The 10 Year Outcomes of Implants Inserted with Dehiscence or Fenestrations in the Rehabilitation of Completely Edentulous Jaws with the All-on-4 Concept
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outcome Measures
2.2. Statistical Evaluation
3. Results
3.1. Sample
3.2. Lost to Follow-Up Rate; Implant Survival, Success, and Failure; Prosthetic Survival
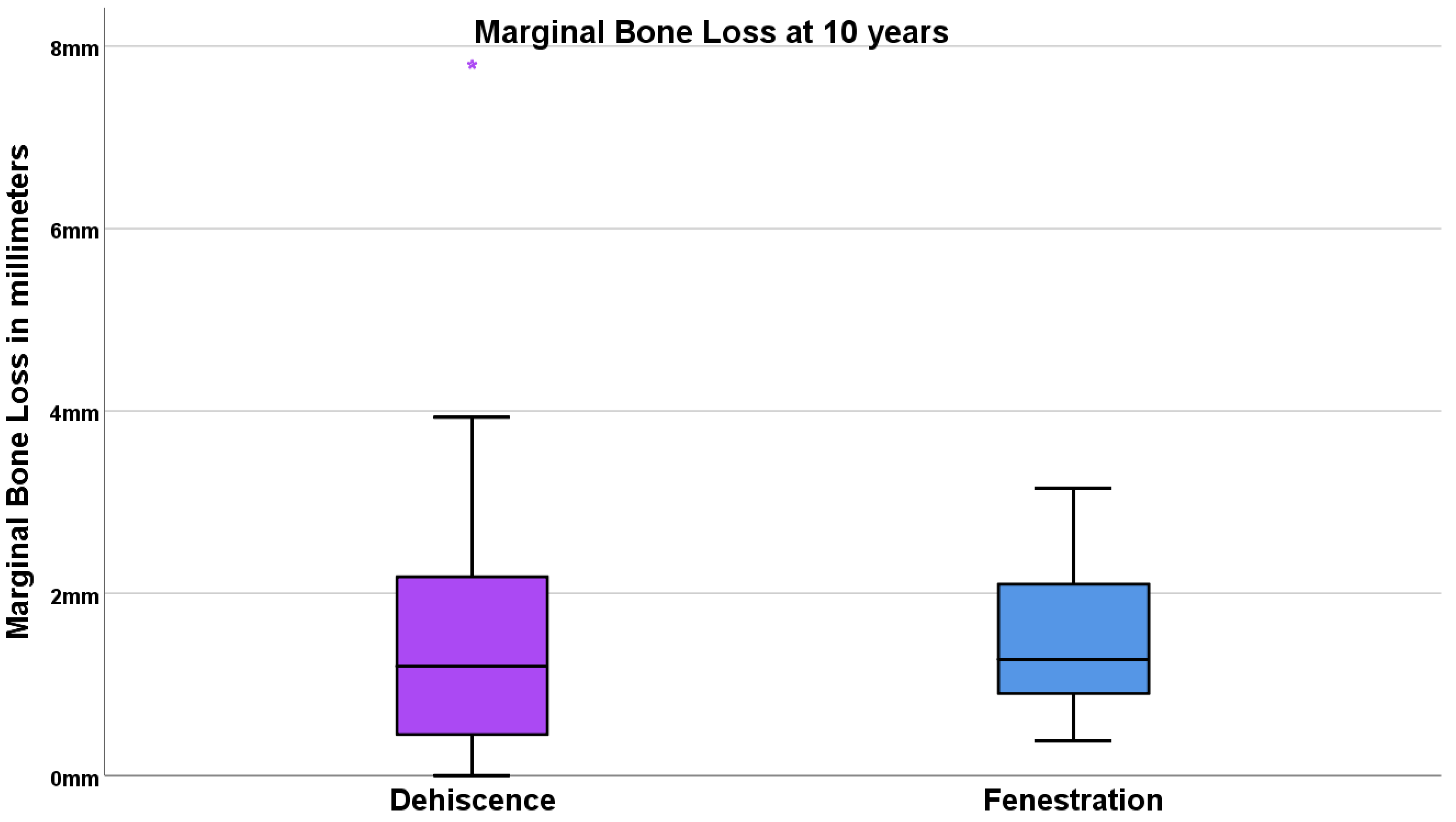
3.3. Marginal Bone Loss
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumie Yaguinuma Gonçalves, G.; Murieli Ferreira de Magalhães, K.; Passos Rocha, E.; Henrique dos Santos, P.; Gonçalves Assunção, W. Oral health-related quality of life and satisfaction in edentulous patients rehabilitated with implant-supported full dentures all-on-four concept: A systematic review. Clin. Oral Investig. 2022, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Testori, T.; Kekovic, V.; Goker, F.; Tumedei, M.; Wang, H.-L. Clinical Medicine A Systematic Review of Survival Rates of Osseointegrated Implants in Fully and Partially Edentulous Patients Following Immediate Loading. J. Clin. Med. 2019, 8, 2142. [Google Scholar] [CrossRef] [Green Version]
- Maló, P.; de Araújo Nobre, M.; Gonçalves, Y.; Lopes, A. Long-Term Outcome of Implant Rehabilitations in Patients with Systemic Disorders and Smoking Habits: A Retrospective Clinical Study. Clin. Implant Dent. Relat. Res. 2016, 18, 649–665. [Google Scholar] [CrossRef]
- Maló, P.; De Araújo Nobre, M.; Lopes, A.; Queridinha, B.; Ferro, A.; Gravito, I. Axial implants in immediate function for partial rehabilitation in the maxilla and mandible: A retrospective clinical study evaluating the long-term outcome (Up to 10 Years). Implant Dent. 2015, 24, 557–564. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Gravito, I. Single-Tooth Rehabilitations Supported by Dental Implants Used in an Immediate-Provisionalization Protocol: Report on Long-Term Outcome with Retrospective Follow-Up. Clin. Implant Dent. Relat. Res. 2015, 17, e511-9. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Nunes, M. The All-on-4 concept for full-arch rehabilitation of the edentulous maxillae: A longitudinal study with 5–13 years of follow-up. Clin. Implant Dent. Relat. Res. 2019, 21, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Ferro, A.; Botto, J. The All-on-4 treatment concept for the rehabilitation of the completely edentulous mandible: A longitudinal study with 10 to 18 years of follow-up. Clin. Implant Dent. Relat. Res. 2019, 21, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, T.; Köndell, P.Å.; Gynther, G.W.; Fredholm, U.; Bolin, A. Implant treatment without bone grafting in severely resorbed edentulous maxillae. J. Oral Maxillofac. Surg. 1999, 57, 281–287. [Google Scholar] [CrossRef]
- Bogaerde, L.; Rangert, B.; Wendelhag, I. Immediate/early function of Brånemark System TiUnite implants in fresh extraction sockets in maxillae and posterior mandibles: An 18-month prospective clinical study. Clin. Implant Dent. Relat. Res. 2005, 7, s121–s130. [Google Scholar] [CrossRef]
- Malo, P.; de Araújo Nobre, M.; Rangert, B. Implants placed in immediate function in periodontally compromised sites: A five-year retrospective and one-year prospective study. J. Prosthet. Dent. 2007, 97, S86–S95. [Google Scholar] [CrossRef]
- Malo, P.; De Araújo Nobre, M.; Lopes, A. Immediate rehabilitation of completely edentulous arches with a four-implant prosthesis concept in difficult conditions: An open cohort study with a mean follow-up of 2 years. Int. J. Oral Maxillofac. Implant. 2012, 27, 1177–1190. [Google Scholar]
- Gross, M.D.; Nissan, J. Stress distribution around maxillary implants in anatomic photoelastic models of varying geometry. Part II. J. Prosthet. Dent. 2001, 85, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterwyck, H.; Duyck, J.; Vander Sloten, J.; Van Der Perre, G.; Naert, I. Peri-implant bone tissue strains in cases of dehiscence: A finite element study. Clin. Oral Implant. Res. 2002, 13, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Herzog, M.; Wolleb, K.; Ramel, C.F.; Thoma, D.S.; Hämmerle, C.H.F. A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clin. Oral Implant. Res. 2017, 28, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Siciliano, V.I.; Salvi, G.E.; Matarasso, S.; Cafiero, C.; Blasi, A.; Lang, N.P. Soft tissues healing at immediate transmucosal implants placed into molar extraction sites with buccal self-contained dehiscences. A 12-month controlled clinical trial. Clin. Oral Implant. Res. 2009, 20, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.; Herzog, M.; Thoma, D.S.; Hüsler, J.; Hämmerle, C.H.F.; Jung, R.E. Long-term clinical and radiographic results after treatment or no treatment of small buccal bone dehiscences at posterior dental implants: A randomized, controlled clinical trial. Clin. Oral Implant. Res. 2020, 31, 517–525. [Google Scholar] [CrossRef]
- Boven, G.C.; Meijer, H.J.A.; Slot, W.; Vissink, A.; Raghoebar, G.M. Does a large dehiscent implant surface at placement affect the 5-year treatment outcome? An assessment of implants placed to support a maxillary overdenture. J. Craniomaxillofac. Surg. 2015, 43, 1758–1762. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: A systematic review. Clin. Oral Implant. Res. 2009, 20, S113–S123. [Google Scholar] [CrossRef]
- Aloy-Prósper, A.; Peñarrocha-Oltra, D.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Dental implants with versus without peri-implant bone defects treated with guided bone regeneration. J. Clin. Exp. Dent. 2015, 7, e361–e368. [Google Scholar] [CrossRef]
- Merli, M.; Merli, I.; Raffaelli, E.; Pagliaro, U.; Nastri, L.; Nieri, M. Bone augmentation at implant dehiscences and fenestrations. A systematic review of randomised controlled trials. Eur. J. Oral Implantol. 2016, 9, 11–32. [Google Scholar]
- Steier, L.; Steier, G. Successful dental implant placement surgeries with buccal bone fenestrations. J. Oral Implantol. 2015, 41, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Maló, P.S.; de Araújo Nobre, M.A.; Ferro, A.S.; Parreira, G.G. Five-year outcome of a retrospective cohort study comparing smokers vs. Nonsmokers with full-arch mandibular implant-supported rehabilitation using the All-on-4 concept. J. Oral Sci. 2018, 60, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Araújo Nobre, M.; Mano Azul, A.; Rocha, E.; Maló, P.; Salvado, F. Attributable fractions, modifiable risk factors and risk stratification using a risk score for peri-implant pathology. J. Prosthodont. Res. 2017, 61, 43–53. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Disease, Version 11. Published 2021. Available online: https://icd.who.int/browse11/l-m/en (accessed on 12 October 2021).
- Annibali, S.; Ripari, M.; La Monaca, G.; Tonoli, F.; Cristalli, M.P. Local accidents in dental implant surgery: Prevention and treatment. Int. J. Periodontics Restor. Dent. 2009, 29, 325–331. [Google Scholar]
- Misch, K.; Wang, H.L. Implant surgery complications: Etiology and treatment. Implant. Dent. 2008, 17, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Piattelli, A.; Scarano, A.; Balleri, P.; Favero, G. Clinical and histologic evaluation of an active “implant periapical lesion”: A case report. Int. J. Oral Maxillofac. Implant. 1998, 13, 713–716. [Google Scholar]
- Alfadda, S.A. Current Evidence on Dental Implants Outcomes in Smokers and Nonsmokers: A Systematic Review and Meta-Analysis. J. Oral Implantol. 2018, 44, 390–399. [Google Scholar] [CrossRef]
- Jung, R.E.; Brügger, L.V.; Bienz, S.P.; Hüsler, J.; Hämmerle, C.H.F.; Zitzmann, N.U. Clinical and radiographical performance of implants placed with simultaneous guided bone regeneration using resorbable and nonresorbable membranes after 22–24 years, a prospective, controlled clinical trial. Clin. Oral Implant. Res. 2021, 32, 1455–1465. [Google Scholar] [CrossRef]
- Skelly, A.; Dettori, J.; Brodt, E. Assessing bias: The importance of considering confounding. Evid. Based Spine Care J. 2012, 3, 9–12. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Nobre, M.A.; Malo, P.; Oliveira, S. The influence of implant location and position characteristics on peri-implant pathology. Eur. J. Prosthodont. Restor. Dent. 2014, 22, 125–129. [Google Scholar]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-Implant Bone Resorption during Healing Abutment Placement: The Effect of a 0.20% Chlorhexidine Gel vs. Placebo—A Randomized Double Blind Controlled Human Study. Biomed. Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef] [PubMed] [Green Version]

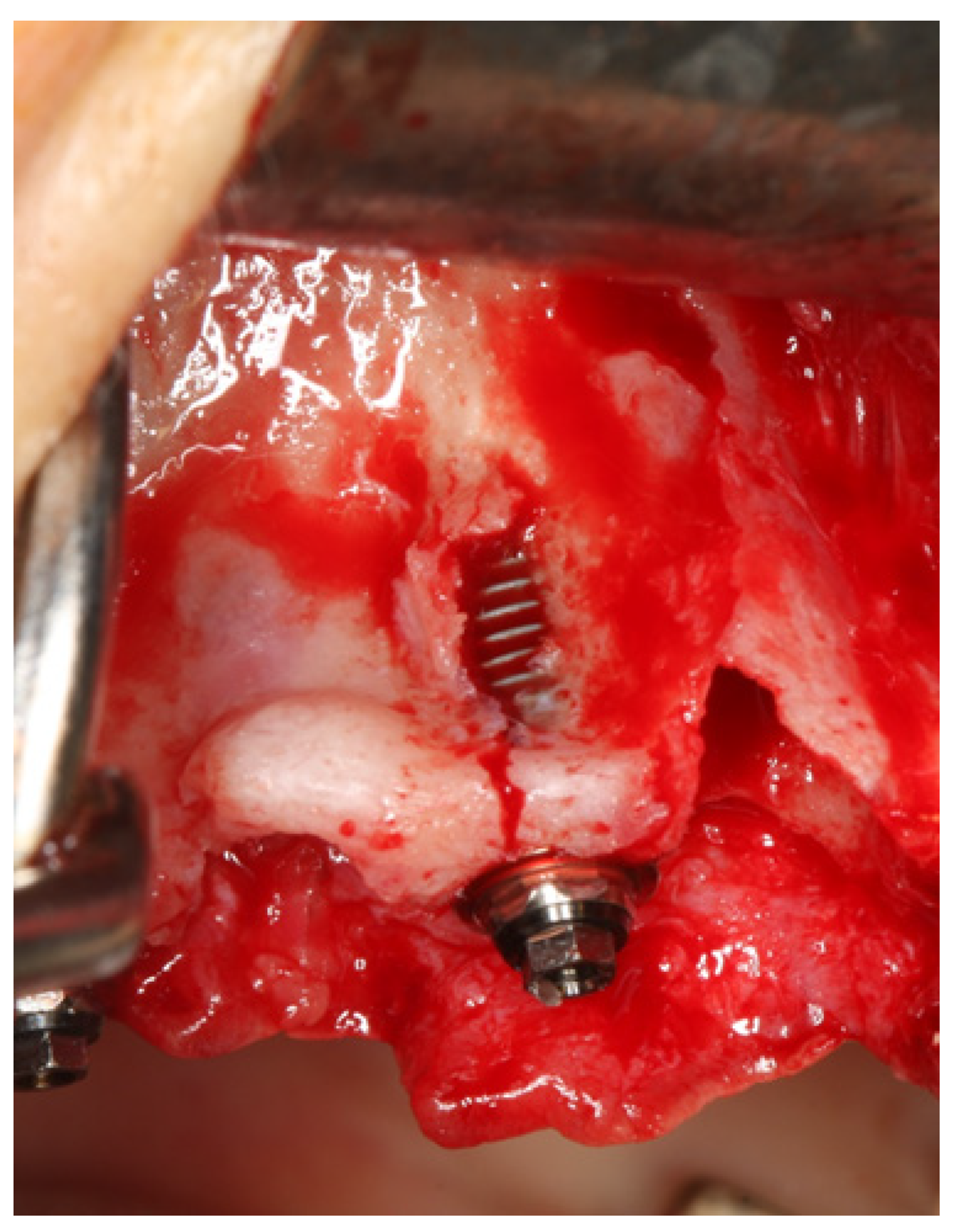
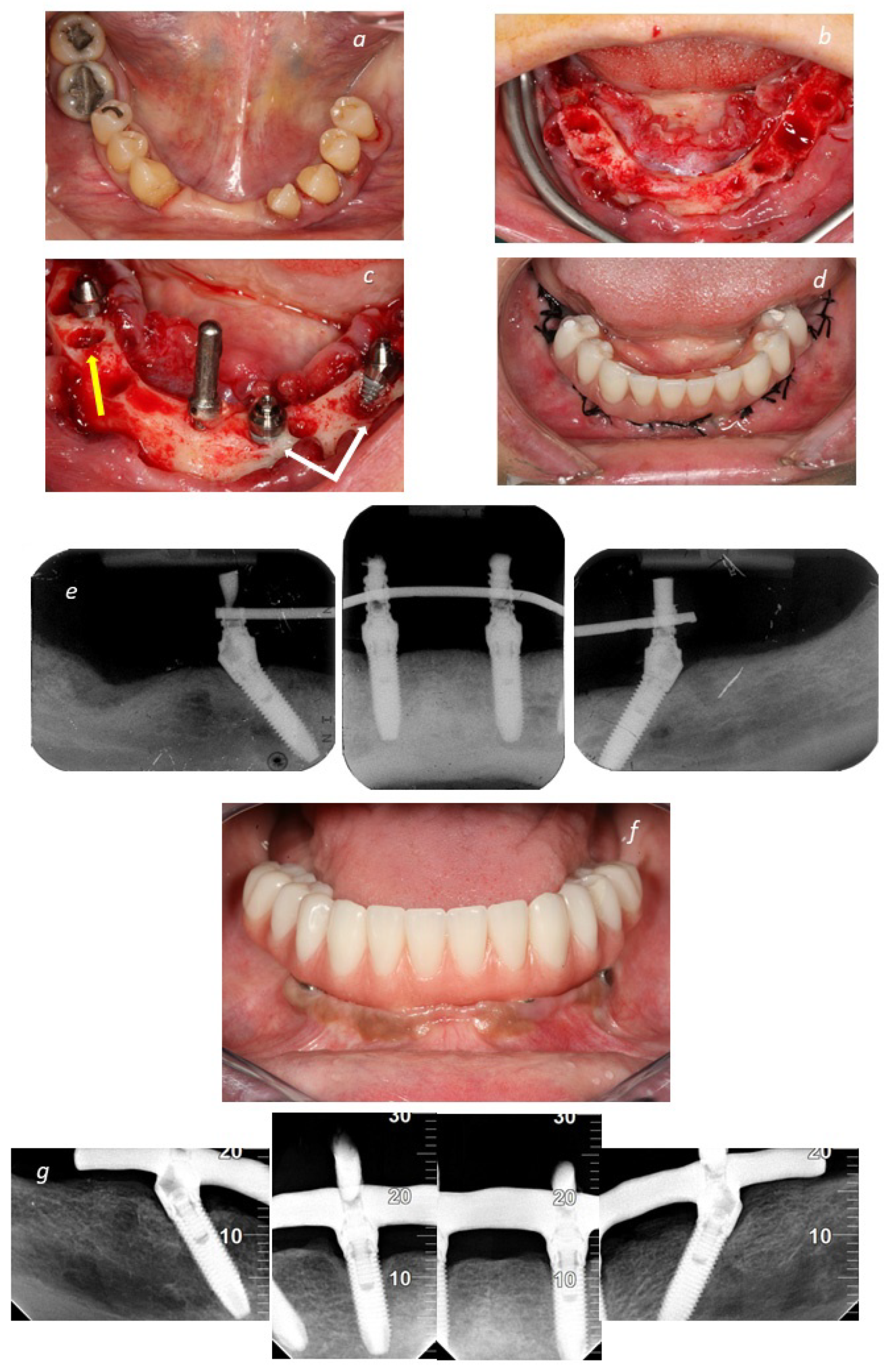
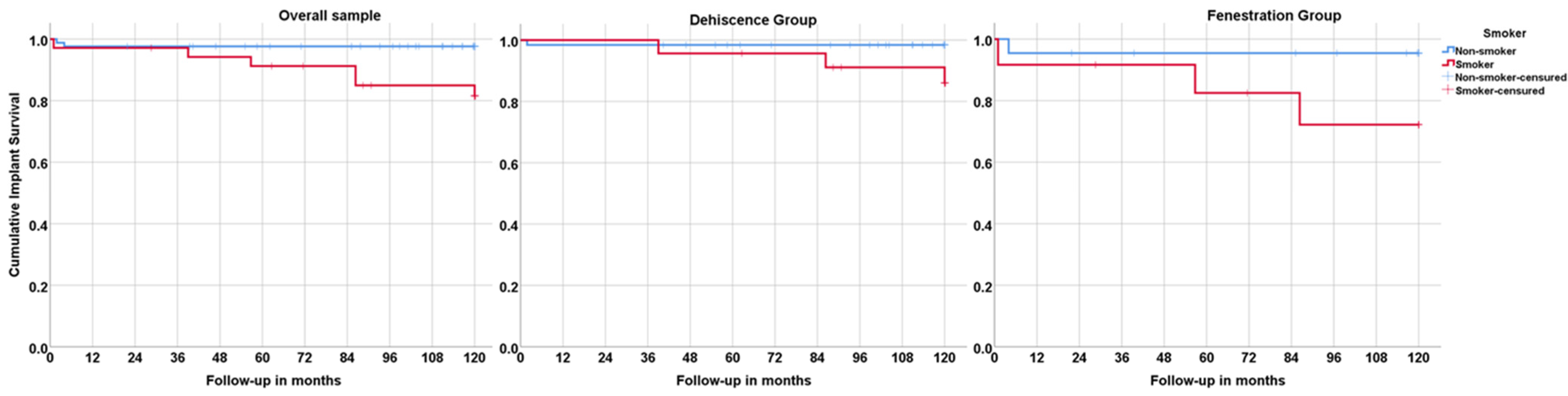
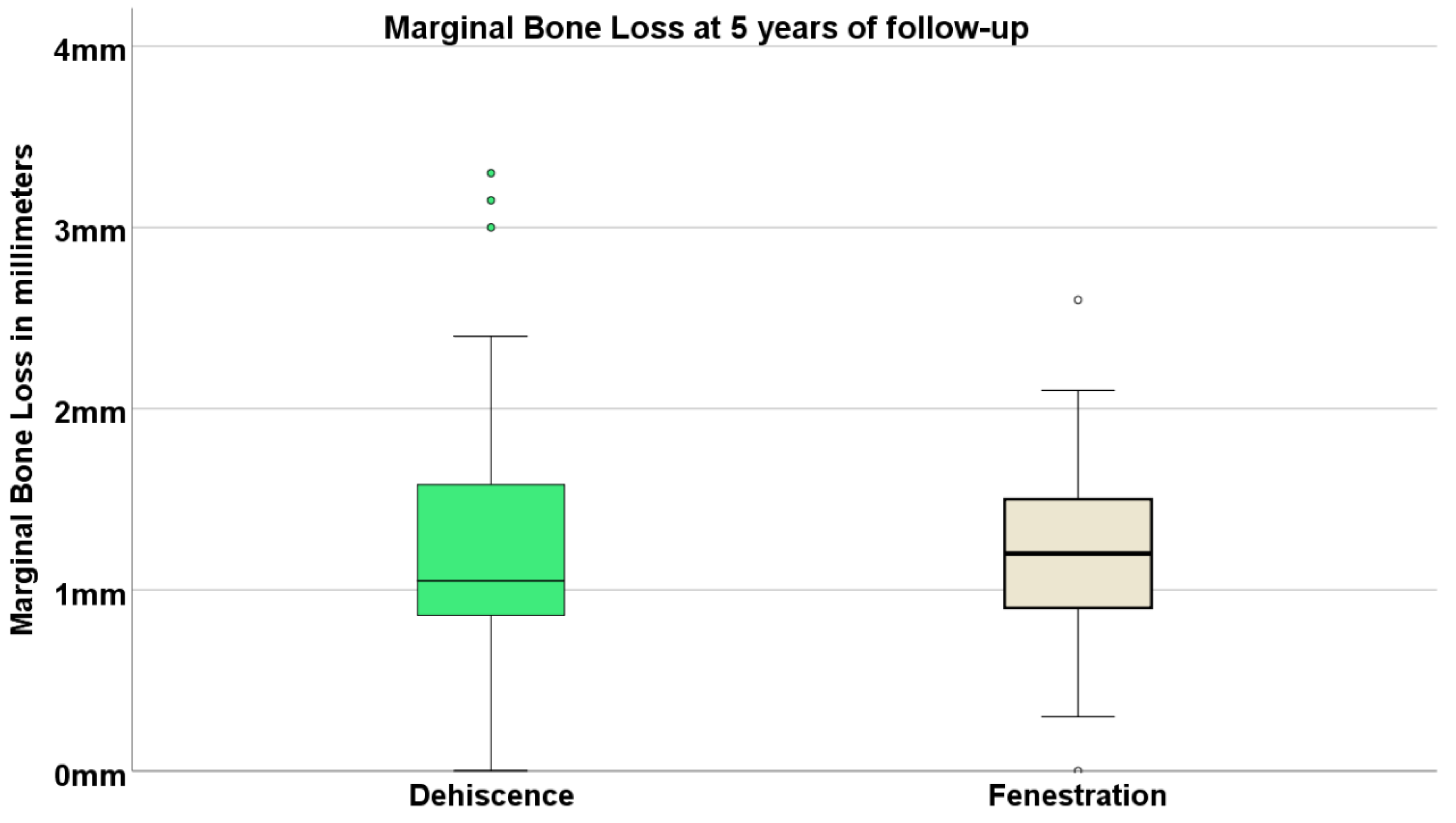
| ICD-11 Classification | ICD-11 Group Description | Examples | n Patients | |
|---|---|---|---|---|
| 1 | Certain infectious or parasitic diseases | (HIV, hepatitis) | 2 | |
| 2 | Neoplasms | (Cancer) | 2 | |
| 4 | Diseases of the immune system | (Lupus) | 1 | |
| 5 | Endocrine, nutritional, or metabolic diseases | (Diabetes, Hyperthyroidism) | 12 | |
| 6 | Mental, behavioral, or neurodevelopmental disorders | (Depressive disorder) | 3 | |
| 8 | Diseases of the nervous system | (Multiple sclerosis, epilepsy) | 2 | |
| 11 | Diseases of the circulatory system | (Hypertension, angina) | 31 | |
| 12 | Diseases of the respiratory system | (Asthma) | 1 | |
| 13 | Diseases of the digestive system | (Heavy bruxer) | 3 | |
| 15 | Diseases of the musculoskeletal system or connective tissue | (Osteoporosis) | 6 | |
| 16 | Diseases of the genitourinary system | (Prostate disorder) | 1 | |
| 18 | Pregnancy, childbirth or the puerperium | (Hysterectomy) | 2 | |
| 24 | Factors influencing health status | (Smoking) | 42 | |
| 26 | Nasal sinusitis disorder | (Sinusitis) | 1 | |
| Healthy | — | 44 | ||
| Totals | * 25 patients presented with more than a single condition in a total of 79 patients. | 123 * | ||
| Implant Distribution According to Platform and Length | ||||
| Type | Diameter | Length | n Implants (n Lost; Position; Follow-Up; Group) | |
| Mk III | 3.75 | 15 mm | 1 (1; #42; 2 months; dehiscence) | |
| 4 | 15 mm | 9 | ||
| Mk IV | 4 | 15 mm | 4 (1; #22; 2 months; fenestration) | |
| NobelSpeedy Replace | 3.5 mm | 15 mm | 1 | |
| NobelSpeedy Groovy | 3.3 | 10 mm | 1 | |
| 11.5 mm | 4 | |||
| 13 mm | 1 | |||
| 15 mm | 1 | |||
| 4 | 8.5 mm | 5 (1; #12; 73 months; dehiscence) | ||
| 10 mm | 3 | |||
| 11.5 mm | 14 | |||
| 13 mm | 23 (1; #44; 86 months; dehiscence-fenestration) | |||
| 15 mm | 72 (1; #44; 4 months; fenestration) | |||
| 18 mm | 53 (2; (#15; 39 months; dehiscence) (#25; 57 months; fenestration)) | |||
| Total | 192 (7) | |||
| Total | Dehiscence | Fenestration | |||||
|---|---|---|---|---|---|---|---|
| Total | Vestibular | Lingual/Palatal | Total | Vestibular | Lingual/Palatal | ||
| Number of patients 1 | 109 | 95 | 46 | 53 | 36 | 28 | 9 |
| Number of patients (smokers) 2 | 35 | 25 | 14 | 12 | 13 | 9 | 4 |
| Number of implants 3 (lost) | 192 | 152 | 71 (4 4) | 83 | 42 | 35 (3) | 7 (2 4) |
| Time (Months) | Status (0 = Survival; 1 = Failure) | Cumulative Proportion Surviving at the Time | N of Cumulative Events | N of Patients at Risk | |
|---|---|---|---|---|---|
| Estimate | Std. Error | ||||
| Total Sample | |||||
| 0 | 0 | . | . | 0 | 123 |
| 1 | 1 | 0.992 | 0.008 | 1 | 122 |
| 2 | 1 | 0.984 | 0.011 | 2 | 121 |
| 4 | 1 | 0.976 | 0.014 | 3 | 120 |
| 24 | 0 | . | . | 3 | 119 |
| 29 | 0 | . | . | 3 | 118 |
| 39 | 1 | 0.967 | 0.016 | 4 | 117 |
| 40 | 0 | . | . | 4 | 115 |
| 48 | 0 | . | . | 4 | 113 |
| 55 | 0 | . | . | 4 | 112 |
| 57 | 1 | 0.959 | 0.018 | 5 | 111 |
| 60 | 0 | . | . | 5 | 110 |
| 72 | 0 | . | . | 5 | 106 |
| 73 | 1 | 0.950 | 0.020 | 6 | 105 |
| 86 | 1 | 0.941 | 0.022 | 7 | 104 |
| 96 | 0 | . | . | 7 | 100 |
| 108 | 0 | . | . | 7 | 95 |
| 120 | 0 | . | . | 7 | 90 |
| Dehiscence Group | |||||
| 0 | 0 | . | . | 0 | 95 |
| 2 | 1 | 0.989 | 0.010 | 1 | 94 |
| 39 | 1 | 0.979 | 0.015 | 2 | 93 |
| 48 | 0 | . | . | 2 | 90 |
| 60 | 0 | . | . | 2 | 88 |
| 72 | 0 | . | . | 2 | 85 |
| 73 | 1 | 0.967 | 0.019 | 3 | 84 |
| 86 | 1 | 0.956 | 0.022 | 4 | 83 |
| 96 | 0 | . | . | 4 | 79 |
| 108 | 0 | . | . | 4 | 75 |
| 111 | 0 | . | . | 4 | 73 |
| 117 | 0 | . | . | 4 | 71 |
| 120 | 0 | . | . | 4 | 70 |
| Fenestration Group | |||||
| 0 | 0 | . | . | 0 | 36 |
| 1 | 1 | 0.972 | 0.027 | 1 | 35 |
| 4 | 1 | 0.944 | 0.038 | 2 | 34 |
| 24 | 0 | . | . | 2 | 33 |
| 36 | 0 | . | . | 2 | 32 |
| 48 | 0 | . | . | 2 | 31 |
| 56 | 1 | 0.914 | 0.048 | 3 | 30 |
| 72 | 0 | . | . | 3 | 29 |
| 85 | 0 | . | . | 3 | 28 |
| 86 | 1 | 0.881 | 0.056 | 4 | 27 |
| 117 | 0 | . | . | 4 | 25 |
| 120 | 0 | . | . | 4 | 24 |
| Dehiscence Group | |||||
| Time (Months) | Number of Implants | Number Lost to Follow-Up | Number of Failures | Survival Rate | Cumulative Survival Rate |
| Baseline | 152 | 0 | 1 | 99.3% | 99.3% |
| 1 year | 151 | 0 | 0 | 100% | 99.3% |
| 2 years | 151 | 0 | 0 | 100% | 99.3% |
| 3 years | 151 | 5 | 1 | 99.3% | 98.7% |
| 4 years | 145 | 4 | 0 | 100% | 98.7% |
| 5 years | 141 | 3 | 0 | 100% | 98.7% |
| 6 years | 138 | 0 | 1 | 99.3% | 98.0% |
| 7 years | 137 | 6 | 1 | 99.3% | 97.2% |
| 8 years | 130 | 5 | 0 | 100% | 97.2% |
| 9 years | 125 | 6 | 0 | 100% | 97.2% |
| 10 years | 119 | 0 | 0 | 100% | 97.2% |
| Fenestration Group | |||||
| Time (Months) | Number of Implants | Number Lost to Follow-Up | Number of Failures | Survival Rate | Cumulative Survival Rate |
| Baseline | 42 | 0 | 2 | 95.2% | 95.2% |
| 1 year | 40 | 1 | 0 | 100% | 95.2% |
| 2 years | 39 | 1 | 0 | 100% | 95.2% |
| 3 years | 38 | 1 | 0 | 100% | 95.2% |
| 4 years | 37 | 0 | 1 | 97.3% | 92.7% |
| 5 years | 36 | 1 | 0 | 100% | 92.7% |
| 6 years | 35 | 0 | 0 | 100% | 92.7% |
| 7 years | 35 | 1 | 1 | 97.1% | 90.0% |
| 8 years | 33 | 1 | 0 | 100% | 90.0% |
| 9 years | 32 | 2 | 0 | 100% | 90.0% |
| 10 years | 30 | 0 | 0 | 100% | 90.0% |
| Dehiscence Group | |||||
| Time (Months) | Number of Implants | Number Lost to Follow-Up | Number of Failures | Survival Rate | Cumulative Survival Rate |
| Baseline | 152 | 0 | 1 | 99.3% | 99.3% |
| 1 year | 151 | 0 | 3 | 98.0% | 97.4% |
| 2 years | 148 | 0 | 1 | 99.3% | 96.7% |
| 3 years | 147 | 5 | 0 | 100% | 96.7% |
| 4 years | 142 | 4 | 1 | 99.3% | 96.0% |
| 5 years | 137 | 3 | 0 | 100% | 96.0% |
| 6 years | 134 | 0 | 1 | 99.3% | 95.3% |
| 7 years | 133 | 6 | 1 | 99.2% | 94.6% |
| 8 years | 126 | 5 | 0 | 100% | 94.6% |
| 9 years | 121 | 5 | 0 | 100% | 94.6% |
| 10 years | 116 | 0 | 0 | 100% | 94.6% |
| Fenestration Group | |||||
| Time (Months) | Number of Implants | Number Lost to Follow-Up | Number of Failures | Survival Rate | Cumulative Survival Rate |
| Baseline | 42 | 0 | 2 | 95.2% | 95.2% |
| 1 year | 40 | 1 | 0 | 100% | 95.2% |
| 2 years | 39 | 1 | 1 | 97.4% | 92.8% |
| 3 years | 37 | 1 | 0 | 100% | 92.8% |
| 4 years | 36 | 0 | 1 | 97.2% | 90.2% |
| 5 years | 35 | 0 | 0 | 100% | 90.2% |
| 6 years | 35 | 1 | 1 | 97.1% | 87.6% |
| 7 years | 33 | 1 | 0 | 100% | 87.6% |
| 8 years | 32 | 1 | 0 | 100% | 87.6% |
| 9 years | 31 | 1 | 0 | 100% | 87.6% |
| 10 years | 30 | 0 | 0 | 100% | 87.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo Nobre, M.; Lopes, A.; Antunes, E. The 10 Year Outcomes of Implants Inserted with Dehiscence or Fenestrations in the Rehabilitation of Completely Edentulous Jaws with the All-on-4 Concept. J. Clin. Med. 2022, 11, 1939. https://doi.org/10.3390/jcm11071939
de Araújo Nobre M, Lopes A, Antunes E. The 10 Year Outcomes of Implants Inserted with Dehiscence or Fenestrations in the Rehabilitation of Completely Edentulous Jaws with the All-on-4 Concept. Journal of Clinical Medicine. 2022; 11(7):1939. https://doi.org/10.3390/jcm11071939
Chicago/Turabian Stylede Araújo Nobre, Miguel, Armando Lopes, and Elsa Antunes. 2022. "The 10 Year Outcomes of Implants Inserted with Dehiscence or Fenestrations in the Rehabilitation of Completely Edentulous Jaws with the All-on-4 Concept" Journal of Clinical Medicine 11, no. 7: 1939. https://doi.org/10.3390/jcm11071939
APA Stylede Araújo Nobre, M., Lopes, A., & Antunes, E. (2022). The 10 Year Outcomes of Implants Inserted with Dehiscence or Fenestrations in the Rehabilitation of Completely Edentulous Jaws with the All-on-4 Concept. Journal of Clinical Medicine, 11(7), 1939. https://doi.org/10.3390/jcm11071939







