Abstract
The diagnosis of alcohol use disorder (AUD) remains a difficult challenge, and some patients may not be adequately diagnosed. This study aims to identify an optimum combination of laboratory markers to detect alcohol consumption, using data science. An analytical observational study was conducted with 337 subjects (253 men and 83 women, with a mean age of 44 years (10.61 Standard Deviation (SD)). The first group included 204 participants being treated in the Addictive Behaviors Unit (ABU) from Albacete (Spain). They met the diagnostic criteria for AUD specified in the Diagnostic and Statistical Manual of mental disorders fifth edition (DSM-5). The second group included 133 blood donors (people with no risk of AUD), recruited by cross-section. All participants were also divided in two groups according to the WHO classification for risk of alcohol consumption in Spain, that is, males drinking more than 28 standard drink units (SDUs) or women drinking more than 17 SDUs. Medical history and laboratory markers were selected from our hospital’s database. A correlation between alterations in laboratory markers and the amount of alcohol consumed was established. We then created three predicted models (with logistic regression, classification tree, and Bayesian network) to detect risk of alcohol consumption by using laboratory markers as predictive features. For the execution of the selection of variables and the creation and validation of predictive models, two tools were used: the scikit-learn library for Python, and the Weka application. The logistic regression model provided a maximum AUD prediction accuracy of 85.07%. Secondly, the classification tree provided a lower accuracy of 79.4%, but easier interpretation. Finally, the Naive Bayes network had an accuracy of 87.46%. The combination of several common biochemical markers and the use of data science can enhance detection of AUD, helping to prevent future medical complications derived from AUD.
1. Introduction
Alcohol dependence is a frequent medical problem in a wide variety of clinical settings and requires attention to reduce medical complications, establish appropriate treatments, and minimize the use of healthcare resources [1]. Alcohol abuse has harmful effects that can cause or contribute to multiple diseases [2] and even increase the risk of cancer [3,4] and deaths [5].
Alcohol has also increased traffic accidents, trauma [6], absenteeism [7] at the workplace and school [8], job loss, violent behavior, and legal [9], family, economic, mental [10], and social problems [5]. Despite the significant benefits of alcohol abuse treatments [11], general practitioners only manage to diagnose a third of these patients [12]. Self-assessment questionnaires (for example, AUDIT (Alcohol Use Disorders Identification Test), CAGE (Cuttingdown, Annoyed, Guilt, Eyeopen), MAST (Michigan Alcohol Screening Test)) are typically used [13] to detect alcohol use disorder [14], but these can be misleading if patients are reluctant to reveal their patterns of alcohol use [15].
Laboratory markers can corroborate clinical suspicion of alcohol abuse and facilitate patient monitoring and compliance with the recommendations. For instance, alcohol biomarkers are physiological indicators of alcohol exposure and can help detect AUD [16]. These biomarkers are more useful when used together with other information such as medical history. Biomarkers can be classified as direct and indirect [17]. Direct markers include detection of alcohol or its metabolites such as ethyl glucuronide, derivatives of acetaldehyde, phosphatidyl ethanol, and ethyl esters of fatty acids. These biomarkers have high specificity but low sensitivity due to their short plasma half-life. However, ethylglucuronide can be detected in urine up to 5 days and can inform us of chronic consumption by measuring it in the hair [18]. Regarding acetaldehyde derivatives, studies have described that they can be used as alcohol use markers in women [19]. Furthermore, the quantification by anti-adduct autoantibodies has been shown to have clinical value, differentiating abstinent users from alcohol users [20,21].
Indirect markers such as aspartate-aminotransferase (GOT/AST), alanine-aminotransferase (GPT/ALT), gamma-glutamyltransferase (GGT), erythrocyte mean cell volume (MCV), uric acid, HDL-cholesterol, triglycerides, cholesteryl ester transfer protein (CETP), and total serum sialic acid (TSA) [22] help identify excessive alcohol use by detecting its toxic effects. They are inexpensive and widely available, but they have the disadvantage of low sensitivity and unspecificity [17]. In addition, the alcohol-induced thrombocytopenia can be the first signal of alcohol use disorder, also being a prognostic factor in the development of alcohol withdrawal syndrome and bleeding [23]. New biomarkers, more sensitive and specific, have been investigated in the last 30 years, with carbohydrate-deficient transferrin (CDT) being the most widely adopted [9,10]. An emerging biomarker is N-acetyl-Beta-Hexosaminidase (Beta-Hex), which could be used for acute alcohol intoxication, but has been used in preclinical research with rats, as well as some patients with Tay–Sachs disease with a diagnostic test. Therefore, alcohol researchers need to investigate more for using beta-Hex as a diagnostic tool [22].
Some studies have used parallel testing, combining two or more laboratory tests to identify alcohol abuse with increased diagnostic accuracy [24,25,26]. For example, CDT and GGT combinations improve the diagnostic yield of any of these markers used alone [27,28]. Similarly, parallel tests with CDT, GGT, and MCV improve detection in women [29], while CDT and the AUDIT help detect alcohol use disorders in a regular workplace [30].
An alternative strategy to specifically using alcohol biomarkers is the use of multiple routine laboratory tests and statistical methods [25,26,31]. For instance, the Early Detection of Alcohol Consumption (EDAC) method uses a linear discriminant function [32] that analyzes 10 routine laboratory tests to generate a score for each subject. Each score and its associated probability value translate into the likelihood that the individual has a specific consumption pattern [25]. Ten laboratory measurements are included in the final regression equation: chloride, sodium, direct-to-total bilirubin ratio, blood urea nitrogen, high-density lipoprotein, monocyte count, phosphorus, platelets, aspartate aminotransferase, and mean corpuscular hemoglobin (HCM). In the validation data for this model, 98% of the 161 heavy drinkers and 95% of the 42 mild drinkers were correctly identified. Widespread adoption of these statistical methods is limited due to limited availability of the software and hardware packages required.
In this study, we describe a laboratory model to predict patients’ risk group of alcohol consumption using discriminant analysis. The laboratory parameters used are routinely included in clinical consultations. Hence, our study can facilitate the early and inexpensive diagnosis of alcohol use, as well as better treatment monitoring.
We hypothesize that a combination of routine biochemical markers can predict the probability of alcohol consumption and improve the results obtained with standardized alcohol use questionnaires in patients with unknown risk [33] of alcohol consumption.
2. Materials and Methods
This study is a retrospective observational study in which the relationship between laboratory test results and patient alcohol consumption was analyzed. Participants were recruited from two different groups: patient from Addictive Behaviors Unit (ABU) and blood donors.
The ABU group (retrospectively recruited) included patients according to the following criteria: (1) Patients who started treatment at ABU from Albacete in the detoxification phase due to alcohol consumption during 2013–2015 (selected retrospectively, from June 2014), at any age. (2) Patients who meet the diagnostic criteria for alcohol use disorder (AUD) specified in the Diagnostic and Statistical Manual of mental disorders fifth edition (DSM-5); they were correctly diagnosed by a mental health expert doctor. (3) Patient’s laboratory test results at the start of treatment in the ABU were available (up to 1 month prior or 90 days after). This information was extracted by consulting the ABU medical records and the laboratories database (in this case, for laboratory test results). Data were pseudo-anonymized by the research team, and participants could only be identified by their assigned code. The exclusion criteria were: (1) Serious mental illness incompatible with participation in the study (with consent). (2) Not having laboratory test results when they started in ABU treatment (up to 1 month before or 90 days after). (3) Patients who followed treatment with any drug that could disturb the blood parameters, from which the prediction was to be made.
The other group (recruited by cross-section) was obtained from a sample of blood donors from Albacete. Serum samples from these donors were used to analyze the same analytical parameters collected (biochemical, hematological, and coagulation) in the ABU participants. The Spanish version of the 10-item AUDIT (Alcohol Use Disorder Identification Test) was administered as a written questionnaire to assess alcohol consumption in these participants, ruling out donors with risky alcohol consumption (AUDIT score > 8 in men or >6 in women) [34]. AUDIT assesses the frequency and quantity of drinking (questions 1–3) and alcohol-related harm (questions 4–10). The study’s objectives were explained to these participants and written informed consents collected.
All the blood parameters studied were: albumin, amylase, activated partial thromboplastin time, uric acid, basophils, basophils_percent, direct bilirubin, indirect bilirubin, total bilirubin, calcium, mean corpuscular hemoglobin concentration, creatine kinase, chlorine, coagulation, cholesterol, creatinine, eosinophils, eosinophils_percent, red blood cells, alkaline phosphatase, ferritin, fibrinogen C, gamma glutamyl transferase, globulins, glucose, aspartate aminotransferase, alanine aminotransferase, hemoglobin, mean corpuscular hemoglobin, hematocrit, high-density lipoprotein cholesterol, red blood cells distribution width, platelet distribution width, International Normalized Ratio, potassium, lactate dehydrogenase, low-density lipoprotein cholesterol, white blood cells, lymphocytes, lymphocytes_percent, large unstained cells percent, large unstained cells, monocytes, monocytes_percent, myeloperoxidase index, neutrophils, neutrophils_percent, phosphorus, C-reactive protein, platelets, total proteins, tryglicerides, transferrin, urea, mean corpuscular volume, mean platelet volume, and sodium.
A total of 337 participants were included in this study after exclusions (due to incomplete data), including 204 in the ABU group and 133 participants in the blood donor group. You can see the flow chart of the study in Figure 1.
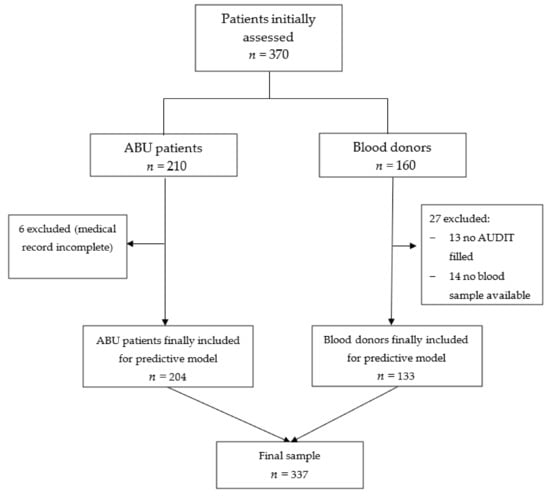
Figure 1.
Flow chart of the study.
Our sample had 253 male participants (75.3%) and 83 female participants (24.7%). The age of the participants ranged from 15 to 78. The mean age in the ABU participants was 45.5 years (Standard Deviation: SD = 10.06), and the mean age in the blood donor participants was 42.5 (SD = 10.89).
We obtained some medical history in the ABU group: age, sex, address (if city or village), study level, marital status, age that alcohol consumption started, and consumption of other drugs (nicotine, cocaine…). The same variables were obtained from the blood donor group, except the last two ones.
For the predictive analysis, participants were classified as alcohol consumption according to their average weekly consumption and gender, as per the WHO classification. More specifically, a value of >28 SDUs (Standard Drink Unit) was used for men and >17 SDUs for women [35]. Alcohol consumption was measured in Standard Drink Units (SDUs), using the equivalence of 10 grams of pure alcohol = 1 SDU [36]. Then, we conducted a bivariate statistical analysis, correlating the lab test parameters analyzed with the number of SDUs consumed. Subsequently, three predictive models were created, using different approaches: logistic regression, classification tree, and Bayesian network.
Complete participant data and variables’ definitions can be found in the supplementary information material. Supplementary Table S1: Variables’ definition used in the predictive model.
We depurated the database, and the statistical analysis was carried out with SPSS program 25.0.0. Values were expressed as mean (SD), and results were considered to be statistically significant with a p value < 0.05. For the execution of the selection of variables and the creation and validation of predictive models, 2 tools were used: the scikit-learn library for Python and the Weka application.
Scikit-learn with the Python [37] programming language is the most widely used data science tool today to perform predictive model building and processing tasks. Normally, it is only usable by people with programming knowledge such as computer engineers or mathematicians. In cases such as this article, the medical doctors contacted an expert in data science to explain and guide them in the process of discovering useful predictive models, with said data science expert taking charge of the programming part, always with the guidance and supervision of the clinician. Supplementary Information Figure S1 shows an extract from the notebook generated with the predictive analysis with Scikit-learn, used for the logistic regression model as well as for the classification tree model.
It should be noted that both the database used and the code generated for this part of the predictive analysis were shared from an institutional repository of the University of Castilla La Mancha (UCLM), specifically in the section corresponding to the SIMD research group (Intelligent Systems and Data Mining), belonging to the contacted machine learning expert. The repository link is: https://github.com/UCLM-SIMD/alcohol_risk_prediction (last access and update 25 October 2021).
Weka [38] is an application developed by the University of Waikato, in New Zealand. This tool allows any trained user to perform various tasks of data science, such as variable selection, normalization, imputation of missing values, and predictive modeling. This is performed relatively easily thanks to its intuitive interface, as can be seen in Figure S2, in the Supplementary Information.
The logistic regression model used automatic variables selection by Incremental Wrapper Subset Selection with replacement (IWSSr) [24]. In IWSSr, even if a variable has been selected, it may cease to be important in the presence of a new candidate variable. When performing a common forward search, the algorithm selects features according to their relevance for the prediction. However, it is possible that a previous relevant feature is discarded if other, more relevant features are included. For instance, if a patient shows symptoms of sinusitis or they feel cold, these might be good variables to predict the probability of having the flu. However, if we also learn that they have high fever, sinusitis is no longer an important feature to predict flu. This property of IWSSr becomes particularly relevant when the cost associated with each feature is considered. That is, if the discarded feature is more expensive than the new one (e.g., in our case, ferritin is more expensive than creatinine), our IWSSr method can lead to improvements both in terms of economic cost and algorithmic complexity.
The classification tree (the second model created) creates easily interpretable models, creating a path from its root node through the value of the case to be classified in the selected variables until it reaches a leaf node, with an associated classification result. The third model-building process captures variables dependencies and builds the simplest and efficient model: a Naive Bayes network. Previously, the variables were discretized following Fayyad and Irani’s method based on entropy (uncertainty) concerning the class (risk).
All the predictive models created were validated with the LOO (leave-one-out validation) method. LOO is a good validation scheme when the number of samples is not large. Give a database with N samples, LOO trains the predictive classifier with N-1 samples and then tests its prediction on the Nth sample, and this is repeated N times, each time with a different test sample. Then, metrics are computed based on the total positives (TP), total negatives (TN), false positives (FP), and false negatives (FN) performed by the classifier during the N train-test steps.
As missing values in the variables were input, the mean of the corresponding analytical variable was applied. This was applied in the logistic regression and classification tree models. Data were normalized to mean 0 and variance 1 for the logistic regression models. The preprocessed imputation of missing values and normalization were always applied to the corresponding training set for each split in the LOO validation.
3. Results
The distribution by sex and age is similar in both groups. In the ABU group, the sample was represented by 78.4% men and 21.6% women. The blood donor group, and therefore participants without risk of exposure to alcohol, was made up of 133 participants, distributed as 70.7% men and 29.3% women.
The mean ages in both groups were comparable, 45 years old for participants in the ABU group, with a standard deviation of 10.05, and a mean of 41 years old for participants in the blood donor group, with a standard deviation of 10.89. They had age ranges of (15–78) and (18–66), respectively, with the variances being homogeneous and reaching statistical significance (p < 0.000).
Regarding the address, 72.7% lived in a town and 27.3% lived in a city.
The marital status of both groups can be seen in Figure 2.
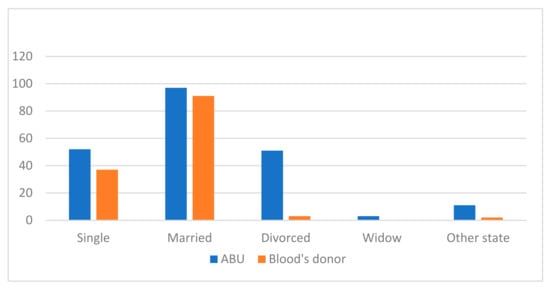
Figure 2.
Comparison of marital status for both groups, ABU and blood donors.
The marital status of the ABU participants was: 52 single (25.5%), 97 married or in a stable union (47.5%), 51 separated or divorced (25%), 3 widowed (1.5%). Within other marital statuses, 5 lived with a partner, 1 had a stable partner but did not live with them, and 5 lived with a common-law partner.
The marital status of the blood donor participants was: 37 single (27.8%), 91 married or in a stable union (68.4%), 3 separated or divorced (2.3%), 2 participants had other marital status (1.5%) (one lived as a couple and the other as a de facto couple).
We also considered it important to observe the study level comparing both groups, which is represented in Figure 3.
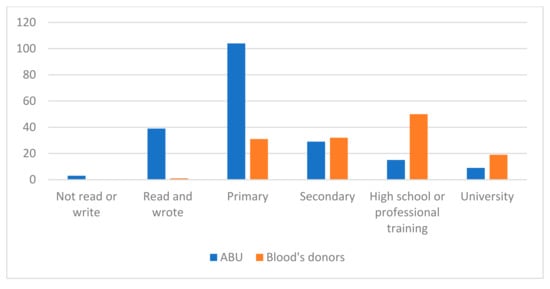
Figure 3.
Comparison of the study level for both groups (ABU and blood donors).
The study level that predominated in users of the ABU was primary studies with 104 participants (51%), 39 participants read and wrote but had no studies (19.1%), 29 participants had secondary studies (14.2%), 15 participants had completed high school or professional training courses (7.4%), 9 had completed university studies (4.4%), and 3 participants did not read or write (1.5%).
The predominant study level of the blood donors in our sample was high school and/or professional training (50 blood donors, 37.6%), followed by 32 blood donors with secondary education (24.1%), 31 blood donors with primary education (23.3%), 19 blood donors with university studies (14.3%), and only 1 blood donor (0.8%) read and write but did not have studies.
In our sample, the age of onset of alcohol consumption had a mean of 17 years old, with a standard deviation of 7.18 and a standard error of 0.507.
Regarding the consumption of other drugs, the ABU’s participants had the following data recorded: tobacco, 135 participants (66.18); cannabis, 38 participants (18.62%), cocaine, 30 participants (14.7%); heroin, 5 participants (2.45%), benzodiazepines, 2 participants; crystal meth, 2 participants; tripis or LSD, 2 participants (1% each one); and Popper and amphetamine, 1 participant each (0.5%).
However, all heroin users reported quitting at the time of the interview, and reported previous use but not current use.
The next section summarizes the main results obtained for the three predictive models created (with logistic regression, classification tree, and Naïve Bayes). Complete data can be found in Supplementary Information.
Supplementary Information (online resource) Table S2 shows the correlation of different lab parameters with the binomial variable alcohol consumption of risk/no risk. S 2.1 all participants, S 2.2 men, and S 2.3 women.
Supplementary Information (online resource) Figure S3 shows the correlation of lab parameters with consumption (S 3.1 all participants, S 3.2 men, and S 3.3 women). Supplementary Information (online resource) Figure S4 shows the rate of participants with risk of alcohol consumption.
3.1. Logistic Regression
We tested three logistic regression models using different subsets of variables, and an example of manual use of these models can be found in Supplementary Material (Example S1). These models were created according to the results of our bivariable analysis.
Table 1 shows the coefficients calculated for all the variables used, together with their confidence interval and their p values.

Table 1.
Coefficients calculated in logistic regression, for all the variables used, together with the confidence interval and its p-value. Statistically significant ones are marked with *.
The first logistic regression model resulted in an accuracy of 83.9%, a sensitivity of 84.4%, and a specificity of 83.3%. Its positive predictive value was 84.4%, and the negative predictive value was 83.3%.
Our statistical analysis revealed fifteen statistically significant variables within the model: mean corpuscular hemoglobin concentration, chlorine, eosinophils, alkaline phosphatase, ferritin, mean corpuscular hemoglobin, red blood cells distribution width, potassium, white blood cells, lymphocytes, monocytes, neutrophils, urea, mean corpuscular volume, and sodium. Therefore, we created a model using only these significant variables. The second simplified model resulted in an accuracy of 85.1%, a sensitivity of 86.7%, and a specificity of 85.4%. Its positive predictive value was 84.8%, and the negative predictive value was 85.4%.
Finally, an automatic variable selection process using IWSSr was tested, which can be seen in Table 2.

Table 2.
Coefficients calculated for logistic regression model for ‘risk’ with automatic selection (IWSSr), for the variables used, together with the confidence interval and its p value.
This resulted in some variables being discarded (one of them was ferritin (more expensive)), resulting in a model with only 8 variables: mean corpuscular hemoglobin, gamma-glutamyl transferase, red blood cells distribution width, creatinine, total bilirubin, mean platelet volume, large unstained cells, and high-density lipoprotein cholesterol. This model provided very similar performance to the prior models, with an accuracy of 84.8%, a sensitivity of 83.8%, and a specificity of 83.2%. Its positive predictive value was 86.3%, and the negative predictive value was 83.2%.
It is remarkable that there were 14 participants followed in the ABU and that they were classified as no risk for the predicted model, due to the number of SDUs declared in their consumption. With the result they obtained, they were considered positive by the model, and when calculating the sensitivity and specificity, they were by both considered as false positives among the 23 total false positives.
3.2. Classification Tree
Figure 4 shows the final classification tree produced. Although this presented a somewhat lower predictive accuracy than the logistic regression, classification trees are easier to interpret, as well as capable of explaining their inference process. Our classification tree resulted in an accuracy of 79.4%, a sensitivity of 78%, and a specificity of 77.5%. Its positive predictive value was 81.3%, and the negative predictive value was 77.5%.
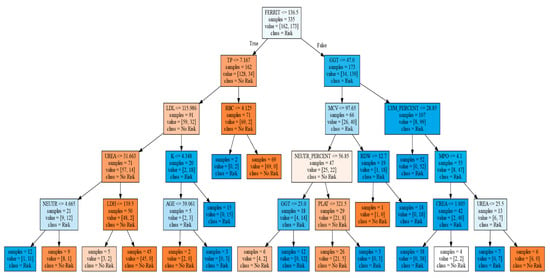
Figure 4.
Classification tree. In that figure, the square brackets are not bibliographic references. It shows the generated tree, whose initial root node is with the variable ferritin, and depending on whether its value is greater or less than 136.5, it continues with total proteins (TP), or gamma-glutamyl transferase (GGT), and later, depending on the value of these, it continues toward different variables, to finally reach the different sheets, in the lower part, that predict whether the participant is at risk or not at risk, with respect to risky alcohol consumption.
It is worth noting that there were 20 participants followed in the ABU and that they were classified as nonrisk due to the number of SDUs declared in their consumption, they were considered as positive by the classification tree, and when calculating the sensitivity and specificity, they were therefore considered as false positives. Furthermore, among the 31 participants classified as false positives in the classification tree and 23 in the logistic regression (IWSSr), there were 14 coincidences.
3.3. Bayesian Network
This network, evaluated with all the variables or the subset resulting from an automatic variable selection process, gave its best results when using the 10 variables selected through the IWSS algorithm, starting with a subset without ferritin, as was the case with the logistic regression. The selected variables were: study level, basophils, creatinine, alkaline phosphatase, gamma-glutamyl transferase, mean corpuscular haemoglobin, hematocrit, red blood cells distribution width, lactate dehydrogenase, and urea. The accuracy was 87.5%, sensitivity was 88.3%, specificity was 86.6%, positive predictive value was 88.3%, and negative predictive value was 86.6%. The network created is shown in Figure 5, whose nodes contain the probability tables for risk and the discretized variables shown in Table 3. An interpretation example for this table is: given a patient at “risk” (according to the cut-off pattern used in this study), their probability of having a GGT value > 46.5 is 42.8%. If you are a “no risk” patient, only 7%. The Supplementary Material (Example S2) shows an example of prediction by using the Bayesian network.
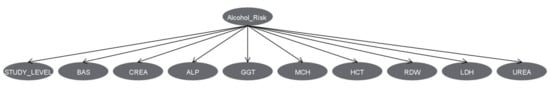
Figure 5.
Prediction of alcohol use by 9 biomarkers and the study level using machine learning, obtained by a Bayesian network. BAS = Basophils, CREA = Creatinine, ALP = Alkaline phosphatase, GGT = Gamma glutamyl transferase, MCH = Mean corpuscular haemoglobin, HCT = Hematocrit, RDW = Red blood cells distribution width, LDH = Lactate Dehydrogenase.

Table 3.
Probability table for risk and the discretized variables in the Bayesian network.
As in the other methods used, the Bayesian network classifies as positive (risk drinkers) some participants followed in the ABU who would belong to the group of no risk by the declared SDUs. Specifically, of the 20 false positives in the sample classified by this method, 14 were participants followed up at the ABU and initially classified as not at risk.
Figure 6 shows the predictive strength of each selected parameter, as measured individually with the Bayesian network classifier.
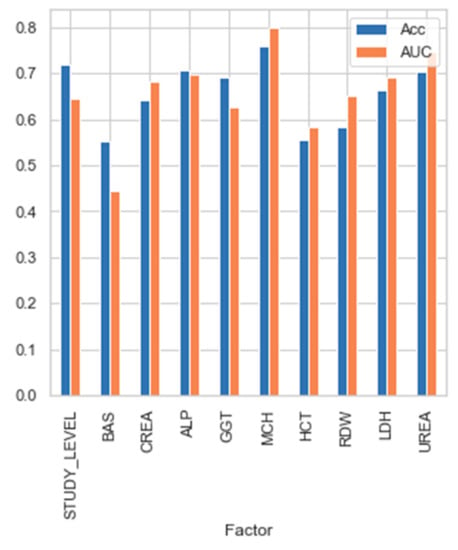
Figure 6.
Predictive strength of each parameter selected in the Bayesian network.
In terms of accuracy, we can see that none of them present such a good value as all the factors together. That is, while selected factors were not capable of providing predictive power individually (ranging from 55% to 75%), when their interaction was used to feed the classifiers, the accuracy improved greatly up to 87.5%.
4. Discussion
This study used three methods to predict alcohol use disorder with a combination of biomarkers and medical information using machine learning and data science. As far as we know, this is one of the few studies to compare the discrimination accuracy of 48 combined biomarkers at the same time. In total, 52 variables were tested (48 numerical variables and 4 categorical variables) in the predictive model for detecting alcohol use.
There is also evidence of the usefulness of some biomarkers individually, for example, the relationship between the lower platelet counts (<119 k/mL) and higher risk for complicated alcohol withdrawal syndrome [39]; however, our best predicted model using the Bayesian network did not select the platelet count with the best variables for combining. Regardless, this parameter was selected in the classification tree, just as the mean platelet volume was necessary for the prediction by logistic regression with IWSSr.
Another study [40], similar to ours, used machine learning and combined psychological variables, substance use history, demographics variables, family history, and three laboratory biomarkers (thyroid stimulating hormone, hematocrit, and hemoglobin A1C percentage).
Some authors used machine learning in the prediction of AUD, with other features, such as electrophysiological (electroencephalogram coherence), psycho-social genetic information [41], and multimodal biomarkers; another study tested if risk factors for alcohol use disorders (family history, male sex, impulsivity, low level of response to alcohol) predicted the rate of binging throughout the alcohol self-intravenous administration session [42]. In the study of [43], they tested 38 features (sex and age), history of smoking, and some biomarkers (chemistry, liver function, hematology, and lipids).
A systematic literature review by Ebrahimi et al. described the more important studies for AUD prediction using machine learning in the last 10 years. It concluded that several features were used for prediction, such as demographics, drinking behavior, family history, and electronic health records, but the lack of deep learning techniques for predicting AUD is evident and they suggested, as future research, challenges for the prediction of AUD [44].
Choosing a combination of variables can improve the predictive value of the variables separately. As can be seen in Figure 6, this clearly proves that predictive features should not be selected by evaluating them in a univariate manner, but multivariate evaluation is most advisable. The parameter predictive power individually is not so high (ranging from 55 to 75%), but when the parameters interact, the accuracy improves greatly up to 87.5%.
Our study selected the optimum combination of biomarkers as objective data by three different types of predicted models to overcome the weakness of self-assessment questionnaires, where people can deny or minimize the quantity or frequency of their patterns of alcohol use [45]. Our tool had a sensitivity from 78% with the classification tree to 88.3% with the Bayesian network, and a specificity from 77.5% with the classification tree to 86.6% with Bayesian network. Although the logistic regression’s accuracy was good, it did not fit the data used excessively well (R2 = 67%). The classification tree, despite its low accuracy (79.4%), had the advantage of interpretability. Our three predicted models (Logistic Regression (IWSSr), Classification Tree, Bayesian network) had positive predicted values of 86.3%, 81.3%, and 88.3% and negative predicted values of 83.2%, 77.5%, and 86.6%, respectively. If we pay attention to the false positives (FP) with the three predictive models, there were some coincidences, regardless of the predictive model used. In the logistic regression model, 14 false positives in ABU participants were present in the 23 participants classified as no risk with our cut point of SDUs at the beginning of the prediction. In the classification tree model, of the 31 FPs, 20 were ABU participants labeled as “No Risk,” and of those 31 FPs of the tree, 13 of the 23 FPs of the logistic regression matched. Of those 13, two different classifiers failed, indicating that perhaps the number of weekly SDUs consumption was not real. Finally, paying attention to the FP with the Bayesian network, of the 20 FP, 14 belonged to ABU participants relabeled as “no risk,” again implying that something was happening with the SDUs recognized for these participants. One of the possible reasons for these numerous coincidences may be that people with alcohol use disorders sometimes minimized their consumption and frequency. Therefore, although according to our cut point, they were labeled as “no risk,” their blood test parameters applied to the regression model predicted that they consume alcohol. We checked the medical histories of these participants, and most of them had alcohol-related medical problems in their personal medical history (trauma, heart attack, transient ischemic accident, cerebral vasculopathy, pancreatitis, legal problems…), so we have the hypothesis that they could have minimized the consumption of alcohol.
Only a few studies have attempted the combined use of biomarkers and data science. Some articles combined screening questionaries with some biomarkers, presenting a sensitive tool for detecting alcohol use. The study of [46] combined CAGE with GGT, and they guessed a good tool for detecting alcohol dependence. Another one of [47] combined two different markers (CDT and GGT) to increase diagnostic accuracy for alcohol use disorder.
Other studies combined biological markers with a questionnaire and clinical parameters for improving the recognition of heavy drinkers [48]. It is similar to our Bayesian network in that one of the selected variables for the best prediction is “educational level,” clinical information that is easy to obtain in a patient’s interview.
A recent study found an association of the elevation of 2-hydroxy-3-methylbutyric with self-reported alcohol intake, and its higher level was associated with the risk of hepatocarcinoma (OR = 2.54; 95%CI = 1.51–4.27) and pancreatic cancer (OR = 1.43; 95% CI 1.03–1.99) [4]. This other study [49] combined two indirect biomarkers (mean corpuscular volume and CDT) with one direct biomarker (Peth) for detection of alcohol ingestion, and they compared the results with a measure of blood alcohol concentration.
This study [50] validated a new method for the simultaneous determination of four alcohol biomarkers, namely, ethyl glucuronide (EtG), ethyl sulfate, N-acetyltaurine, and 16:0/18:1-phosphatidylethanol (Peth). It was developed and validated using human whole blood. Although the N-acetyltaurine needs further studies for global use, and an adequate cut-off concentration has to be defined, the parallel detection of EtG and Peth in one chromatographic method contributes to a more precise result than detection separately.
This study serves as a focus point to search for efficient combinations of direct markers, and thus in the future, studying their pharmacokinetics and pharmacodynamics could help to better understand the different patterns of alcohol consumption, as well as being able to prevent the complicated alcohol withdrawal syndrome. Nowadays, these blood determinations are not universalized and are not accessible to most hospitals, so for now, we can use the combinations of indirect markers to improve diagnosis.
The present study had some strengths, including a sufficient sample size (n = 334) and a clinically strict diagnosis for AUD by experts in the field, from addictive unit care. We checked all drugs [51] people took as chronic treatment and the presence of active viral hepatitis that could affect the biomarkers values (transaminases). We excluded people from blood donors with AUDIT score > 8 for men and >6 for women to ensure that almost every patient from that group was not exposed or minimally exposed to alcohol. Furthermore, we divided the two groups for the predicting model to avoid that bias, depending on the cut point of SDUs. For that reason, in the predicted model, some people from the ABU group received the label “no risk,” but none of the blood donors received the label “risk.”
However, this study had several limitations. First, the retrospective part was based on the clinical history of ABU participants; thus, we consider that the predicted model´s sensitivity and specificity would have been better if we had an objective measure of SDUs, and not only the patient’s version. In the future, we plan to conduct a prospective study, with an alcohol test (breathalyze) of participants, to not need the SDU cut-point.
Secondly, we did not use the CDT, which is one of the most specific biomarkers [52], but this was because our laboratory does not use this biomarker routinely, and we wanted a tool that could be used in any consultation at primary care or hospitalized participants with the standard basic blood test.
Thus far, as there is no ideal validated biomarker, which is essential to explore new combinations of biomarkers [10] and discover new ways to detect alcohol use with inexpensive and accessible tools, using data science combined with medical knowledge. We are currently working on a project, creating an app for smartphones or computers, where you can fill in the biomarker value in each box to obtain the result of the predicted model (Bayesian network), so it will be easy to use and you will be able to do it at the patient’s bedside.
A future study will be to validate the predictive model by a computer or smartphone app, where it will be possible to write the biomarker value and receive from the application the predicted label. Secondly, another project will be to create a system for detecting when a patient underestimates the alcohol quantity; for example, 14 participants were labeled as no risk, and the predicted model classified them as at risk. Is the predictive model making mistakes, or did the patient not say the correct alcohol quantity consumed? In addition, 10 of them were labelled as at risk for the three predicted models, so something about it has to be resolved in the future.
5. Conclusions
By combining several biochemical markers, clinical history (study level), and machine learning, we can enhance the detection of AUD, helping to prevent future complications from alcohol use. Our best predictive model to predict risky alcohol consumption, with an accuracy of 87.5%, using a Bayesian network, selected the best combination of nine biomarkers (basophils, creatinine, alkaline phosphatase, gamma-glutamyl transferase, mean corpuscular haemoglobin, hematocrit, red blood cells distribution width, lactate dehydrogenase, and urea) and the study’s level, being able to predict if the biomarkers alterations of our patients could be secondary to alcohol consumption. It had a sensitivity of 88.3% and a specificity of 86.6%. The selected variables were easy to obtain, even in emergency services, so if the model was validated, it could be an accessible tool for several doctors in many departments (emergencies, traumatology, psychiatry, internal medicine, general practice doctor), advocating for an early diagnosis of AUD and anticipating the possible problems derived from its consumption.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11072061/s1. Table S1. Variables´s definition used in the predictive model. Figure S1. Excerpt from the notebook generated with the predictive analysis with Scikit-learn. Figure S2. Use of the Weka application for the creation of predictive models. Table S2. Correlation of different laboratory parameters with the binomial variable risk/no risk (alcohol consume). Figure S3. Correlation of lab parameters with alcohol consumption (in Standard Drink Units (SDU). Figure S4. Rate of participants with alcohol consumption risk. Example S1. Example of manual use of the logistic regression model to make a prediction. Example S2. Example of manual use for bayesian network for prediction.
Author Contributions
Conceptualization, J.P.-S., P.B.L., B.N.B., L.N.C., J.J.S.S. and J.S.G.D.P.; data curation, J.P.-S., P.B.L., B.N.B., F.A.-P. and J.R.-R.; formal analysis, J.P.-S., P.B.L., B.N.B. and J.S.G.D.P.; funding acquisition, B.N.B.; investigation, J.P.-S., B.N.B., M.L.C.B., M.E.M., S.G.R., J.R.-R. and J.S.G.D.P.; methodology, J.P.-S., P.B.L., B.N.B., M.L.C.B., M.E.M., S.G.R., L.N.C. and J.S.G.D.P.; project administration, J.P.-S. and B.N.B.; resources, J.P.-S., B.N.B. and L.N.C.; software, P.B.L.; supervision, P.B.L., B.N.B. and J.S.G.D.P.; visualization, J.P.-S., P.B.L., B.N.B. and J.S.G.D.P.; writing—original draft, J.P.-S.; writing—review and editing, J.P.-S., P.B.L., B.N.B., J.R.-R. and J.S.G.D.P. We confirm that all authors have been personally and actively involved in the substantive work leading to the report, and will hold themselves jointly and individually responsible for its content. All authors have read and agreed to the published version of the manuscript.
Funding
Pablo Bermejo designed the machine learning part. This work has been partially funded by the Spanish Government (AEI), FEDER funds, and the JCCM through the projects PID2019-106758GB-C33/AEI/10.13039/501100011033 and SBPLY/17/180501/000493. Beatriz Navarro’s contract is co-financed by the European Development Fund Regional (Feder) in accordance with the Operational Program of the Region of Castilla-La Mancha for Feder 2014-2020, and for the University of Castilla-La Mancha’s own Research Plan.
Institutional Review Board Statement
The Ethical Committee of Drug research from Gerencia de Atención Integrada de Albacete, from Albacete, approved this study protocol on 26 June 2014 (nº 06/2014), and this study was conducted following the ethical principles for medical research of Helsinki’s Declaration.
Informed Consent Statement
The study’s objectives were explained to the participants and written informed consents were collected.
Data Availability Statement
Here, you can access to the dataset for the predictive model. It is the public repository link from the official account of Intelligent Systems and Data Mining Group (Grupo Sistemas Inteligentes y Minería de datos (SIMD)) from the University of Castilla La Mancha (Universidad de Castilla La Mancha (UCLM)). https://github.com/UCLM-SIMD/alcohol_risk_prediction. (last access and update 25 October 2021).
Acknowledgments
We would like to thank Diego Martínez Plasencia (University College London (UCL) and Alicia Colino Acevedo (Internal Medicine Specialist in University Hospitals Sussex) for correcting the translation of the article into English.
Conflicts of Interest
All authors have not potential conflict of interest.
References
- Friedmann, P.D. Alcohol use in adults. N. Engl. J. Med. 2013, 368, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Villa, J.L.; Fernández-Cáceres, C. Comorbilidad Médica Asociada al Abuso y Dependencia de Alcohol. Revisión Documental. Revista Internacional de Investigación en Adicciones. Medical Comorbidity Associated with Alcohol Abuse and Dependence. Document Review. Int. J. Addict. Res. 2019. Available online: http://riiad.org/index.php/riiad/article/view/riiad.2019.1.06/269#toc (accessed on 16 October 2021).
- International Agency for Research on Cancer. Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024. IARC Monogr Eval Carcinog Risks to Humans. 2020, (April 2014):i-ix+1-390. Available online: http://monographs.iarc.fr/ENG/Monographs/vol83/mono83-1.pdf (accessed on 16 October 2021).
- Loftfield, E.; Stepien, M.; Viallon, V.; Trijsburg, L. Novel Biomarkers of Habitual Alcohol Intake and Associations with Risk of Pancreatic and Liver Cancers and Liver Disease Mortality; Oxford University Press: Oxford, UK, 2021; pp. 1–33. [Google Scholar]
- Esser, M.B.; Sherk, A.; Liu, Y.; Naimi, T.S.; Stockwell, T.; Stahre, M. Deaths and Years of Potential Life Lost From Excessive Alcohol Use—United States, 2011–2015. Morb. Mortal. Wkly. Rep. 2020, 69, 981. [Google Scholar] [CrossRef] [PubMed]
- Whitty, M.; Nagel, T.; Jayaraj, R.; Kavanagh, D. Development and evaluation of training in culturally specific screening and brief intervention for hospital patients with alcohol-related injuries. Aust. J. Rural Health 2016, 24, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Parsley, I.C.; Dale, A.M.; Fisher, S.L.; Mintz, C.M.; Hartz, S.M.; Evanoff, B.A.; Bierut, L.J. Association Between Workplace Absenteeism and Alcohol Use Disorder From the National Survey on Drug Use and Health, 2015–2019. JAMA Netw. Open. 2022, 5, e222954. [Google Scholar] [CrossRef]
- Gakh, M.; Coughenour, C.; Assoumou, B.O.; Vanderstelt, M. The Relationship between School Absenteeism and Substance Use: An Integrative Literature Review. Subst. Use Misuse 2020, 55, 491–502. [Google Scholar] [CrossRef]
- Van Loon, M.; Van Der Mast, R.C.; Van Der Linden, M.C.; Van Gaalen, F.A. Routine alcohol screening in the ED: Unscreened patients have an increased risk for hazardous alcohol use. Emerg. Med. J. 2019, 37, 206–211. [Google Scholar] [CrossRef]
- Nowakowska-Domagała, K.; Jabłkowska-Górecka, K.; Mokros, Ł.; Koprowicz, J.; Pietras, T. Differences in the verbal fluency, working memory and executive functions in alcoholics: Short-term vs. long-term abstainers. Psychiatry Res 2017, 249, 1–8. [Google Scholar] [CrossRef]
- Torrens, M.; Domingo-salvany, A. Patología dual: Una perspectiva europea Dual diagnosis: An European perspective. Adicciones 2017, 29, 2016–2018. [Google Scholar] [CrossRef]
- Rehm, J.; Anderson, P.; Manthey, J.; Shield, K.D.; Struzzo, P.; Wojnar, M.; Gual, A. Alcohol Use Disorders in Primary Health Care: What Do We Know and Where Do We Go? Alcohol Alcohol. 2015, 51, 422–427. [Google Scholar] [CrossRef]
- Knightly, R.; Tadros, G.; Sharma, J.; Duffield, P.; Carnall, E.; Fisher, J.; Salman, S. Alcohol screening for older adults in an acute general hospital: FAST v. MAST-G assessments. BJPsych Bull. 2016, 40, 72–76. BJPsych Bull. 2016, 40, 72–76. [Google Scholar] [CrossRef]
- Rosón, B.; Corbella, X.; Perney, P.; Santos, A.; Stauber, R.; Lember, M.; Arutyunov, A.; Ruza, I.; Vaclavik, J.; García, L.; et al. Prevalence, Clinical Characteristics, and Risk Factors for Non-recording of Alcohol Use in Hospitals across Europe: The ALCHIMIE Study. Alcohol Alcohol. 2016, 51, 457–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brime, N.G.B. Observatorio Español de las Drogas y las Adicciones. Informe 2021. Alcohol, Tabaco y Drogas Ilegales en España; Spanish Observatory of Drugs and Addictions. Report 2021. Alcohol, Tobacco and Illegal Drugs in Spain; Ministry of Health, Government Delegation for the National Plan on Drugs: Madrid, Spain, 2021; pp. 6–65. [Google Scholar]
- Marcos Martín, M.; Pastor Encinas, I.; Laso, F.J. Marcadores biológicos del alcoholismo. [Alcoholism’s biomarkers]. Rev. Clín. Esp. 2005, 205, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, R.; Moonka, D.; Nunes, F. Using biomarkers to quantify problematic alcohol use. J. Fam. Pract. 2021, 70, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, H.R.; Hull, M.; Okasinski, L.M. Review of current clinical biomarkers for the detection of alcohol dependence. Innov. Clin. Neurosci. 2011, 8, 26–33. [Google Scholar]
- Hurme, L.; Seppä, K.; Rajaniemi, H.; Sillanaukee, P. Chromatographically identified alcohol-induced haemoglobin adducts as markers of alcohol abuse among women. Eur. J. Clin. Investig. 1998, 28, 87–94. [Google Scholar] [CrossRef]
- Israel, Y.; Hurwitzt, E.; Niemela, O.; Arnont, R. Monoclonal and polyclonal antibodies against acetaldehyde–containing epitopes in acetaldehyde-protein adducts. Proc. Natl. Acad. Sci. USA 1986, 83, 7923–7927. [Google Scholar] [CrossRef]
- Koivisto, H.; Hietala, J.; Anttila, P.; Niemelä, O. Co-Occurrence of IgA Antibodies Against Ethanol Metabolites and Tissue Transglutaminase in Alcohol Consumers: Correlation with Proinflammatory Cytokines and Markers of Fibrogenesis. Dig. Dis. Sci. 2008, 53, 500–505. [Google Scholar] [CrossRef]
- Harris, J.C.; Leggio, L.; Farokhnia, M. Blood Biomarkers of Alcohol Use: A Scoping Review. Curr. Addict Rep. 2021, 8, 500–508. [Google Scholar] [CrossRef]
- Silczuk, A.; Habrat, B. Alcohol-induced thrombocytopenia: Current review. Alcohol 2020, 86, 9–16. [Google Scholar] [CrossRef]
- Sillanaukee, P. The diagnostic Value of a Discriminant Score in the Detection of Alcohol Use. Arch. Pathol. Lab. Med. 1992, 116, 924–929. [Google Scholar]
- Hartz, A.J.; Guse, C.; Kajdacsy-Balla, A. Identification of heavy drinkers using a combination of laboratory tests. J. Clin. Epidemiol. 1997, 50, 1357–1368. [Google Scholar] [CrossRef]
- Harasymiw, J.; Bean, P. The combined use of the early detection of alcohol consumption (EDAC) test and carbohydrate-deficient transferrin to identify heavy drinking behaviour in males. Alcohol Alcohol. 2001, 36, 349–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anton, R.F.; Moak, D.H. Carbohydrate-Deficient Transferrin and γ-Glutamyltransferase as Markers of Heavy Alcohol Consumption: Gender Differences. Alcohol. Clin. Exp. Res. 1994, 18, 747–754. [Google Scholar] [CrossRef]
- van Pelt, J.; Leusink, G.L.; van Nierop, P.W.N.; Keyzer, J.J. Carbohydrate- Deficient Transferrin and gammaglutamyltransferase in alcohol-using perimenopausal women. Alcohol. Clin. Exp. Res. 2000, 24, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Mundle, G.; Munkes, J.; Ackermann, K.; Mann, K. Sex Differences of Carbohydrate-Deficient Transferrin, gamma-Glutamyltransferase, and Mean Corpuscular Volume in Alcohol-Dependent Patients. Alcohol. Clin. Exp. Res. 2000, 24, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, U.; Helander, A.; Brandt, L.; Huss, A.; Rönnberg, S. The alcohol use disorders identification test and carbohydrate-deficient transferrin in alcohol-related sickness absence. Alcohol. Clin. Exp. Res. 2002, 26, 28–35. [Google Scholar] [CrossRef]
- Bean, P.; Harasymiw, J.; Peterson, C.M.; Javors, M. Innovative technologies for the diagnosis of alcohol abuse and monitoring abstinence. Alcohol. Clin. Exp. Res. 2001, 25, 309–316. [Google Scholar] [CrossRef]
- Multivariate, A.; Analysis, S.; Johnson, R.A.; Wichern, D.W. Reviewed Work: Applied Multivariate Statistical Analysis. Biometrics 1998, 54, 1203. [Google Scholar]
- Ministerio de Sanidad. Límites de Consumo de Bajo Riesgo de Alcohol; [Ministry of Health. Low-Risk Alcohol Consumption]; Ministry of Health: Madrid, Spain, 2020.
- Ángel, M.; Carretero, G.; Pedro, J.; Ruiz, N.; Martínez, J.M.; González, C.O.F. Validation of the Alcohol Use Disorders Identification Test in university students: AUDIT and AUDIT-C Validación del test para la identificación de trastornos por uso de alcohol en población universitaria: AUDIT y AUDIT-C. Adicciones 2016, 4, 194–204. [Google Scholar]
- Stockwell, T.; Chikritzhs, T.; Holder, H.; Single, E.; Elena, M.; Jernigan, D. International Guide for Monitoring Alcohol Consumption and Harm; World Health Organization: Geneva, Switzerland, 2000; pp. 1–193. Available online: http://apps.who.int/iris/bitstream/10665/66529/1/WHO_MSD_MSB_00.4.pdf (accessed on 24 January 2021).
- Gual, A.; Martos, A.R.; Lligoña, A.; Llopis, J.J. Does the concept of a standard drink apply to viticultural societies? Alcohol Alcohol. 1999, 34, 153–160. [Google Scholar] [CrossRef]
- Bac, J.; Mirkes, E.M.; Gorban, A.N.; Tyukin, I.; Zinovyev, A. Scikit-dimension: A python package for intrinsic dimension estimation. Entropy 2021, 23, 1368. [Google Scholar] [CrossRef] [PubMed]
- Weka 3—Data Mining with Open Source Machine Learning Software in Java. Available online: https://www.cs.waikato.ac.nz/ml/weka/ (accessed on 28 November 2021).
- Silczuk, A.; Habrat, B.; Lew-Starowicz, M. Thrombocytopenia in Patients Hospitalized for Alcohol Withdrawal Syndrome and Its Associations to Clinical Complications. Alcohol Alcohol. 2019, 54, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Gowin, J.L.; Manza, P.; Ramchandani, V.A.; Volkow, N.D. Neuropsychosocial Markers of Binge Drinking in Young Adults. Mol. Psychiatry 2021, 26, 4931–4943. [Google Scholar] [CrossRef]
- Kinreich, S.; Meyers, J.L.; Maron-Katz, A.; Kamarajan, C.; Pandey, A.K.; Chorlian, D.B.; Zhang, J.; Pandey, G.; de Viteri, S.S.-S.; Pitti, D.; et al. Predicting risk for Alcohol Use Disorder using longitudinal data with multimodal biomarkers and family history: A machine learning study. Mol. Psychiatry 2021, 26, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Gowin, J.L.; Sloan, M.E.; Stangl, B.L.; Vatsalya, V.; Ramchandani, V.A. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am. J. Psychiatry 2017, 174, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Bonnell, L.N.; Littenberg, B.; Wshah, S.R.; Rose, G.L. A Machine Learning Approach to Identification of Unhealthy Drinking. J. Am. Board Fam. Med. 2020, 33, 397–406. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Wiil, U.K.; Schmidt, T.; Naemi, A.; Nielsen, A.S.; Shaikh, G.M.; Mansourvar, M. Predicting the Risk of Alcohol Use Disorder Using Machine Learning: A Systematic Literature Review. IEEE Access 2021, 9, 151697–151712. [Google Scholar] [CrossRef]
- Pérez-Milena, A.; Redondo-Olmedilla, M.d.D.; Martínez-Fernández, M.L.; Jiménez-Pulido, I.; Mesa-Gallardo, I.; Leal-Helmling, F.J. Changes in hazardous drinking in Spanish adolescent population in the last decade (2004–2013) using a quantitative and qualitative design. Aten. Primaria 2017, 49, 525–533. [Google Scholar] [CrossRef]
- Choe, Y.M.; Lee, B.C.; Choi, I.G.; Suh, G.H.; Lee, D.Y.; Kim, J.W. Combination of the CAGE and serum gamma-glutamyl transferase: An effective screening tool for alcohol use disorder and alcohol dependence. Neuropsychiatr. Dis. Treat. 2019, 15, 1507–1515. [Google Scholar] [CrossRef]
- Hietala, J.; Koivisto, H.; Anttila, P.; Niemelä, O. Comparison of the combined marker GGT-CDT and the conventional laboratory markers of alcohol abuse in heavy drinkers, moderate dinkers and abstainers. Alcohol Alcohol. 2006, 41, 528–533. [Google Scholar] [CrossRef]
- Bataille, V.; Ruidavets, J.B.; Arveiler, D.; Amouyel, P.; Ducimetière, P.; Perret, B.; Ferrières, J. Joint use of clinical parameters, biological markers and cage questionnaire for the identification of heavy drinkers in a large population-based sample. Alcohol Alcohol. 2003, 38, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Aboutara, N.; Müller, A.; Jungen, H.; Szewczyk, A.; van Rüth, V.; Bertram, F.; Püschel, K.; Heinrich, F.; Iwersen-Bergmann, S. Investigating the use of PEth, CDT and MCV to evaluate alcohol consumption in a cohort of homeless individuals—A comparison of different alcohol biomarkers. Forensic Sci. Int. 2022, 331, 111147. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, V.; Sundermann, T.R.; Schmitt, G.; Bartel, M. Development and validation of an analytical method for the simultaneous determination of the alcohol biomarkers ethyl glucuronide, ethyl sulfate, N-acetyltaurine, and 16:0/18:1-phosphatidylethanol in human blood. Drug Test. Anal. 2022, 14, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Alonso, A.; Stephens, C.; Lucena, M.I.; Andrade, R.J. Case characterization, clinical features and risk factors in drug-induced liver injury. Int. J. Mol. Sci. 2016, 17, 714. [Google Scholar] [CrossRef] [PubMed]
- Fagan, K.J.; Irvine, K.M.; Mcwhinney, B.C.; Fletcher, L.M.; Horsfall, L.U.; Johnson, L.; O’Rourke, P.; Martin, J.; Scott, I.; Pretorius, C.J.; et al. Diagnostic sensitivity of carbohydrate deficient transferrin in heavy drinkers. BMC Gastroenterol. 2014, 14, 97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).