Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
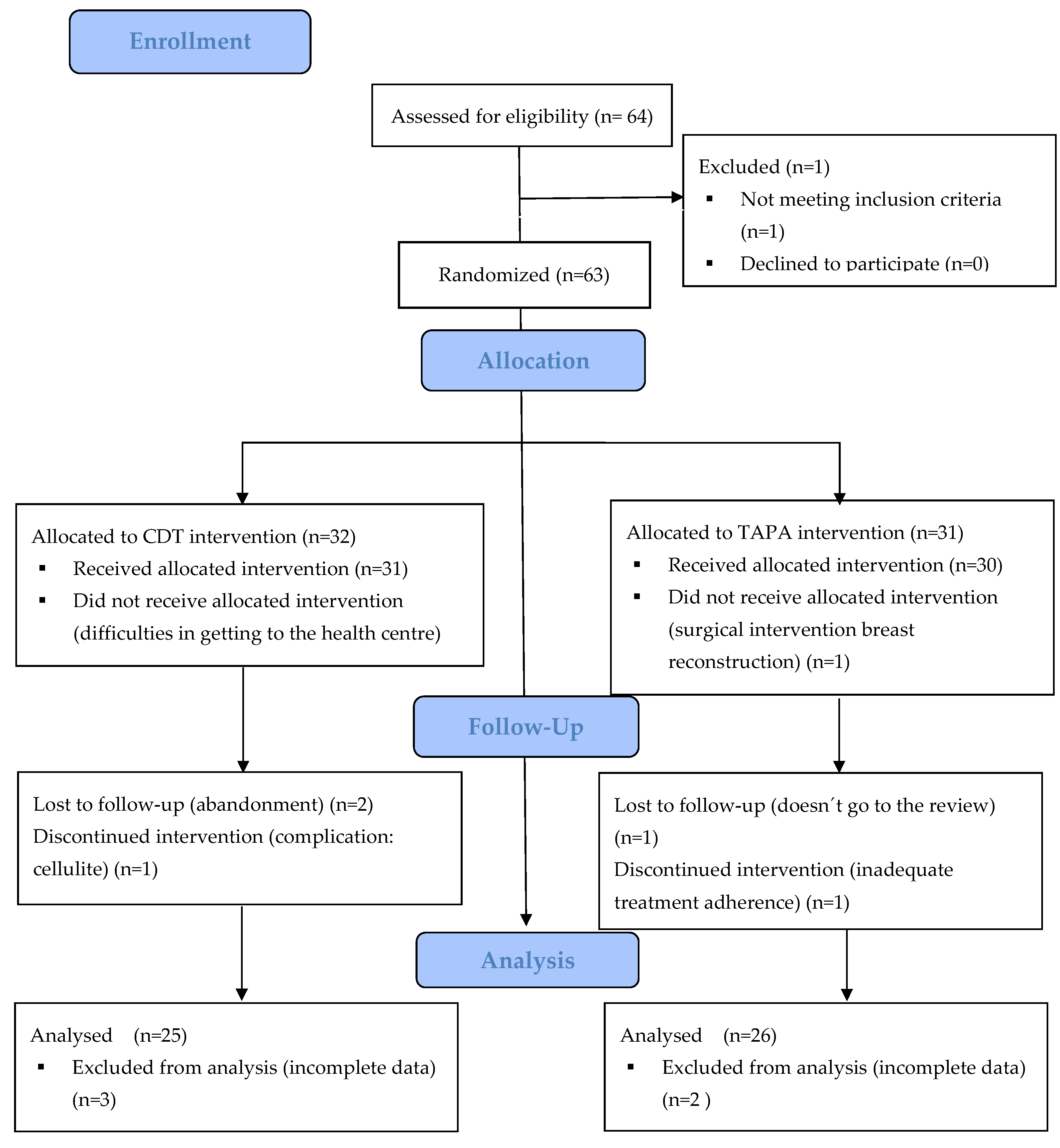
2.3. Procedure and Randomization
2.4. Main Outcomes
2.5. Statistical Analysis
2.6. Intervention
3. Results
3.1. Main Characteristics of the Participants
3.2. Volume, Joint Balance and Upper Limb Function: Differences between Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- 2022 ICD-10-CM Diagnosis Code I97.2: Postmastectomy Lymphedema Syndrome. Available online: https://www.icd10data.com/ICD10CM/Codes/I00-I99/I95-I99/I97-/I97.2 (accessed on 23 February 2022).
- Congress, I. The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the international society of lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Cristina Puigdellivol Serafí; Belén Alonso Álvarez. Guía de Práctica Clínica-Orientación Diagnóstica y Terapéutica del Linfedema, 2nd ed.; Sociedad Española de Rehabilitación y Medicina Física: Madrid, Spain, 2017; ISBN 9788469743294. [Google Scholar]
- Breast Cancer Awareness Month 2021—IARC. Available online: https://iarc.who.int/featured-news/breast-cancer-awareness-month-2021/ (accessed on 12 December 2021).
- De Britto, L.R.P.B.; de Souza, E.C.; Ribeiro, L.T.; Schwingel, P.A.; de Melo, A.R.A.; do Nascimento, P.L.; Portela, G.J.S. The impact of sensory alterations on upper limb function after a mastectomy. Mastology 2017, 27, 287–292. [Google Scholar] [CrossRef]
- Braveman, B.; Newman, R. Cancer and Occupational Therapy: Enabling Performance and Participation Across the Lifespan. Available online: https://eds-s-ebscohost-com.bvsspa.idm.oclc.org/eds/ebookviewer/ebook/ZTAwMHh3d19fMjUwOTAzNV9fQU41?sid=ad4e45d6-b6dc-470f-bc26-2b570c5523b7@redis&vid=1&format=EB (accessed on 19 December 2021).
- Viehoff, P.B.; Gielink, P.D.C.; Damstra, R.J.; Heerkens, Y.F.; Van Ravensberg, D.C.; Neumann, M.H.A. Functioning in lymphedema from the patients’ perspective using the International Classification of Functioning, Disability and health (ICF) as a reference. Acta Oncol. 2015, 54, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, L.H.; Narkthong, N.; Hulett, J.M. Psychosocial Issues Associated with Breast Cancer-Related Lymphedema: A Literature Review. Curr. Breast Cancer Rep. 2020, 12, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Musa, A.N. Methods to improve rehabilitation of patients following breast cancer surgery: A review of systematic reviews. Breast Cancer Targets Ther. 2015, 7, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Olsson Möller, U.; Beck, I.; Rydén, L.; Malmström, M. A comprehensive approach to rehabilitation interventions following breast cancer treatment—A systematic review of systematic reviews. BMC Cancer 2019, 19, 472. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, T.F.; Allison, G.M.; Iafrati, M.D. A systematic review of guidelines for lymphedema and the need for contemporary intersocietal guidelines for the management of lymphedema. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 676–684. [Google Scholar] [CrossRef]
- Fish, M.L.; Grover, R.; Schwarz, G.S. Quality-of-life outcomes in surgical vs nonsurgical treatment of breast cancer-related lymphedema: A systematic review. JAMA Surg. 2020, 155, 513–519. [Google Scholar] [CrossRef]
- Longhurst, E.; Dylke, E.S.; Kilbreath, S.L. Use of compression garments by women with lymphoedema secondary to breast cancer treatment. Support. Care Cancer 2018, 26, 2625–2632. [Google Scholar] [CrossRef]
- Rabe, E.; Partsch, H.; Morrison, N.; Meissner, M.H.; Mosti, G.; Lattimer, C.R.; Carpentier, P.H.; Gaillard, S.; Jünger, M.; Urbanek, T.; et al. Risks and contraindications of medical compression treatment—A critical reappraisal. An international consensus statement. Phlebology 2020, 35, 447–460. [Google Scholar] [CrossRef] [Green Version]
- Woods, M. Using compression garments in the management of lymphoedema. Br. J. Nurs. 2019, 28, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Al Onazi, M.; Dolgoy, N.; Parkinson, J.; McNeely, M.L. Exploring adherence to daytime compression in women with breast cancer related lymphedema: A multi-methods study. Plast. Aesthetic Res. 2020, 2020, 23. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Palma, S.; Ramazanova, D.; Kölbl, H.; Dorner, T.E.; Keilani, M.; Crevenna, R. Resistance exercise and breast cancer–related lymphedema—A systematic review update and meta-analysis. Support. Care Cancer 2020, 28, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Keesing, S.; Wallis, A.; Russell, B.; Smith, A.; Grant, R. Occupational therapy intervention for cancer patients following hospital discharge: How and when should we intervene? A systematic review. Aust. Occup. Ther. J. 2021, 68, 546–562. [Google Scholar] [CrossRef]
- Hunter, E.G.; Gibson, R.W.; Arbesman, M.; D’Amico, M. Systematic Review of Occupational Therapy and Adult Cancer Rehabilitation: Part 2. Impact of Multidisciplinary Rehabilitation and Psychosocial, Sexuality, and Return-to-Work Interventions. Am. J. Occup. Ther. 2017, 71, 7102100040p1–7102100040p8. [Google Scholar] [CrossRef]
- Scheiman, N.R. Comprehensive Occupational Therapy in a Breast Cancer Program; Nova Southeastern University: Davie, FL, USA, 2018; p. 1. [Google Scholar]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef]
- Maldonado, A.A.; Ramos, E.; García-Alonso, P.; Jover, J.J.; Holguín, P.; Fernández-Cañamaque, J.L.; Cristóbal, L. Multidisciplinary approach in the lymphedema patient: From rehabilitation to microsurgery. Rehabilitacion, 2021; in press. [Google Scholar] [CrossRef]
- Pilot Study with Myolymphokinetic Activities in the Treatment of Lymphedema after Breast Cancer. Available online: https://www.researchgate.net/publication/283599950_Pilot_study_with_Myolymphokinetic_activities_in_the_treatment_of_lymphedema_after_breast_cancer (accessed on 24 March 2021).
- Activities and Myolymphokinetic Exercises: Therapeutic Approach to Lymphedema—Kindle Edition by de Godoy, José Maria Pereira, Hewitt, David. Professional & Technical Kindle eBooks@Amazon.com. Available online: https://www.amazon.com/Activities-Myolymphokinetic-Exercises-therapeutic-lymphedema-ebook/dp/B00H9K3MG8 (accessed on 25 July 2021).
- Drenaje Linfático Global: Concepto Godoy & Godoy. Available online: https://www.researchgate.net/publication/305607823_DRENAJE_LINFATICO_GLOBAL_Concepto_Godoy_Godoy (accessed on 25 July 2021).
- Luctkar-Flude, M.; Aiken, A.; McColl, M.A.; Tranmer, J. What do primary care providers think about implementing breast cancer survivorship care? Curr. Oncol. 2018, 25, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.N. Lymphedema Self-Management by Breast Cancer Survivors: A Focus on Psychosocial and Occupational Performance Factors. Ph.D. Thesis, St. Catherine University, Saint Paul, MN, USA, 2020. [Google Scholar]
- Douglass, J.; Graves, P.; Gordon, S. Self-Care for Management of Secondary Lymphedema: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004740. [Google Scholar] [CrossRef]
- Pollán, M.; Casla-Barrio, S.; Alfaro, J.; Esteban, C.; Segui-Palmer, M.A.; Lucia, A.; Martín, M. Exercise and cancer: A position statement from the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2020, 22, 1710–1729. [Google Scholar] [CrossRef] [Green Version]
- Yildiz Kabak, V.; Gursen, C.; Aytar, A.; Akbayrak, T.; Duger, T. Physical activity level, exercise behavior, barriers, and preferences of patients with breast cancer–related lymphedema. Support. Care Cancer 2020, 29, 3593–3602. [Google Scholar] [CrossRef] [PubMed]
- Smoot, B.; Boyd, B.S.; Byl, N.; Dodd, M. Mechanosensitivity in the upper extremity following breast cancer treatment. J. Hand Ther. 2014, 27, 4–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Rosa Díaz, I.; Torres Lacomba, M.; Cerezo Téllez, E.; Díaz del Campo Gómez-Rico, C.; Gutiérrez Ortega, C. Accessory Joint and Neural Mobilizations for Shoulder Range of Motion Restriction After Breast Cancer Surgery: A Pilot Randomized Clinical Trial. J. Chiropr. Med. 2017, 16, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen Hammond, E.; Pitz, M.; Steinfeld, K.; Lambert, P.; Shay, B. An Exploratory Randomized Trial of Physical Therapy for the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Neurorehabilit. Neural Repair 2020, 34, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Lee, M.S.S. The Effects of Proprioceptive Neuromuscular Facilitation (PNF) Using Elastic Bands on Edema, Range of Motion, and Pain in Post-Mastectomy Patients with Upper Limb. Ph.D. Thesis, Korea Proprioceptive Neuromuscular Facilitation Association, Busan, Korea, 2020; pp. 1–10. [Google Scholar]
- Ha, K.J.; Lee, S.Y.; Lee, H.; Choi, S.J. Synergistic effects of proprioceptive neuromuscular facilitation and manual lymphatic drainage in patients with mastectomy-related lymphedema. Front. Physiol. 2017, 8, 959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, W.T.; Chung, S.H.; Chung, M.S.; Lee, K.H.; Kim, T. Effect of proprioceptive neuromuscular facilitation D2 flexion and breathing exercises on lymphedema without a short stretch compression bandage. J. Phys. Ther. Sci. 2015, 27, 3341–3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borman, P.; Koyuncu, E.G.; Yaman, A.; Calp, E.; Koç, F.; Sargut, R.; Karahan, S. The Comparative Efficacy of Conventional Short-Stretch Multilayer Bandages and Velcro Adjustable Compression Wraps in Active Treatment Phase of Patients with Lower Limb Lymphedema. Lymphat. Res. Biol. 2021, 19, 286–294. [Google Scholar] [CrossRef]
- Yaman, A.; Borman, P.; Inanli, A.; Kul, F.; Karahan, S. The efficacy of different bandaging methods in patients with breast cancer-related lymphedema: A prospective, randomized study. Turk. J. Phys. Med. Rehabil. 2021, 67, 155–166. [Google Scholar] [CrossRef]
- Muñoz-Alcaraz, M.N.; Pérula-de-Torres, L.Á.; Serrano-Merino, J.; Jiménez-Vílchez, A.J.; Olmo-Carmona, M.V.; Muñoz-García, M.T.; Bartolomé-Moreno, C.; Oliván-Blázquez, B.; Magallón-Botaya, R. Efficacy and efficiency of a new therapeutic approach based on activity-oriented proprioceptive antiedema therapy (TAPA) for edema reduction and improved occupational performance in the rehabilitation of breast cancer-related arm lymphedema in women: A controlled, randomized clinical trial. BMC Cancer 2020, 20, 1074. [Google Scholar] [CrossRef]
- Johnson, K.C.; Kennedy, A.G.; Henry, S.M. Clinical Measurements of Lymphedema. Lymphat. Res. Biol. 2014, 12, 216–221. [Google Scholar] [CrossRef]
- Kizil, R.; Dilek, B.; Şahin, E.; Engin, O.; Soylu, A.C.; Akalin, E.; Alper, S. Is Continuous Passive Motion Effective in Patients with Lymphedema? A Randomized Controlled Trial. Lymphat. Res. Biol. 2018, 16, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.L.; Hernández, M.A.; Avendaño, C.; Rodríguez, F.; Martínez, H. Manual lymphatic drainage therapy in patients with breast cancer related lymphoedema. BMC Cancer 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleiss, J.L. The Design and Analysis of Clinical Experiments. Des. Anal. Clin. Exp. 1999. [Google Scholar] [CrossRef]
- Romero Cullerés, G.; Almendáriz Juárez, A. Linfedema después de la cirugía por cáncer de mama. Estudio de la incidencia y factores de riesgo en 113 pacientes. Rehabilitación 2004, 38, 72–77. [Google Scholar] [CrossRef]
- Cuadrado, A.; Vázquez, A. Rehabilitación del linfedema. In Actualización y Protocolo; Medicina, 72; Servicio de Medicina Física y Rehabilitación del Servicio Gallego de Salud: Galicia, Spain, 2008; Volume 72, Available online: http://www.sld.cu/galerias/pdf/sitios/rehabilitacion/tratamiento_del_linfedema.pdf (accessed on 25 July 2021).
- López Martín, M.; Valencia Álvarez, F.J.; González González, R.; Rodríguez Salvanés, F.J.; Crespo Cobo, P.; Hernández García, M.A. Validación de herramienta informática para el cálculo de linfedema en pacientes con afectación unilateral de extremidad superior. Rehabilitación 2011, 45, 127–133. [Google Scholar] [CrossRef]
- Arias-Cuadrado, A.; Lvarez-Vzquez, M.J.; Martn-Mourelle, R.; Villarino-Daz Jimnez, C. Clnica, clasificacin y estadiaje del linfedema. Rehabilitacion 2010, 44, 29–34. [Google Scholar] [CrossRef]
- Forner Cordero, I.; Maldonado Garrido, D.; Muñoz Langa, J. Necesidad de información para la prevención del linfedema posmastectomía. Rehabilitación 2003, 37, 141–144. [Google Scholar] [CrossRef]
- Hervás, M.T.; Navarro Collado, M.J.; Peiró, S.; Rodrigo Pérez, J.L.; López Matéu, P.; Martínez Tello, I. Versión Española del cuestionario DASH. Adaptación transcultural, fiabilidad, validez y sensibilidad a los cambios. Med. Clin. 2006, 127, 441–447. [Google Scholar] [CrossRef]
- Beaton, D.E.; Wright, J.G.; Katz, J.N.; Amadio, P.; Bombardier, C.; Cole, D.; Davis, A.; Hudak, P.; Marx, R.; Hawker, G.; et al. Development of the QuickDASH: COmparison of three item-reduction approaches. J. Bone Jt. Surg.-Ser. A 2005, 87, 1038–1046. [Google Scholar] [CrossRef]
- Ferguson, C.J. Una cartilla de tamaño de efecto: Una guía para médicos e investigadores. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Alba Conejo, E.; Alvarez, M.; Bayo, E.; Del Río, S.; Doctor, M.; Dueñas, B.; Fernandez, R. Proceso Asistencial Integrado Cáncer de Mama. 2011. ISBN 8474681375. Available online: https://www.juntadeandalucia.es/export/drupaljda/salud_5af1956e6a064_mama_deteccion_3e_nuevo.pdf (accessed on 19 December 2021).
- Ezzo, J.; Manheimer, E.; Mcneely, M.L.; Howell, D.M.; Weiss, R.; Johansson, K.I.; Bao, T.; Bily, L.; Tuppo, C.M.; Williams, A.F.; et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst. Rev. 2015, 5, CD003475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangon, F.B.; da Silva, J.; Dibai-Filho, A.V.; Guirro, R.R.d.J.; Guirro, E.C.d.O. Effects of Complex Physical Therapy and Multimodal Approaches on Lymphedema Secondary to Breast Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Arch. Phys. Med. Rehabil. 2022, 103, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Marchica, P.; Darpa, S.; Magno, S.; Rossi, C.; Forcina, L.; Capizzi, V.; Oieni, S.; Amato, C.; Piazza, D.; Gebbia, V. Integrated treatment of breast cancer-related lymphedema: A descriptive review of the state of the art. Anticancer Res. 2021, 41, 3233–3246. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, R.; Messaggi-Sartor, M.; Ferrer, M.; Pont, A.; Escalada, F. Prospective study of shoulder strength, shoulder range of motion, and lymphedema in breast cancer patients from pre-surgery to 5 years after ALND or SLNB. Support. Care Cancer 2018, 26, 3277–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baran, E.; Yildiz, T.İ.; Gursen, C.; Üzelpasaci, E.; Özgül, S.; Düzgün, İ.; Akbayrak, T. The association of breast cancer-related lymphedema after unilateral mastectomy with shoulder girdle kinematics and upper extremity function. J. Biomech. 2021, 121, 110432. [Google Scholar] [CrossRef]
- Oosterwijk, A.M.; Nieuwenhuis, M.K.; van der Schans, C.P.; Mouton, L.J. Shoulder and elbow range of motion for the performance of activities of daily living: A systematic review. Physiother. Theory Pract. 2018, 34, 505–528. [Google Scholar] [CrossRef]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef]
| Variable | Type of Treatment Received | n | Mean | SD | SEM |
|---|---|---|---|---|---|
| Age | Experimental | 31 | 58.00 | 9.71 | 1.74 |
| Control | 32 | 63.66 | 9.96 | 1.76 | |
| Body mass index | Experimental | 30 | 28.76 | 4.73 | 0.86 |
| Control | 31 | 27.59 | 4.91 | 0.88 | |
| Number of nodes removed | Experimental | 29 | 14.00 | 9.73 | 1.80 |
| Control | 24 | 12.20 | 7.21 | 1.47 | |
| Pain, VAS scale 0–10 | Experimental | 31 | 3.77 | 2.82 | 0.50 |
| Control | 32 | 3.88 | 3.11 | 0.55 | |
| Heaviness, VAS scale 0–10 | Experimental | 31 | 4.87 | 2.79 | 0.50 |
| Control | 32 | 4.88 | 2.25 | 0.39 | |
| Tightness, VAS scale 0–10 | Experimental | 31 | 4.19 | 2.67 | 0.48 |
| Control | 32 | 4.38 | 2.72 | 0.48 | |
| Health Questionnaire | Experimental | 31 | 67.10 | 18.42 | 3.31 |
| Control | 30 | 64.83 | 16.47 | 3.00 |
| Variable | Source | Type III Sum of Square | df | MS | F | p-Value | η2 |
|---|---|---|---|---|---|---|---|
| Differential Vol% | Vol% pre-test | 194,568,957 | 1 | 194,568,957 | 1,809,568 | <0.001 | 0.974 |
| CG/EG | 82,518 | 1 | 82,518 | 0.767 | 0.385 | 0.016 | |
| Error | 5,161,072 | 48 | 107,522 | ||||
| Differential Vol mL | Vol mL pre-test | 8,698,016 | 1 | 8,698,016 | 0.478 | 0.493 | 0.010 |
| CG/EG | 10,305,528 | 1 | 10,305,528 | 0.567 | 0.455 | 0.012 | |
| Error | 872,983,058 | 48 | 18,187,147 | ||||
| Differential QDASH | QDASH pre-test | 3325 | 1 | 3.325 | 0.019 | 0.890 | 0.000 |
| CG/EG | 94,477 | 1 | 94.477 | 0.545 | 0.464 | 0.011 | |
| Error | 8,317,476 | 48 | 173.281 | ||||
| Differential JBER | JBER pre-test | 200,036 | 1 | 200,036 | 6289 | 0.016 | 0.114 |
| CG/EG | 135,014 | 1 | 135,014 | 4245 | 0.045 | 0.080 | |
| Error | 1,558,617 | 49 | 31,809 | ||||
| Differential JBF | JBF pre-test | 624,712 | 1 | 624,712 | 6723 | 0.013 | 0.121 |
| CG/EG | 128,549 | 1 | 128,549 | 1383 | 0.245 | 0.027 | |
| Error | 4,553,173 | 49 | 92,922 | ||||
| Differential JBABD | JBABD pre-test | 239,799 | 1 | 239,799 | 1171 | 0.284 | 0.023 |
| CG/EG | 477,384 | 1 | 477,384 | 2331 | 0.133 | 0.045 | |
| Error | 10,034,239 | 49 | 204,780 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Alcaraz, M.N.; Jiménez-Vílchez, A.J.; Santamaría-Peláez, M.; Pérula-de Torres, L.A.; Olmo-Carmona, M.V.; Muñoz-García, M.T.; Jorge-Gutiérrez, P.; Serrano-Merino, J.; Romero-Rodríguez, E.; Rodríguez-Elena, L.; et al. Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial. J. Clin. Med. 2022, 11, 2234. https://doi.org/10.3390/jcm11082234
Muñoz-Alcaraz MN, Jiménez-Vílchez AJ, Santamaría-Peláez M, Pérula-de Torres LA, Olmo-Carmona MV, Muñoz-García MT, Jorge-Gutiérrez P, Serrano-Merino J, Romero-Rodríguez E, Rodríguez-Elena L, et al. Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial. Journal of Clinical Medicine. 2022; 11(8):2234. https://doi.org/10.3390/jcm11082234
Chicago/Turabian StyleMuñoz-Alcaraz, María Nieves, Antonio José Jiménez-Vílchez, Mirian Santamaría-Peláez, Luis A. Pérula-de Torres, María Victoria Olmo-Carmona, María Teresa Muñoz-García, Presentación Jorge-Gutiérrez, Jesús Serrano-Merino, Esperanza Romero-Rodríguez, Lorena Rodríguez-Elena, and et al. 2022. "Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial" Journal of Clinical Medicine 11, no. 8: 2234. https://doi.org/10.3390/jcm11082234
APA StyleMuñoz-Alcaraz, M. N., Jiménez-Vílchez, A. J., Santamaría-Peláez, M., Pérula-de Torres, L. A., Olmo-Carmona, M. V., Muñoz-García, M. T., Jorge-Gutiérrez, P., Serrano-Merino, J., Romero-Rodríguez, E., Rodríguez-Elena, L., Refusta-Ainaga, R., Lahoz-Sánchez, M. P., Miró-Palacios, B., Medrano-Cid, M., Magallón-Botaya, R., Mínguez-Mínguez, L. A., González-Santos, J., & González-Bernal, J. J. (2022). Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial. Journal of Clinical Medicine, 11(8), 2234. https://doi.org/10.3390/jcm11082234










