Clinical Features of Anti-Synthetase Syndrome Associated with Prognosis in Patients with Dermatomyositis and Polymyositis
Abstract
1. Introduction
2. Materials and Methods
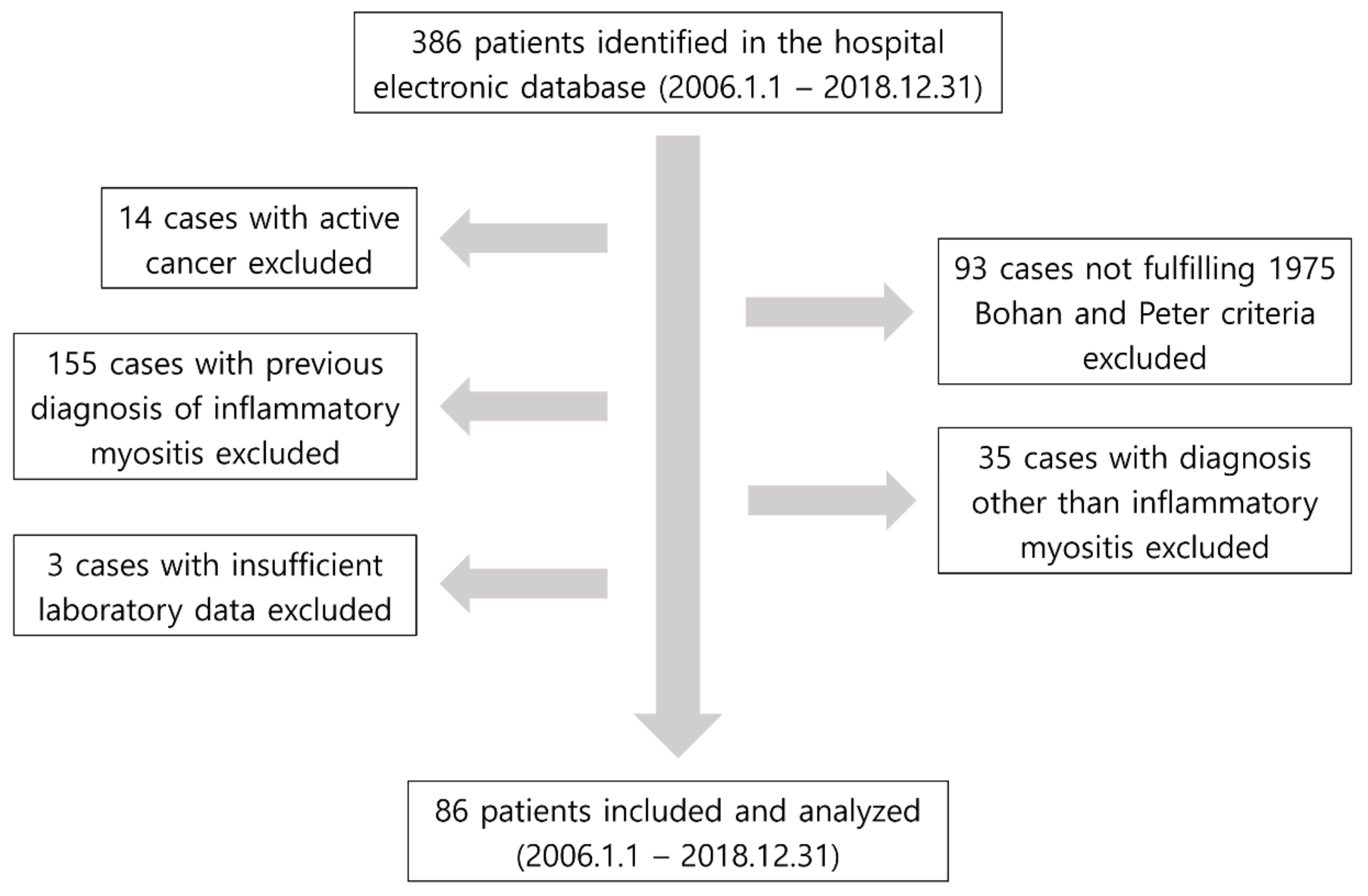
2.1. Study Design and Patient Selection
2.2. Collection of Baseline Patient Data
2.3. Clinical Features of Anti-Synthetase Syndrome, Definition of Clinical Outcomes and Treatment
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes Based on the Presence of Fever, Interstitial Lung Disease and Anti-Jo-1 Antibody
3.3. Clinical and Laboratory Features Associated with All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, S.; Prayson, R.; Farver, C. Antisynthetase syndrome: Not just an inflammatory myopathy. Cleve Clin. J. Med. 2013, 80, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Connors, G.R.; Christopher-Stine, L.; Oddis, C.V.; Danoff, S.K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in the past 35 years? Chest 2010, 138, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Witt, L.J.; Curran, J.J.; Strek, M.E. The Diagnosis and Treatment of Antisynthetase Syndrome. Clin. Pulm. Med. 2016, 23, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kronzer, V.L.; Dellaripa, P.F.; Deane, K.D.; Bolster, M.B.; Nagaraja, V.; Khanna, D.; Doyle, T.J.; Sparks, J.A. Rheumatoid arthritis-associated interstitial lung disease: Current update on prevalence, risk factors, and pharmacologic treatment. Curr. Treatm. Opt. Rheumatol. 2020, 6, 337–353. [Google Scholar] [CrossRef]
- Reiseter, S.; Gunnarsson, R.; Mogens Aaløkken, T.; Lund, M.B.; Mynarek, G.; Corander, J.; Haydon, J.; Molberg, Ø. Progression and mortality of interstitial lung disease in mixed connective tissue disease: A long-term observational nationwide cohort study. Rheumatology 2018, 57, 255–262. [Google Scholar] [CrossRef]
- Katsumata, Y.; Kawaguchi, Y.; Yamanaka, H. Interstitial Lung Disease with ANCA-associated Vasculitis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2015, 9 (Suppl. S1), 51–56. [Google Scholar] [CrossRef]
- Sikora, K.A.; Grom, A.A. Update on the pathogenesis and treatment of systemic idiopathic arthritis. Curr. Opin. Pediatr. 2011, 23, 640–646. [Google Scholar] [CrossRef]
- Ahn, S.S.; Yoo, B.W.; Jung, S.M.; Lee, S.W.; Park, Y.B.; Song, J.J. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Semin. Arthritis Rheum. 2017, 47, 216–221. [Google Scholar] [CrossRef]
- Cook, R.J.; Gladman, D.D.; Pericak, D.; Urowitz, M.B. Prediction of short term mortality in systemic lupus erythematosus with time dependent measures of disease activity. J. Rheumatol. 2000, 27, 1892–1895. [Google Scholar]
- Dalakas, M.C. Inflammatory muscle diseases. N. Engl. J. Med. 2015, 372, 1734–1747. [Google Scholar] [CrossRef]
- Yang, S.H.; Chang, C.; Lian, Z.X. Polymyositis and dermatomyositis—Challenges in diagnosis and management. J. Transl. Autoimmun. 2019, 2, 100018. [Google Scholar] [CrossRef]
- Bronner, I.M.; van der Meulen, M.F.; de Visser, M.; Kalmijn, S.; van Venrooij, W.J.; Voskuyl, A.E.; Dinant, H.J.; Linssen, W.H.J.P.; Wokke, J.H.J.; Hoogendijk, J.E. Long-term outcome in polymyositis and dermatomyositis. Ann. Rheum. Dis. 2006, 65, 1456–1461. [Google Scholar] [CrossRef]
- Airio, A.; Kautiainen, H.; Hakala, M. Prognosis and mortality of polymyositis and dermatomyositis patients. Clin. Rheumatol. 2006, 25, 234–239. [Google Scholar] [CrossRef]
- Marie, I.; Hachulla, E.; Hatron, P.Y.; Hellot, M.F.; Levesque, H.; Devulder, B.; Courtois, H. Polymyositis and dermatomyositis: Short term and longterm outcome, and predictive factors of prognosis. J. Rheumatol. 2001, 28, 2230–2237. [Google Scholar] [CrossRef]
- Schiopu, E.; Phillips, K.; MacDonald, P.M.; Crofford, L.J.; Somers, E.C. Predictors of survival in a cohort of patients with polymyositis and dermatomyositis: Effect of corticosteroids, methotrexate and azathioprine. Arthritis Res. Ther. 2012, 14, R22. [Google Scholar] [CrossRef]
- Yang, X.; Hao, Y.; Zhang, X.; Geng, Y.; Ji, L.; Li, G.; Zhang, Z. Mortality of Chinese patients with polymyositis and dermatomyositis. Clin. Rheumatol. 2020, 39, 1569–1579. [Google Scholar] [CrossRef]
- Marie, I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr. Rheumatol. Rep. 2012, 14, 275–285. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef]
- Fathi, M.; Lundberg, I.E. Interstitial lung disease in polymyositis and dermatomyositis. Curr. Opin. Rheumatol. 2005, 17, 701–706. [Google Scholar] [CrossRef]
- Marie, I.; Hatron, P.Y.; Cherin, P.; Hachulla, E.; Diot, E.; Vittecoq, O.; Menard, J.-F.; Jouen, F.; Dominique, S. Functional outcome and prognostic factors in anti-Jo1 patients with antisynthetase syndrome. Arthritis Res. Ther. 2013, 15, R149. [Google Scholar] [CrossRef]
- Watanabe, E.; Gono, T.; Kuwana, M.; Terai, C. Predictive factors for sustained remission with stratification by myositis-specific autoantibodies in adult polymyositis/dermatomyositis. Rheumatology 2020, 59, 586–593. [Google Scholar] [CrossRef]
- Lee, L.W.; Narang, N.S.; Postolova, A.; Seminara, N.; Kantor, M.A. Anti-MDA5-Positive Dermatomyositis Presenting as Fever of Unknown Origin. J. Gen. Intern. Med. 2016, 31, 1530–1536. [Google Scholar] [CrossRef][Green Version]
- Nakashima, R. Clinical significance of myositis-specific autoantibodies. Immunol. Med. 2018, 41, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Opinc, A.H.; Makowska, J.S. Antisynthetase syndrome—Much more than just a myopathy. Semin. Arthritis Rheum. 2020, 51, 72–83. [Google Scholar] [CrossRef]
- Douglas, W.W.; Tazelaar, H.D.; Hartman, T.E.; Hartman, R.P.; Decker, P.A.; Schroeder, D.R.; Ryu, J. Polymyositis-dermatomyositis-associated interstitial lung disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1182–1185. [Google Scholar] [CrossRef]
- Aggarwal, R.; Cassidy, E.; Fertig, N.; Koontz, D.C.; Lucas, M.; Ascherman, D.P.; Oddis, C.V. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann. Rheum. Dis. 2014, 73, 227–232. [Google Scholar] [CrossRef]
- Fathi, M.; Vikgren, J.; Boijsen, M.; Tylen, U.; Jorfeldt, L.; Tornling, G.; Lundberg, I.E. Interstitial lung disease in polymyositis and dermatomyositis: Longitudinal evaluation by pulmonary function and radiology. Arthritis Rheum. 2008, 59, 677–685. [Google Scholar] [CrossRef]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.M. Interleukin 1 and interferon-gamma: Cytokines that provide reciprocal regulation of macrophage and T cell function. Toxicol. Pathol. 1987, 15, 333–337. [Google Scholar] [CrossRef]
- Moran, E.M.; Mastaglia, F.L. Cytokines in immune-mediated inflammatory myopathies: Cellular sources, multiple actions and therapeutic implications. Clin. Exp. Immunol. 2014, 178, 405–415. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Values |
|---|---|
| Demographics | |
| Age | 51.0 (29.0) |
| Male sex | 28 (32.6) |
| Diagnosis | |
| Polymyositis | 38 (44.2) |
| Dermatomyositis | 37 (43.0) |
| Juvenile dermatomyositis | 11 (12.8) |
| Previous comorbidities present ‡ | |
| Hypertension | 19 (22.1) |
| Diabetes mellitus | 13 (15.1) |
| Dyslipidemia | 5 (5.8) |
| Laboratory results | |
| White blood cell count (/mm3) | 7375.0 (3330.0) |
| Neutrophil count (/mm3) | 4340.0 (2867.5) |
| Platelet count (×1000/mm3) | 274.5 (130.0) |
| Erythrocyte sedimentation rate (mm/h) | 32.0 (34.0) |
| C-reactive protein (mg/L) (n = 81) | 2.6 (8.1) |
| Aspartate aminotransferase (IU/L) | 115.0 (192.0) |
| Alanine aminotransferase (IU/L) | 88.5 (144.0) |
| Creatinine kinase (IU/L) | 1884.0 (6684.0) |
| Anti-Jo-1 antibody positivity (n = 67) † | 20 (29.9) |
| Clinical features of anti-synthetase syndrome present | |
| Fever | 31 (36.0) |
| Raynaud’s phenomenon | 9 (10.5) |
| Mechanic’s hand | 5 (5.8) |
| Arthritis | 11 (12.8) |
| Interstitial lung disease | 23 (26.7) |
| Outcomes | |
| All-cause mortality | 12 (14.0) |
| Intensive care unit admission | 11 (12.8) |
| Remission at 1 year (n = 58) ¶ | 33 (56.9) |
| Follow-up duration (months) | 34.2 (61.1) |
| Anti-Jo-1 Antibody (+) Group (n = 20) | Anti-Jo-1 Antibody (−) Group (n = 47) | p-Value | |
|---|---|---|---|
| Fever | 9 (45.0) | 20 (42.6) | 0.854 |
| Raynaud’s phenomenon | 4 (20.0) | 4 (8.5) | 0.226 |
| Mechanic’s hand | 2 (10.0) | 3 (6.4) | 0.631 |
| Arthritis | 3 (15.0) | 7 (14.9) | 0.999 |
| Interstitial lung disease | 10 (50.0) | 12 (25.5) | 0.053 |
| All Patients (n = 86) | Fever (n = 86) | Interstitial Lung Disease (n = 86) | Anti-Jo-1 Antibody (n = 67) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 31) | No (n = 55) | p-Value | Yes (n = 23) | No (n = 63) | p-Value | Yes (n = 20) | No (n = 47) | p-Value | |
| All-cause mortality (+) | 8 (25.8) | 4 (7.3) | 0.024 | 6 (26.1) | 6 (9.5) | 0.051 | 5 (25.0) | 6 (12.8) | 0.220 |
| All-cause mortality (−) | 23 (74.2) | 51 (92.7) | 17 (73.9) | 57 (90.5) | 15 (75.0) | 41 (87.2) | |||
| Intensive care unit admission (+) | 7 (22.6) | 4 (7.3) | 0.051 | 5 (21.7) | 6 (9.5) | 0.136 | 5 (25.0) | 5 (10.6) | 0.134 |
| Intensive care unit admission (−) | 24 (77.4) | 51 (92.7) | 18 (78.3) | 57 (90.5) | 15 (75.0) | 42 (89.4) | |||
| Patients with follow-up ≥1 year (n = 58) | Fever (n = 58) | Interstitial lung disease (n = 58) | Anti-Jo-1 antibody (n = 48) | ||||||
| Yes (n = 17) | No (n = 41) | p-value | Yes (n = 13) | No (n = 45) | p-value | Yes (n = 13) | No (n = 35) | p-value | |
| Remission at 1 year (+) † (n = 58) | 14 (82.4) | 19 (46.3) | 0.019 | 5 (38.5) | 28 (62.2) | 0.131 | 4 (30.8) | 22 (62.9) | 0.059 |
| Remission at 1 year (−) † (n = 58) | 3 (17.6) | 22 (53.7) | 8 (61.5) | 17 (37.8) | 9 (69.2) | 13 (37.1) | |||
| Patients with Interstitial Lung Disease (n = 23) | Patients without Interstitial Lung Disease (n = 63) | p-Value | |
|---|---|---|---|
| Glucocorticoid | 23 (100.0) | 63 (100.0) | 0.999 |
| Methotrexate | 7 (30.4) | 36 (57.1) | 0.029 |
| Azathioprine | 13 (56.5) | 16 (25.4) | 0.007 |
| Intravenous immunoglobulin | 8 (34.8) | 19 (30.2) | 0.684 |
| Rituximab | 1 (4.3) | 4 (6.3) | 0.999 |
| Hydroxychloroquine | 5 (21.7) | 20 (31.7) | 0.369 |
| Cyclophosphamide | 3 (13.0) | 5 (7.9) | 0.436 |
| Tacrolimus | 3 (13.0) | 3 (4.8) | 0.336 |
| Mycophenolate mofetil | 2 (8.7) | 4 (6.3) | 0.656 |
| Patients with Mortality (n = 12) | Patients without Mortality (n = 74) | p-Value | |
|---|---|---|---|
| Glucocorticoid | 12 (100.0) | 74 (100.0) | 0.999 |
| Methotrexate | 3 (25.0) | 40 (54.1) | 0.117 |
| Azathioprine | 3 (25.0) | 26 (35.1) | 0.743 |
| Intravenous immunoglobulin | 5 (41.7) | 22 (29.7) | 0.411 |
| Rituximab | 2 (16.7) | 3 (4.1) | 0.141 |
| Hydroxychloroquine | 2 (16.7) | 23 (31.1) | 0.496 |
| Cyclophosphamide | 2 (16.7) | 6 (8.1) | 0.309 |
| Tacrolimus | 1 (8.3) | 5 (6.8) | 0.999 |
| Mycophenolate mofetil | 1 (8.3) | 5 (6.8) | 0.999 |
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.04 | 1.01, 1.08 | 0.02 | |||
| Male sex | 5.54 | 1.66, 18.56 | <0.01 | 5.53 | 1.65, 18.49 | <0.01 |
| Hypertension | 1.99 | 0.60, 6.62 | 0.26 | |||
| Diabetes mellitus | 3.57 | 1.07, 11.93 | 0.04 | |||
| Dyslipidemia ¶ | n/a | |||||
| White blood cell count | 1.00 | 1.00, 1.00 | 0.30 | |||
| Neutrophil count | 1.00 | 1.00, 1.00 | 0.96 | |||
| Platelet count | 1.00 | 1.00, 1.00 | 0.95 | |||
| Erythrocyte sedimentation rate | 1.02 | 1.00, 1.03 | 0.04 | |||
| Aspartate aminotransferase | 1.00 | 1.00, 1.00 | 0.77 | |||
| Alanine aminotransferase | 1.00 | 0.99, 1.00 | 0.37 | |||
| Creatinine kinase | 1.00 | 1.00, 1.00 | 0.68 | |||
| Fever | 4.20 | 1.26, 13.97 | 0.02 | 4.20 | 1.26, 14.01 | 0.02 |
| Raynaud’s phenomenon | 0.87 | 0.11, 6.76 | 0.90 | |||
| Mechanic’s hand ¶ | n/a | |||||
| Arthritis | 0.49 | 0.06, 3.79 | 0.49 | |||
| Interstitial lung disease | 3.06 | 0.98, 9.49 | 0.05 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.S.; Park, Y.-B.; Lee, S.-W. Clinical Features of Anti-Synthetase Syndrome Associated with Prognosis in Patients with Dermatomyositis and Polymyositis. J. Clin. Med. 2022, 11, 2052. https://doi.org/10.3390/jcm11072052
Ahn SS, Park Y-B, Lee S-W. Clinical Features of Anti-Synthetase Syndrome Associated with Prognosis in Patients with Dermatomyositis and Polymyositis. Journal of Clinical Medicine. 2022; 11(7):2052. https://doi.org/10.3390/jcm11072052
Chicago/Turabian StyleAhn, Sung Soo, Yong-Beom Park, and Sang-Won Lee. 2022. "Clinical Features of Anti-Synthetase Syndrome Associated with Prognosis in Patients with Dermatomyositis and Polymyositis" Journal of Clinical Medicine 11, no. 7: 2052. https://doi.org/10.3390/jcm11072052
APA StyleAhn, S. S., Park, Y.-B., & Lee, S.-W. (2022). Clinical Features of Anti-Synthetase Syndrome Associated with Prognosis in Patients with Dermatomyositis and Polymyositis. Journal of Clinical Medicine, 11(7), 2052. https://doi.org/10.3390/jcm11072052






