma, A.A.; et al. Coronary microvascular resistance: Methods for its quantification in humans. Basic Res. Cardiol. 2009, 104, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Low, A.F.; Yong, A.S.; McGeoch, R.; Berry, C.; Shah, M.G.; Ho, M.Y.; Kim, H.-S.; Loh, J.P.; Oldroyd, K.G. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation 2013, 127, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, G.; Wolfrum, M.; De Maria, G.L.; Cuculi, F.; Dawkins, S.; Alkhalil, M.; Patel, N.; Forfar, J.C.; Prendergast, B.D.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights From the OxAMI (Oxford Study in Acute Myocardial Infarction) Cohort. J. Am. Heart Assoc. 2017, 6, e005409. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.O.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- De Maria, G.L.; Scarsini, R.; Shanmuganathan, M.; Kotronias, R.A.; Terentes-Printzios, D.; Borlotti, A.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.; et al. Angiography-derived index of microcirculatory resistance as a novel, pressure-wire-free tool to assess coronary microcirculation in ST elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1395–1406. [Google Scholar] [CrossRef]
- Ciofani, J.L.; Allahwala, U.K.; Scarsini, R.; Ekmejian, A.; Banning, A.P.; Bhindi, R.; De Maria, G.L. No-reflow phenomenon in ST-segment elevation myocardial infarction: Still the Achilles’ heel of the interventionalist. Future Cardiol. 2021, 17, 383–397. [Google Scholar] [CrossRef]
- Gragnano, F. Microvascular Obstruction Treatment Efficacy Measured Using Dynamic Microvascular Resistance (dMVR): First-in-Man Study Diagnosis Sequence Patient Cohort, EuroPCR e-Course, Paris 2020. Available online: https://media.pcronline.com/diapos/PCReCourse2020/226-20200627_0900_Hotline_and_Innovation_Channel_Gragnano_Felice_0000_ (accessed on 25 June 2020).
- Lima, J.A.; Judd, R.M.; Bazille, A.; Schulman, S.P.; Atalar, E.; Zerhouni, E.A. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation 1995, 92, 1117–1125. [Google Scholar] [CrossRef]
- Wu, K.C.; Kim, R.J.; Bluemke, D.A.; Rochitte, C.E.; Zerhouni, E.A.; Becker, L.C.; Lima, J.A. Quantification and time course of microvascular obstruction by contrast-enhanced echocardiography and magnetic resonance imaging following acute myocardial infarction and reperfusion. J. Am. Coll. Cardiol. 1998, 32, 1756–1764. [Google Scholar] [CrossRef]
- Ganame, J.; Messalli, G.; Dymarkowski, S.; Rademakers, F.E.; Desmet, W.; Van de Werf, F.; Bogaert, J. Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur. Heart J. 2009, 30, 1440–1449. [Google Scholar] [CrossRef]
- Zia, M.I.; Ghugre, N.R.; Connelly, K.A.; Strauss, B.H.; Sparkes, J.D.; Dick, A.J.; Wright, G.A. Characterizing myocardial edema and hemorrhage using quantitative T2 and T2* mapping at multiple time intervals post ST-segment elevation myocardial infarction. Circ. Cardiovasc. Imaging 2012, 5, 566–572. [Google Scholar] [CrossRef]
- García-Dorado, D.; Oliveras, J.; Gili, J.; Sanz, E.; Pérez-Villa, F.; Barrabés, J.; Carreras, M.J.; Solares, J.; Soler-Soler, J. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc. Res. 1993, 27, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Croisille, P.; Revel, D.; Saeed, M. Contrast agents and cardiac MR imaging of myocardial ischemia: From bench to bedside. Eur. Radiol. 2006, 16, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Tjandrawidjaja, M.C.; Fu, Y.; Westerhout, C.M.; White, H.D.; Todaro, T.G.; Van de Werf, F.; Mahaffey, K.W.; Wagner, G.S.; Granger, C.B.; Armstrong, P.W.; et al. Resolution of ST-segment depression: A new prognostic marker in ST-segment elevation myocardial infarction. Eur. Heart J. 2010, 31, 573–581. [Google Scholar] [CrossRef]
- Nijveldt, R.; van der Vleuten, P.A.; Hirsch, A.; Beek, A.M.; Tio, R.A.; Tijssen, J.G.P.; Piek, J.J.; van Rossum, A.C.; Zijlstra, F. Early electrocardiographic findings and MR imaging-verified microvascular injury and myocardial infarct size. JACC Cardiovasc. Imaging 2009, 2, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Lautamäki, R.; Schuleri, K.H.; Sasano, T.; Javadi, M.S.; Youssef, A.; Merrill, J.; Nekolla, S.G.; Abraham, M.R.; Lardo, A.C.; Bengel, F.M. Integration of infarct size, tissue perfusion, and metabolism by hybrid cardiac positron emission tomography/computed tomography: Evaluation in a porcine model of myocardial infarction. Circ. Cardiovasc. Imaging 2009, 2, 299–305. [Google Scholar] [CrossRef]
- Porter, T.R.; Li, S.; Oster, R.; Deligonul, U. The clinical implications of no reflow demonstrated with intravenous perfluorocarbon containing microbubbles following restoration of Thrombolysis in Myocardial Infarction (TIMI) 3 flow in patients with acute myocardial infarction. Am. J. Cardiol. 1998, 82, 1173–1177. [Google Scholar] [CrossRef]
- Kaul, S. Myocardial contrast echocardiography: A 25-year retrospective. Circulation 2008, 118, 291–308. [Google Scholar] [CrossRef]
- Galiuto, L.; Garramone, B.; Scarà, A.; Rebuzzi, A.G.; Crea, F.; La Torre, G.; Funaro, S.; Madonna, M.; Fedele, F.; Agati, L.; et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: Results of the multicenter AMICI study. J. Am. Coll. Cardiol. 2008, 51, 552–559. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef]
- Van’t Hof, A.W.J.; Ten Berg, J.; Heestermans, T.; Dill, T.; Funck, R.C.; van Werkum, W.; Dambrink, J.-H.E.; Suryapranata, H.; van Houwelingen, G.; Ottervanger, J.P.; et al. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIME 2): A multicentre, double-blind, randomised controlled trial. Lancet Lond. Engl. 2008, 372, 537–546. [Google Scholar] [CrossRef]
- Park, S.-D.; Baek, Y.-S.; Lee, M.-J.; Kwon, S.W.; Shin, S.-H.; Woo, S.-I.; Kim, D.-H.; Kwan, J.; Park, K.-S. Comprehensive assessment of microcirculation after primary percutaneous intervention in ST-segment elevation myocardial infarction: Insight from thermodilution-derived index of microcirculatory resistance and coronary flow reserve. Coron. Artery Dis. 2016, 27, 34–39. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; Carberry, J.; Yue May, V.T.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Hood, S.; et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation 2016, 134, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Faruk Akturk, I.; Arif Yalcin, A.; Biyik, I.; Sarikamis, C.; Turhan Caglar, N.; Erturk, M.; Celik, O.; Uzun, F.; Murat Caglar, I.; Oner, E. Effects of verapamil and adenosine in an adjunct to tirofiban on resolution and prognosis of noreflow phenomenon in patients with acute myocardial infarction. Minerva Cardioangiol. 2014, 62, 389–397. [Google Scholar] [PubMed]
- Ibanez, B.; Prat-González, S.; Speidl, W.S.; Vilahur, G.; Pinero, A.; Cimmino, G.; García, M.J.; Fuster, V.; Sanz, J.; Badimon, J.J. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: Analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 2007, 115, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Macaya, C.; Sánchez-Brunete, V.; Pizarro, G.; Fernández-Friera, L.; Mateos, A.; Fernández-Ortiz, A.; García-Ruiz, J.M.; García-Álvarez, A.; Iñiguez, A.; et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013, 128, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Montone, R.A.; Ibanez, B.; Thiele, H.; Crea, F.; Heusch, G.; Bulluck, H.; Hausenloy, D.J.; Berry, C.; Stiermaier, T.; et al. Optimized Treatment of ST-Elevation Myocardial Infarction. Circ. Res. 2019, 125, 245–258. [Google Scholar] [CrossRef]
- Roolvink, V.; Ibáñez, B.; Ottervanger, J.P.; Pizarro, G.; van Royen, N.; Mateos, A.; Dambrink, J.-H.E.; Escalera, N.; Lipsic, E.; Albarran, A.; et al. Early Intravenous Beta-Blockers in Patients With ST-Segment Elevation Myocardial Infarction Before Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2016, 67, 2705–2715. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Z.; Gu, Y.; Peng, D. Short-Term Effects of Verapamil and Diltiazem in the Treatment of No Reflow Phenomenon: A Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2015, 2015, 382086. [Google Scholar] [CrossRef]
- Michaels, A.D.; Appleby, M.; Otten, M.H.; Dauterman, K.; Ports, T.A.; Chou, T.M.; Gibson, C.M. Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: Results of the randomized, controlled vasodilator prevention on no-reflow (VAPOR) trial. J. Invasive Cardiol. 2002, 14, 299–302. [Google Scholar]
- Huang, R.I.; Patel, P.; Walinsky, P.; Fischman, D.L.; Ogilby, J.D.; Awar, M.; Frankil, C.; Savage, M.P. Efficacy of intracoronary nicardipine in the treatment of no-reflow during percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2006, 68, 671–676. [Google Scholar] [CrossRef]
- Fischell, T.A.; Haller, S.; Pulukurthy, S.; Virk, I.S. Nicardipine and adenosine “flush cocktail” to prevent no-reflow during rotational atherectomy. Cardiovasc. Revascularization Med. Mol. Interv. 2008, 9, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Grygier, M.; Araszkiewicz, A.; Lesiak, M.; Grajek, S. Role of adenosine as an adjunct therapy in the prevention and treatment of no-reflow phenomenon in acute myocardial infarction with ST segment elevation: Review of the current data. Kardiol. Pol. 2013, 71, 115–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Lo Schiavo, P.; et al. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: The REOPEN-AMI study (Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial Infarction). JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Puma, J.A.; Barbagelata, N.A.; DiCarli, M.F.; Leesar, M.A.; Browne, K.F.; Eisenberg, P.R.; Bolli, R.; Casas, A.C.; Molina-Viamonte, V.; et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: Results of a multicenter, randomized, placebo-controlled trial: The Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J. Am. Coll. Cardiol. 1999, 34, 1711–1720. [Google Scholar] [CrossRef]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W.; AMISTAD-II Investigators. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.A.; Khan, J.N.; Mahmoud, I.Z.; Greenwood, J.P.; Blackman, D.J.; Kunadian, V.; Been, M.; Abrams, K.R.; Wilcox, R.; Adgey, A.J.; et al. The REFLO-STEMI (REperfusion Facilitated by Local Adjunctive Therapy in ST-Elevation Myocardial Infarction) Trial: A Randomised Controlled Trial Comparing Intracoronary Administration of Adenosine or Sodium Nitroprusside with Control for Attenuation of Microvascular Obstruction during Primary Percutaneous Coronary Intervention; Efficacy and Mechanism Evaluation; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar]
- Wang, H.-J.; Lo, P.-H.; Lin, J.-J.; Lee, H.; Hung, J.-S. Treatment of slow/no-reflow phenomenon with intracoronary nitroprusside injection in primary coronary intervention for acute myocardial infarction. Catheter. Cardiovasc. Interv. 2004, 63, 171–176. [Google Scholar] [CrossRef]
- Parham, W.A.; Bouhasin, A.; Ciaramita, J.P.; Khoukaz, S.; Herrmann, S.C.; Kern, M.J. Coronary hyperemic dose responses of intracoronary sodium nitroprusside. Circulation 2004, 109, 1236–1243. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Fu, X.-H.; Ma, X.-X.; Wang, D.-Y.; Dong, Q.-L.; Wang, Y.-B.; Li, W.; Xing, K.; Gu, X.-S.; Jiang, Y.-F. Intracoronary fixed dose of nitroprusside via thrombus aspiration catheter for the prevention of the no-reflow phenomenon following primary percutaneous coronary intervention in acute myocardial infarction. Exp. Ther. Med. 2013, 6, 479–484. [Google Scholar] [CrossRef]
- Amit, G.; Cafri, C.; Yaroslavtsev, S.; Fuchs, S.; Paltiel, O.; Abu-Ful, A.; Weinstein, J.M.; Wolak, A.; Ilia, R.; Zahger, D. Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. Am. Heart J. 2006, 152, 887.e9–887.e14. [Google Scholar] [CrossRef]
- Skelding, K.A.; Goldstein, J.A.; Mehta, L.; Pica, M.C.; O’Neill, W.W. Resolution of refractory no-reflow with intracoronary epinephrine. Catheter. Cardiovasc. Interv. 2002, 57, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Colak, A.; Baysal, E.; Durukan, M.; Sen, T.; Guray, U. Intracoronary epinephrine in the treatment of refractory no-reflow after primary percutaneous coronary intervention: A retrospective study. BMC Cardiovasc. Disord. 2015, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Muçaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: The RESTORE observational study. Catheter. Cardiovasc. Interv. 2021, 97, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients With Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, e011408. [Google Scholar] [CrossRef]
- Case, B.C.; Satler, L.F. The challenges of coronary no-reflow phenomenon. Catheter. Cardiovasc. Interv. 2021, 97, 612–613. [Google Scholar] [CrossRef]
- Iwakura, K.; Ito, H.; Okamura, A.; Koyama, Y.; Date, M.; Higuchi, Y.; Inoue, K.; Kimura, R.; Nagai, H.; Imai, M.; et al. Nicorandil treatment in patients with acute myocardial infarction: A meta-analysis. Circ. J. 2009, 73, 925–931. [Google Scholar] [CrossRef]
- Steg, P.G.; James, S.; Harrington, R.A.; Ardissino, D.; Becker, R.C.; Cannon, C.P.; Emanuelsson, H.; Finkelstein, A.; Husted, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation 2010, 122, 2131–2141. [Google Scholar] [CrossRef]
- Montalescot, G.; van ’t Hof, A.W.; Lapostolle, F.; Silvain, J.; Lassen, J.F.; Bolognese, L.; Cantor, W.J.; Cequier, Á.; Chettibi, M.; Goodman, S.G.; et al. Prehospital Ticagrelor in ST-Segment Elevation Myocardial Infarction. N. Engl. J. Med. 2014, 371, 1016–1027. [Google Scholar] [CrossRef]
- van Leeuwen, M.A.H.; van der Hoeven, N.W.; Janssens, G.N.; Everaars, H.; Nap, A.; Lemkes, J.S.; de Waard, G.A.; van de Ven, P.M.; van Rossum, A.C.; ten Cate, T.J.F.; et al. Evaluation of Microvascular Injury in Revascularized Patients With ST-Segment–Elevation Myocardial Infarction Treated With Ticagrelor Versus Prasugrel: The REDUCE-MVI Trial. Circulation 2019, 139, 636–646. [Google Scholar] [CrossRef]
- Park, K.; Cho, Y.-R.; Park, J.-S.; Park, T.-H.; Kim, M.-H.; Kim, Y.-D. Comparison of the Effects of Ticagrelor and Clopidogrel on Microvascular Dysfunction in Patients With Acute Coronary Syndrome Using Invasive Physiologic Indices. Circ. Cardiovasc. Interv. 2019, 12, e008105. [Google Scholar] [CrossRef]
- Dai, W.; Ye, Z.; Li, L.; Su, Q. Effect of preoperative loading dose ticagrelor and clopidogrel on no-reflow phenomenon during intervention in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: A systematic review and meta-analysis. Drug Des. Devel. Ther. 2018, 12, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Chan, M.H.H.; Bryant, J.A.; Chai, P.; Chawla, A.; Chua, T.S.; Chung, Y.-C.; Fei, G.; Ho, H.H.; Ho, A.F.W.; et al. Platelet inhibition to target reperfusion injury trial: Rationale and study design. Clin. Cardiol. 2019, 42, 5–12. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Navarese, E.; Marino, P. Risk profile and benefits from Gp IIb-IIIa inhibitors among patients with ST-segment elevation myocardial infarction treated with primary angioplasty: A meta-regression analysis of randomized trials. Eur. Heart J. 2009, 30, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.L.; Kampinga, M.A.; Wieringa, W.G.; Fokkema, M.L.; Nijsten, M.W.; Hillege, H.L.; van den Heuvel, A.F.M.; Tan, E.-S.; Pundziute, G.; van der Werf, R.; et al. Intracoronary Versus Intravenous Administration of Abciximab in Patients With ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention With Thrombus Aspiration: The Comparison of Intracoronary Versus Intravenous Abciximab Administration During Emergency Reperfusion of ST-Segment Elevation Myocardial Infarction (CICERO) Trial. Circulation 2010, 122, 2709–2717. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Witzenbichler, B.; Godlewski, J.; Parise, H.; Dambrink, J.-H.E.; Ochala, A.; Carlton, T.W.; Cristea, E.; Wolff, S.D.; et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA 2012, 307, 1817–1826. [Google Scholar] [CrossRef]
- Thiele, H.; Wöhrle, J.; Neuhaus, P.; Brosteanu, O.; Sick, P.; Prondzinsky, R.; Birkemeyer, R.; Wiemer, M.; Kerber, S.; Schuehlen, H.; et al. Intracoronary compared with intravenous bolus abciximab application during primary percutaneous coronary intervention: Design and rationale of the Abciximab Intracoronary versus intravenously Drug Application in ST-Elevation Myocardial Infarction (AIDA STEMI) trial. Am. Heart J. 2010, 159, 547–554. [Google Scholar] [CrossRef]
- Potdar, A.; Sharma, S. The “MAP strategy” (Maximum aspiration of atherothrombus and adjunctive glycoprotein IIb/IIIa inhibitor utilization combined with prolonged inflation of balloon/stent) for preventing no-reflow in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention: A retrospective analysis of seventy-one cases. Indian Heart J. 2015, 67 (Suppl. 3), S43–S46. [Google Scholar] [CrossRef]
- Chen, L.; Shi, L.; Tian, W.; Zhao, S. Intracoronary Thrombolysis in Patients With ST-Segment Elevation Myocardial Infarction: A Meta-Analysis of Randomized Controlled Trials. Angiology 2021, 72, 679–686. [Google Scholar] [CrossRef]
- McCartney, P.J.; Eteiba, H.; Maznyczka, A.M.; McEntegart, M.; Greenwood, J.P.; Muir, D.F.; Chowdhary, S.; Gershlick, A.H.; Appleby, C.; Cotton, J.M.; et al. Effect of Low-Dose Intracoronary Alteplase During Primary Percutaneous Coronary Intervention on Microvascular Obstruction in Patients With Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA 2019, 321, 56–68. [Google Scholar] [CrossRef]
- Alyamani, M.; Campbell, S.; Navarese, E.; Welsh, R.C.; Bainey, K.R. Safety and Efficacy of Intracoronary Thrombolysis as Adjunctive Therapy to Primary PCI in STEMI: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2021, 37, 339–346. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, J.; Choi, D.; Lee, C.J.; Lee, S.H.; Ko, Y.-G.; Hong, M.-K.; Kim, B.-K.; Oh, S.J.; Jeon, D.W.; et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: The STATIN STEMI trial. JACC Cardiovasc. Interv. 2010, 3, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Berwanger, O.; Santucci, E.V.; de Barros E Silva, P.G.M.; de Andrade Jesuíno, I.; Damiani, L.P.; Barbosa, L.M.; Santos, R.H.N.; Laranjeira, L.N.; de Mattos Egydio, F.; Borges de Oliveira, J.A.; et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. JAMA 2018, 319, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef]
- Ma, M.; Wang, L.; Diao, K.-Y.; Liang, S.-C.; Zhu, Y.; Wang, H.; Wang, M.; Zhang, L.; Yang, Z.-G.; He, Y. A randomized controlled clinical trial of prolonged balloon inflation during stent deployment strategy in primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A pilot study. BMC Cardiovasc. Disord. 2022, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Rezkalla, S.H.; Stankowski, R.V.; Hanna, J.; Kloner, R.A. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 215–223. [Google Scholar] [CrossRef]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef]
- Egred, M.; Bagnall, A.; Spyridopoulos, I.; Purcell, I.F.; Das, R.; Palmer, N.; Grech, E.D.; Jain, A.; Stone, G.W.; Nijveldt, R.; et al. Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. Int. J. Cardiol. Heart Vasc. 2020, 28, 100526. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction–Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, e352–e359. [Google Scholar] [CrossRef]
- El Farissi, M.; Mast, T.P.; van de Kar, M.R.D.; Dillen, D.M.M.; Demandt, J.P.A.; Vervaat, F.E.; Eerdekens, R.; Dello, S.A.G.; Keulards, D.C.; Zelis, J.M.; et al. Hypothermia for Cardioprotection in Patients with St-Elevation Myocardial Infarction: Do Not Give It the Cold Shoulder Yet! J. Clin. Med. 2022, 11, 1082. [Google Scholar] [CrossRef]
- Erlinge, D.; Götberg, M.; Lang, I.; Holzer, M.; Noc, M.; Clemmensen, P.; Jensen, U.; Metzler, B.; James, S.; Bötker, H.E.; et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: A randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Nichol, G.; Strickland, W.; Shavelle, D.; Maehara, A.; Ben-Yehuda, O.; Genereux, P.; Dressler, O.; Parvataneni, R.; Nichols, M.; McPherson, J.; et al. Prospective, multicenter, randomized, controlled pilot trial of peritoneal hypothermia in patients with ST-segment- elevation myocardial infarction. Circ. Cardiovasc. Interv. 2015, 8, e001965. [Google Scholar] [CrossRef] [PubMed]
- Keeble, T.R.; Karamasis, G.V.; Noc, M.; Sredniawa, B.; Aradi, D.; Neskovic, A.N.; Arheden, H.; Erlinge, D.; Holzer, M. Effect of Intravascular Cooling on Microvascular Obstruction (MVO) in Conscious Patients with ST-Elevation Myocardial Infarction Undergoing Primary PCI: Results from the COOL AMI EU Pilot Study. Cardiovasc. Revascularization Med. 2019, 20, 799–804. [Google Scholar] [CrossRef] [PubMed]
- El Farissi, M.; Keulards, D.C.J.; van ’t Veer, M.; Zelis, J.M.; Berry, C.; De Bruyne, B.; Engstrøm, T.; Fröbert, O.; Piroth, Z.; Oldroyd, K.G.; et al. Selective intracoronary hypothermia in patients with ST-elevation myocardial infarction. Rationale and design of the EURO-ICE trial. EuroIntervention 2021, 16, 1444–1446. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef]
- Stone, G.W.; Martin, J.L.; de Boer, M.-J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef]
- David, S.W.; Khan, Z.A.; Patel, N.C.; Metzger, D.C.; Wood, F.O.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; et al. Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter. Cardiovasc. Interv. 2019, 93, 882–890. [Google Scholar] [CrossRef]
- O’Farrell, F.M.; Attwell, D. A role for pericytes in coronary no-reflow. Nat. Rev. Cardiol. 2014, 11, 427–432. [Google Scholar] [CrossRef]
- Elbaz-Greener, G.; Sud, M.; Tzuman, O.; Leitman, M.; Vered, Z.; Ben-Dov, N.; Oron, U.; Blatt, A. Adjunctive laser-stimulated stem-cells therapy to primary reperfusion in acute myocardial infarction in humans: Safety and feasibility study. J. Intervent. Cardiol. 2018, 31, 711–716. [Google Scholar] [CrossRef]
| Medication | Dosage | Side Effects |
|---|---|---|
| Adenosine | Intravenous: 70 μg/kg/min infusion Intracoronary: 100–200 μg bolus | Bradycardia, hypotension, chest pain, dyspnea |
| Sodium Nitroprusside | Intracoronary: 60–100 μg bolus | Bradycardia and hypotension |
| Verapamil | Intracoronary: 100–500 μg bolus (max 1 mg) | Bradycardia, transient heart block |
| Diltiazem | Intracoronary: 400 μg bolus (max 5 mg) | Bradycardia, hypotension |
| Nicardipine | Intracoronary: 200 μg (max 1 mg) | Bradycardia, hypotension |
| Epinephrine | Intracoronary: 80–100 μg bolus | Malignant arrhythmias |
| Nicorandil | 500 μg (max: 5 mg) | Malignant arrhythmias |
| Streptokinase | 250 kU over 3 min | Bleeding |
| Tenecteplase | 5 mg (max: 25 mg) | Bleeding |
| Tissue plasminogen activator (tPA) | 0.025–0.5 mg/kg/h | Bleeding |
| Abciximab | 0.25 mg/kg bolus, then 0.125 μg/kg/min (max 10 μg/min) infusion for 12 h | Bleeding |
| Eptifibatide | 180 μg/kg bolus, then further 180 μg/kg bolus 10 min later, then 2 μg/kg/min infusion for up to 18 h. If CrCl < 50 mL/min, reduce infusion by 50% | Bleeding |
| Tirofiban | 25 μg/kg over 3 min, then 0.15 μg/kg/min infusion for up to 18 h If CrCl < 30 mL/min, reduce infusion by 50% | Bleeding |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” Phenomenon: A Contemporary Review. J. Clin. Med. 2022, 11, 2233. https://doi.org/10.3390/jcm11082233
Annibali G, Scrocca I, Aranzulla TC, Meliga E, Maiellaro F, Musumeci G. “No-Reflow” Phenomenon: A Contemporary Review. Journal of Clinical Medicine. 2022; 11(8):2233. https://doi.org/10.3390/jcm11082233
Chicago/Turabian StyleAnnibali, Gianmarco, Innocenzo Scrocca, Tiziana Claudia Aranzulla, Emanuele Meliga, Francesco Maiellaro, and Giuseppe Musumeci. 2022. "“No-Reflow” Phenomenon: A Contemporary Review" Journal of Clinical Medicine 11, no. 8: 2233. https://doi.org/10.3390/jcm11082233
APA StyleAnnibali, G., Scrocca, I., Aranzulla, T. C., Meliga, E., Maiellaro, F., & Musumeci, G. (2022). “No-Reflow” Phenomenon: A Contemporary Review. Journal of Clinical Medicine, 11(8), 2233. https://doi.org/10.3390/jcm11082233





