Update of Pediatric Lipomatous Lesions: A Clinicopathological, Immunohistochemical and Molecular Overview
Abstract
1. Introduction
2. Lipoblastoma and Lipoblastomatosis
3. Lipomatosis
3.1. Congenital Infiltrating Lipomatosis of the Face (CILF)
3.2. Diffuse Lipomatosis (DL)
3.3. Encephalocraniocutaneous Lipomatosis (ECCL)
3.4. Michelin Tire Baby Syndrome (MTBS)
3.5. PTEN Hamartoma of Soft Tissue (PHOST)
3.6. PIK3CA-Related Overgrowth Spectrum (PROS)
3.7. Nevus Lipomatosis Superficialis (NLS)
3.8. Lipomatosis of Nerve (LN)
4. Liposarcoma
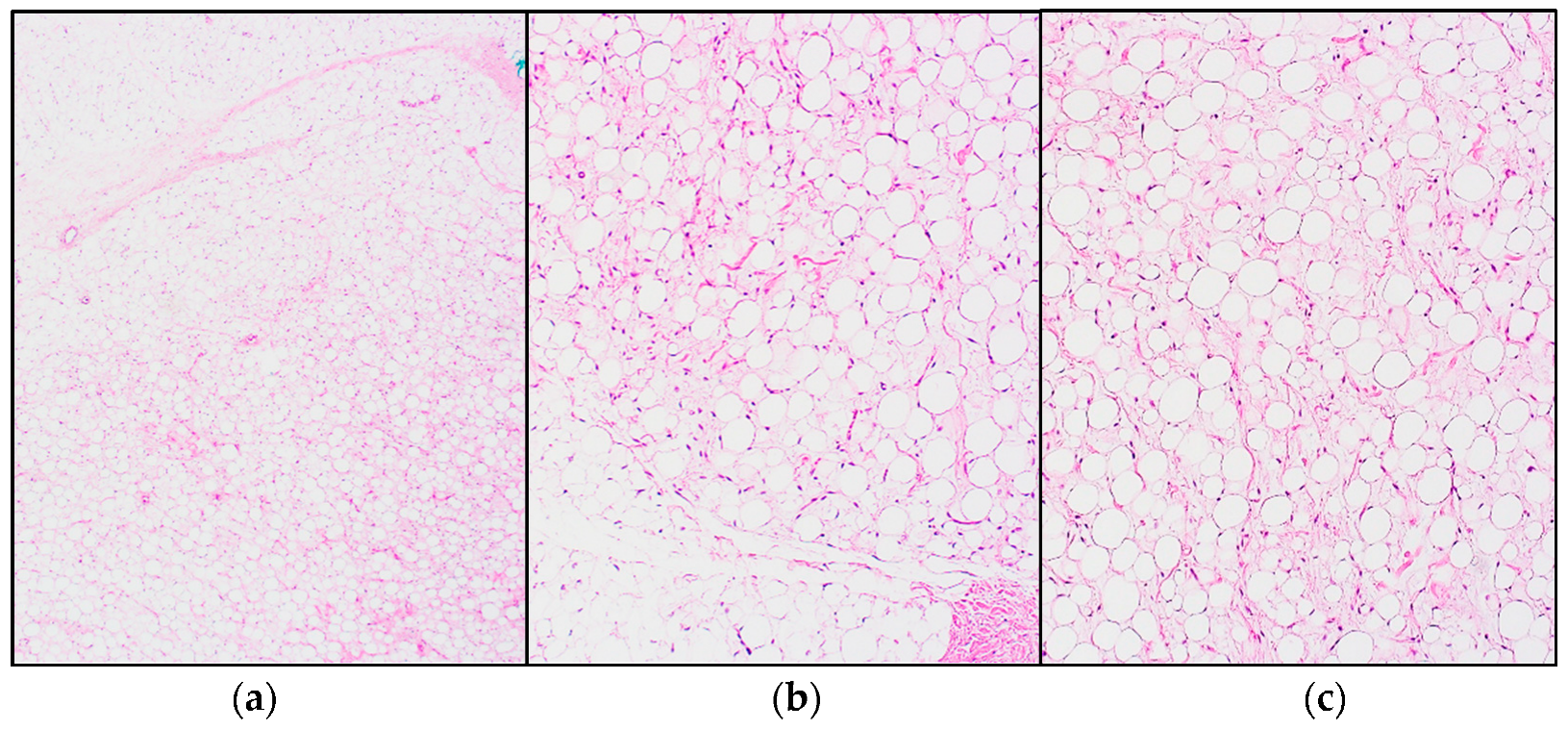
4.1. Well-Differentiated Liposaroma (WDLPS)/Atypical Lipomatous Tumor (ALT)
- Adipocytic (lipoma-like) is the most frequent subtype. It is composed of mature adipocytes with substantial variation in cell size, as well as cytonuclear atypia in adipocytes and/or stromal spindle cells. MDM2 and CDK4 immunohistochemical expression are typical, though in some cases difficult to evaluate, making fluorescence in situ hybridization (FISH) a valid alternative [10].
- The sclerosing subtype is most often seen in cases located in the retroperitoneum or spermatic cord. Scattered, bizarre stromal cells with marked nuclear hyperchromasia are seen, set in an extensive fibrillary collagenous stroma. The fibrous component may overshadow lipogenic areas, making it easy to miss in a small (biopsy) sample [10].
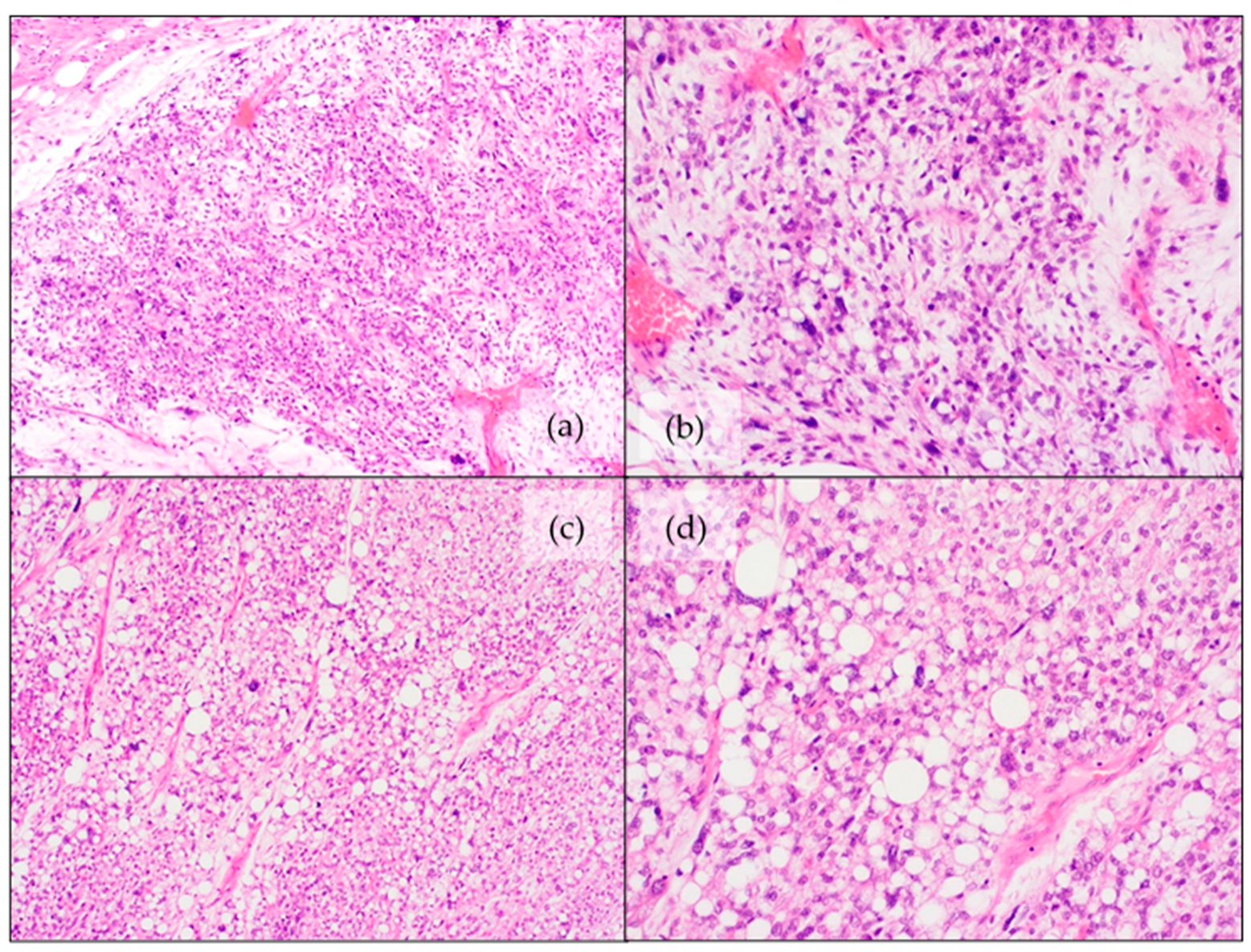
4.2. Dedifferentiated Liposarcoma (DDLPS)
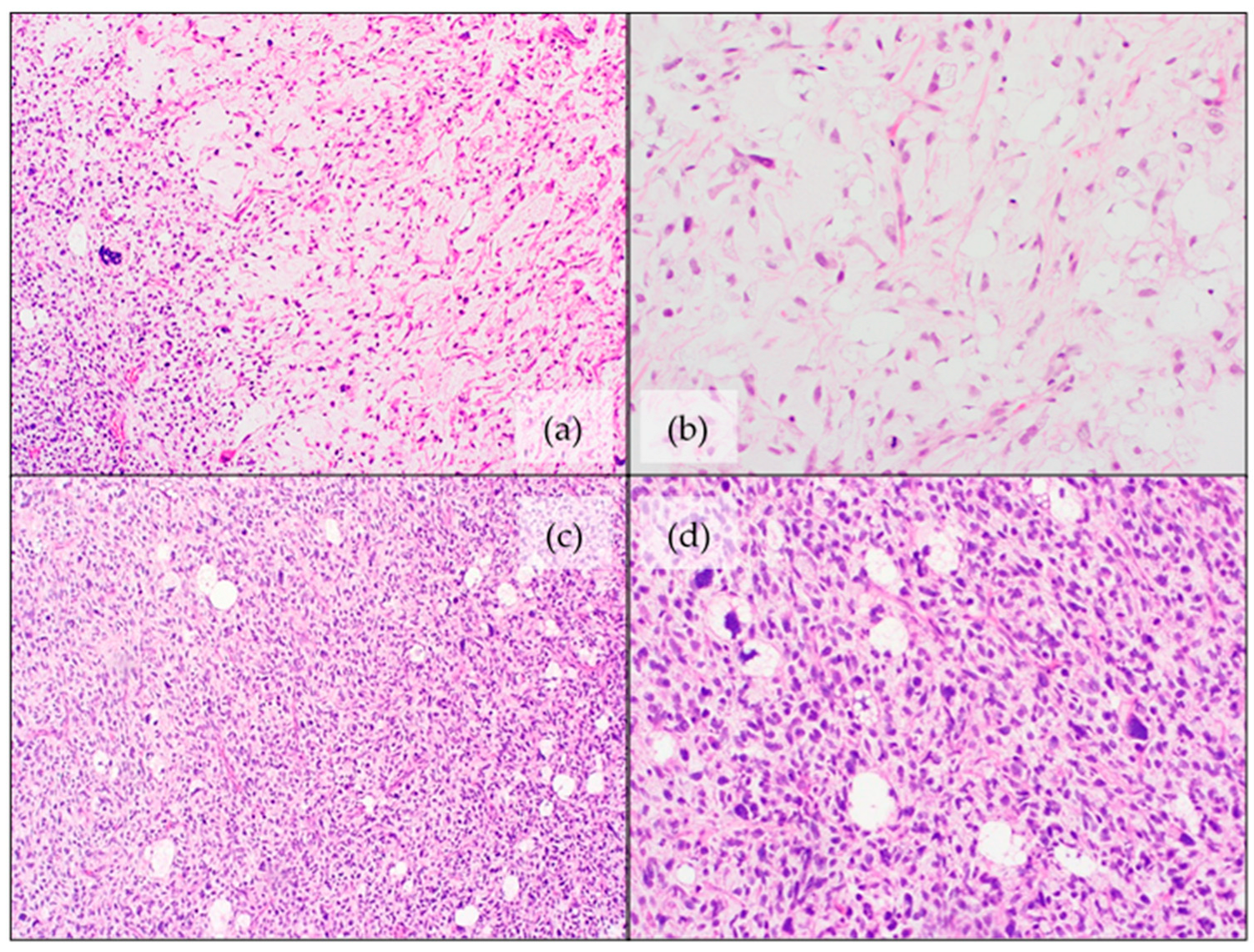
4.3. Myxoid Liposarcoma (MLPS)
4.4. Pleomorphic Liposarcoma (PLPS)
- Pleomorphic/spindle cell areas with “malignant fibrous histiocytoma-like” appearance with scattered lipoblasts. This is the most common pattern, found in approximately two-thirds of the cases.
- Approximately one quarter of the cases show an epithelioid morphology with scattered lipoblasts.
4.5. Myxoid Pleomorphic Liposarcoma (MPLPS)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffin, C.M.; Alaggio, R. Adipose and Myxoid Tumors of Childhood and Adolescence. Pediatr. Dev. Pathol. 2012, 15, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-W.; Kim, H.; Youn, J.K.; Oh, C.; Jung, S.-E.; Park, K.-W.; Lee, S.-C.; Kim, H.-Y. Analysis of clinical features of lipoblastoma in children. Pediatr. Hematol. Oncol. 2017, 34, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L.; Inwards, C.Y. Bone and Soft Tissue Pathology E-Book: A Volume in the Foundations in Diagnostic Pathology Series, 2nd ed.; Goldblum, J.R., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2022; p. 544. [Google Scholar]
- Coffin, M.C.; Lowichik, A.; Putnam, A. Lipoblastoma (LPB): A clinicopathologic and immunohistochemical analysis of 59 cases. Am. J. Surg. Pathol. 2009, 33, 1705–1712. [Google Scholar] [CrossRef]
- Mentzel, T.; Calonje, E.; Fletcher, C.D. Lipoblastoma and lipoblastomatosis: A clinicopathological study of 14 cases. Histopathology 1993, 23, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghafar, J.; Ahmad, Z.; Tariq, M.U.; Kayani, N.; Uddin, N. Lipoblastoma: A clinicopathologic review of 23 cases from a major tertiary care center plus detailed review of literature. BMC Res. Notes 2018, 11, 42. [Google Scholar] [CrossRef]
- Collins, M.H.; Chatten, J. Lipoblastoma/Lipoblastomatosis: A Clinicopathologic Study of 25 Tumors. Am. J. Surg. Pathol. 1997, 21, 1131–1137. [Google Scholar] [CrossRef]
- Vellios, F.; Baez, J.; Shumacker, H.B. Lipoblastomatosis: A tumor of fetal fat different from hibernoma; report of a case, with observations on the embryogenesis of human adipose tissue. Am. J. Pathol. 1958, 34, 1149–1159. [Google Scholar]
- Dehner, L.P.; Gru, A.A. Nonepithelial Tumors and Tumor-like Lesions of the Skin and Subcutis in Children. Pediatr. Dev. Pathol. 2018, 21, 150–207. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. New WHO Classification of Pediatric Tumours; WHO: Geneva, Switzerland, 2022; Forthcoming. [Google Scholar]
- Ozsen, M.; Yalcinkaya, U.; Yazici, Z.; Sarisozen, M.B. Lipomatous Tumors in Pediatric Patients: A Retrospective Analysis of 50 cases. Turk. J. Pathol. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Sen, H.S.; Yalcin, B.; Kutluk, T.; Tanyel, F.C.; Haliloglu, M.; Orhan, D.; Aydin, B.; Kurucu, N.; Varan, A.; Akyuz, C.; et al. Lipoblastoma in children: Review of 12 cases. Pediatr. Int. 2017, 59, 545–550. [Google Scholar] [CrossRef]
- Eyssartier, E.; Villemagne, T.; Maurin, L.; Machet, M.C.; Lardy, H. Intrascrotal lipoblastoma: A report of two cases and a review of the literature. J. Pediatr. Urol. 2013, 9, e151–e154. [Google Scholar] [CrossRef] [PubMed]
- Amra, N.K.; Amr, S.S. Mediastinal Lipoblastomatosis: Report of a Case with Complex Karyotype and Review of the Literature. Pediatr. Dev. Pathol. 2009, 12, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Fanna, M.; Rougemont, A.-L.; Arni, D.; Toso, C.; Anooshiravani-Dumont, M.; Wildhaber, B.E. Giant Intrahepatic Lipoblastoma in a Child. J. Pediatr. 2019, 210, 235–236.e1. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shakya, U.; Sayami, G.; Rajbanshi, B.G. Lipoblastoma: An unusual tumour of the left ventricle. Eur. J. Cardio-Thorac. Surg. 2016, 49, e147–e148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, S.-M.; Chang, P.-Y.; Luo, C.-C.; Huang, C.-S.; Lai, J.-Y.; Hsueh, C. Lipoblastoma/lipoblastomatosis: A clinicopathologic study of 16 cases in Taiwan. Pediatr. Surg. Int. 2005, 21, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Dishop, M.K.; O’Connor, W.N.; Abraham, S.; Cottrill, C.M. Primary cardiac lipoblastoma. Pediatr. Dev. Pathol. 2001, 4, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Kanu, A.; Oermann, C.M.; Malicki, D.; Wagner, M.; Langston, C. Pulmonary lipoblastoma in an 18-month-old child: A unique tumor in children. Pediatr. Pulmonol. 2002, 34, 150–154. [Google Scholar] [CrossRef]
- Mathew, J.; Sen, S.; Chandi, S.M.; Kumar, N.K.S.; Zachariah, N.; Chacko, J.; Thomas, G. Pulmonary lipoblastoma: A case report. Pediatr. Surg. Int. 2001, 17, 543–544. [Google Scholar] [CrossRef]
- Nagano, Y.; Uchida, K.; Inoue, M.; Ide, S.; Shimura, T.; Hashimoto, K.; Koike, Y.; Kusunoki, M. Mesenteric lipoblastoma presenting as a small intestinal volvulus in an infant: A case report and literature review. Asian J. Surg. 2017, 40, 70–73. [Google Scholar] [CrossRef]
- Brundler, M.-A.; Kurek, K.C.; Patel, K.; Jester, I. Submucosal Colonic Lipoblastoma Presenting With Colo-colonic Intussusception in an Infant. Pediatr. Dev. Pathol. 2017, 21, 401–405. [Google Scholar] [CrossRef]
- Jaafar, R.; Tang, I.P.; Jong, D.E.; Narihan, M.Z. Cervical Lipoblastoma: An Uncommon Presentation. Ear. Nose Throat J. 2015, 94, E8–E10. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Kundu, S.; Roy, S.; Mukhopadhyay, S. Lipoblastomatosis of the retropharyngeal space: Pathogenesis, presentation, and management, with a focus on head-neck lipoblastoma(toses). B-ENT 2016, 12, 33–39. [Google Scholar]
- Pham, N.S.; Poirier, B.; Fuller, S.C.; Dublin, A.B.; Tollefson, T.T. Pediatric lipoblastoma in the head and neck: A systematic review of 48 reported cases. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.; Grenda, E.; Hatziagorou, E.; Tsikopoulos, G.; Foroulis, C.N.; Georgopoulou, V.; Anastasiou, A.; Tsiviki, E.; Tsanakas, J. Chest wall lipoblastoma in a 3 year-old boy. Respir. Med. Case Rep. 2019, 26, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Miyano, G.; Hayashi, T.; Arakawa, A.; Goto, S.; Lane, G.J.; Okazaki, T.; Yamataka, A. Giant omental lipoblastoma and CD56 expression. Afr. J. Paediatr. Surg. 2013, 10, 32–34. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, G.; Wadhwa, C.; Bansal, H.; Kaushal, R. A rare primary dumbbell lipoblastoma. Asian J. Neurosurg. 2018, 13, 83–85. [Google Scholar] [CrossRef]
- Peter, S.; Matevž, S.; Borut, P. Spinal dumbbell lipoblastoma: A case-based update. Child’s Nerv. Syst. 2016, 32, 2069–2073. [Google Scholar] [CrossRef]
- Reiseter, T.; Nordshus, T.; Borthne, A.; Roald, B.; Naess, P.; Schistad, O. Lipoblastoma: MRI appearances of a rare paediatric soft tissue tumour. Pediatr. Radiol. 1999, 29, 542–545. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chang, W.-C.; Lee, H.-S.; Ko, K.-H.; Chang, C.-C.; Huang, G.-S. MRI features of lipoblastoma: Differentiating from other palpable lipomatous tumor in pediatric patients. Clin. Imaging 2010, 34, 453–457. [Google Scholar] [CrossRef]
- Chung, E.B.; Enzinger, F.M. Benign lipoblastomatosis. An analysis of 35 cases. Cancer 1973, 32, 482–492. [Google Scholar] [CrossRef]
- Putra, J.; Al-Ibraheemi, A. Adipocytic tumors in Children: A contemporary review. Semin. Diagn. Pathol. 2019, 36, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bolen, J.W.; Thorning, D. Benign lipoblastoma and myxoid liposarcoma: A comparative light- and electron-microscopic study. Am. J. Surg. Pathol. 1980, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, T.; Masumoto, K.; Ono, K.; Yano, E.; Kobayashi, C.; Fukushima, T.; Sumazaki, R.; Satomi, K.; Noguchi, M. A case of unusual histology of infantile lipoblastoma confirmed by PLAG1 rearrangement. Surg. Case Rep. 2015, 1, 42. [Google Scholar] [CrossRef][Green Version]
- Craver, R.D.; Henrich, S.; Kao, Y.S. Fibrous lipoblastoma with 8q11.2 abnormality. Cancer Genet. Cytogenet. 2006, 171, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Fritchie, K.; Wang, L.; Yin, Z.; Nakitandwe, J.; Hedges, D.; Horvai, A.; Mora, J.T.; Folpe, A.L.; Bahrami, A. Lipoblastomas presenting in older children and adults: Analysis of 22 cases with identification of novel PLAG1 fusion partners. Mod. Pathol. 2021, 34, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nunez, O.; Alaggio, R.; Ranganathan, S.; Schmitt, L.; John, I.; Church, A.J.; Picarsic, J. New molecular insights into the pathogenesis of lipoblastomas: Clinicopathologic, immunohistochemical, and molecular analysis in pediatric cases. Hum. Pathol. 2020, 104, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; D’Amore, E.S.; Dall’Igna, P.; Guzzardo, V.; Vassarotto, E.; Rugge, M.; Alaggio, R. Immunohistochemical expression of p16 in lipoblastomas. Hum. Pathol. 2016, 47, 64–69. [Google Scholar] [CrossRef]
- Fallon, S.C.; Brandt, M.L.; Rodriguez, J.R.; Vasudevan, S.A.; Lopez, M.E.; Hicks, M.J.; Kim, E.S. Cytogenetic analysis in the diagnosis and management of lipoblastomas: Results from a single institution. J. Surg. Res. 2013, 184, 341–346. [Google Scholar] [CrossRef]
- Bartuma, H.; Domanski, H.; Von Steyern, F.V.; Kullendorff, C.-M.; Mandahl, N.; Mertens, F. Cytogenetic and molecular cytogenetic findings in lipoblastoma. Cancer Genet. Cytogenet. 2008, 183, 60–63. [Google Scholar] [CrossRef]
- Dadone, B.; Refae, S.; Lemarié-Delaunay, C.; Bianchini, L.; Pedeutour, F. Molecular cytogenetics of pediatric adipocytic tumors. Cancer Genet. 2015, 208, 469–481. [Google Scholar] [CrossRef]
- Gisselsson, D.; Hibbard, M.K.; Cin, P.D.; Sciot, R.; Hsi, B.-L.; Kozakewich, H.P.; Fletcher, J.A. PLAG1 Alterations in Lipoblastoma: Involvement in Varied Mesenchymal Cell Types and Evidence for Alternative Oncogenic Mechanisms. Am. J. Pathol. 2001, 159, 955–962. [Google Scholar] [CrossRef]
- Meloni-Ehrig, A.M.; Riggott, L.; Christacos, N.C.; Mowrey, P.N.; Johal, J. A case of lipoblastoma with seven copies of chromosome 8. Cancer Genet. Cytogenet. 2009, 190, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Somerhausen, N.D.S.A.; Coindre, J.M.J.; Debiec-Rychter, M.; Delplace, J.; Sciot, R. Lipoblastoma in adolescents and young adults: Report of six cases with FISH analysis. Histopathology 2008, 52, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.; Rademaker, M. A collection of rare anomalies: Multiple digital glomuvenous malformations, epidermal naevus, temporal alopecia, heterochromia and abdominal lipoblastoma. Clin. Exp. Dermatol. 2009, 34, e862–e864. [Google Scholar] [CrossRef] [PubMed]
- Nitta, Y.; Miyachi, M.; Tomida, A.; Sugimoto, Y.; Nakagawa, N.; Yoshida, H.; Ouchi, K.; Tsuchiya, K.; Iehara, T.; Konishi, E.; et al. Identification of a novel BOC-PLAG1 fusion gene in a case of lipoblastoma. Biochem. Biophys. Res. Commun. 2019, 512, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Miyachi, M.; Ouchi, K.; Kuwahara, Y.; Tsuchiya, K.; Iehara, T.; Konishi, E.; Yanagisawa, A.; Hosoi, H. Identification ofCOL3A1andRAB2Aas novel translocation partner genes ofPLAG1in lipoblastoma. Genes Chromosom. Cancer 2014, 53, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Deen, M.; Ebrahim, S.; Schloff, D.; Mohamed, A.N. A novel PLAG1-RAD51L1 gene fusion resulting from a t(8;14)(q12;q24) in a case of lipoblastoma. Cancer Genet. 2013, 206, 233–237. [Google Scholar] [CrossRef]
- Brinkman, A.S.; Maxfield, B.; Gill, K.; Patel, N.J.; Gosain, A. A novel t(3;8)(p13;q21.1) translocation in a case of lipoblastoma. Pediatr. Surg. Int. 2012, 28, 737–740. [Google Scholar] [CrossRef]
- Krsková, L.; Němečková, T.; Balko, J.; Brož, P.; Vícha, A. Novel ZEB2-PLAG1 fusion gene identified by RNA sequencing in a case of lipoblastoma. Pediatr. Blood Cancer 2021, 68, 28691. [Google Scholar] [CrossRef]
- Giovannoni, I.; Barresi, S.; Rossi, S.; Stracuzzi, A.; Argentieri, M.G.; Alaggio, R. Pediatric lipoblastoma with a novel EEF1A1-PLAG1 fusion. Genes Chromosom. Cancer 2021, 60, 525–526. [Google Scholar] [CrossRef]
- Hibbard, M.K.; Kozakewich, H.P.; Cin, P.D.; Sciot, R.; Tan, X.; Xiao, S.; A Fletcher, J. PLAG1 fusion oncogenes in lipoblastoma. Cancer Res. 2000, 60, 4869–4872. [Google Scholar] [PubMed]
- Morerio, C.; Rapella, A.; Rosanda, C.; Tassano, E.; Gambini, C.; Romagnoli, G.; Panarello, C. PLAG1-HAS2 fusion in lipoblastoma with masked 8q intrachromosomal rearrangement. Cancer Genet. Cytogenet. 2005, 156, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.; Turpin, B.K.; Mark, M.; Smolarek, T.A.; Li, X. Undifferentiated myxoid lipoblastoma with PLAG1–HAS2 fusion in an infant; morphologically mimicking primitive myxoid mesenchymal tumor of infancy (PMMTI)—Diagnostic importance of cytogenetic and molecular testing and literature review. Cancer Genet. 2016, 209, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Speer, A.L.; Schofield, D.E.; Wang, K.S.; Shin, C.E.; Stein, J.E.; Shaul, D.B.; Mahour, G.H.; Ford, H.R. Contemporary management of lipoblastoma. J. Pediatr. Surg. 2008, 43, 1295–1300. [Google Scholar] [CrossRef]
- Séguier-Lipszyc, E.; Baazov, A.; Fichman, S.; Ash, S.; Freud, E. Current management of lipoblastoma. Eur. J. Pediatr. 2018, 177, 237–241. [Google Scholar] [CrossRef]
- Sun, R.; Sun, L.; Li, G.; Sun, Z.; Zhao, Y.; Ma, X.; Sun, C. Congenital infiltrating lipomatosis of the face: A subtype of hemifacial hyperplasia. Int. J. Pediatr. Otorhinolaryngol. 2019, 125, 107–112. [Google Scholar] [CrossRef]
- Loconte, D.C.; Grossi, V.; Bozzao, C.; Forte, G.; Bagnulo, R.; Stella, A.; Lastella, P.; Cutrone, M.; Benedicenti, F.; Susca, F.C.; et al. Molecular and Functional Characterization of Three Different Postzygotic Mutations in PIK3CA-Related Overgrowth Spectrum (PROS) Patients: Effects on PI3K/AKT/mTOR Signaling and Sensitivity to PIK3 Inhibitors. PLoS ONE 2015, 10, e0123092. [Google Scholar] [CrossRef]
- Pagliazzi, A.; Oranges, T.; Traficante, G.; Trapani, C.; Facchini, F.; Martin, A.; Semeraro, A.; Perrone, A.; Filippeschi, C.; Giglio, S. PIK3CA-Related Overgrowth Spectrum From Diagnosis to Targeted Therapy: A Case of CLOVES Syndrome Treated With Alpelisib. Front. Pediatr. 2021, 9, 732836. [Google Scholar] [CrossRef]
- Sahai, S.; Rajan, S.; Singh, N.; Arora, H. Congenital infiltrating lipomatosis of the face with exophytic temporomandibular joint ankylosis: A case report and review of the literature. Dentomaxillofac. Radiol. 2013, 42, 16128745. [Google Scholar] [CrossRef]
- Kamal, D.; Breton, P.; Bouletreau, P. Congenital infiltrating lipomatosis of the face: Report of three cases and review of the literature. J. Cranio-Maxillofac. Surg. 2010, 38, 610–614. [Google Scholar] [CrossRef]
- Blackburn, P.R.; Milosevic, D.; Marek, T.; Folpe, A.L.; Howe, B.M.; Spinner, R.J.; Carter, J.M. PIK3CA mutations in lipomatosis of nerve with or without nerve territory overgrowth. Mod. Pathol. 2020, 33, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Barr, R.J. Diffuse lipomatosis and tuberous sclerosis. Arch. Dermatol. 1986, 122, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Happle, R.; Torrelo, A. Superimposed mosaicism in tuberous sclerosis complex: A key to understanding all of the manifold manifestations? J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Vinay, K.; De, D.; Handa, S.; Sinha, A. Tuberous Sclerosis Complex and Diffuse Lipomatosis: Case Report of a Rare Association. Indian Dermatol. Online J. 2018, 9, 37–39. [Google Scholar]
- Bavle, A.; Shah, R.; Gross, N.; Gavula, T.; Ruiz-Elizalde, A.; Wierenga, K.; McNall-Knapp, R. Encephalocraniocutaneous Lipomatosis. J. Pediatric Hematol./Oncol. 2018, 40, 553–554. [Google Scholar] [CrossRef]
- Siddiqui, S.; Naaz, S.; Ahmad, M.; Khan, Z.A.; Wahab, S.; Rashid, B.A. Encephalocraniocutaneous lipomatosis: A case report with review of literature. Neuroradiol. J. 2017, 30, 578–582. [Google Scholar] [CrossRef]
- Valera, E.T.; McConechy, M.K.; Gayden, T.; Rivera, B.; Jones, D.T.W.; Wittmann, A.; Han, H.; Bareke, E.; Nikbakht, H.; Mikael, L.; et al. Methylome analysis and whole-exome sequencing reveal that brain tumors associated with encephalocraniocutaneous lipomatosis are midline pilocytic astrocytomas. Acta Neuropathol. 2018, 136, 657–660. [Google Scholar] [CrossRef]
- Han, J.-Y.; Yum, M.-S.; Kim, E.-H.; Hong, S.; Ko, T.-S. A rare case of dysembryoplastic neuroepithelial tumor combined with encephalocraniocutaneous lipomatosis and intractable seizures. Korean J. Pediatr. 2016, 59, S139–S144. [Google Scholar] [CrossRef]
- Rothman, I.L. Michelin Tire Baby Syndrome: A Review of the Literature and a Proposal for Diagnostic Criteria with Adoption of the Name Circumferential Skin Folds Syndrome. Pediatr. Dermatol. 2014, 31, 659–663. [Google Scholar] [CrossRef]
- Kurek, K.C.; Howard, E.; Tenant, L.B.; Upton, J.; Alomari, A.I.; Burrows, P.E.; Chalache, K.; Harris, D.J.; Trenor, C.C.; Eng, C.; et al. PTEN hamartoma of soft tissue: A distinctive lesion in PTEN syndromes. Am. J. Surg. Pathol. 2012, 36, 671–687. [Google Scholar] [CrossRef]
- Keppler-Noreuil, K.M.; Sapp, J.C.; Lindhurst, M.J.; Parker, V.E.; Blumhorst, C.; Darling, T.; Tosi, L.L.; Huson, S.M.; Whitehouse, R.W.; Jakkula, E.; et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am. J. Med. Genet. Part A 2014, 164, 1713–1733. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Rios, J.J.; Parker, V.E.; Semple, R.; Lindhurst, M.J.; Sapp, J.; Alomari, A.; Ezaki, M.; Dobyns, W.; Biesecker, L.G. PIK3CA -related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am. J. Med. Genet. Part A 2015, 167, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.A.; Konczyk, D.; Vivero, M.P.; Kozakewich, H.P.; Upton, J.; Fu, X.; Padwa, B.L.; Mulliken, J.B.; Warman, M.L.; Greene, A.K. Somatic PIK3CA mutations are present in multiple tissues of facial infiltrating lipomatosis. Pediatr. Res. 2017, 82, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Goucha, S.; Khaled, A.; Zéglaoui, F.; Rammeh, S.; Zermani, R.; Fazaa, B. Nevus lipomatosus cutaneous superficialis: Report of eight cases. Dermatol. Ther. 2011, 1, 25–30. [Google Scholar] [CrossRef]
- Peng, R.; Li, N.; Lan, T.; Chen, H.; Du, T.; He, X.; Chen, M.; Xie, Y.; Zhang, Z.; Zhao, W.; et al. Liposarcoma in children and young adults: A clinicopathologic and molecular study of 23 cases in one of the largest institutions of China. Virchows Arch. 2021, 479, 537–549. [Google Scholar] [CrossRef]
- Huh, W.W.; Yuen, C.; Munsell, M.; Hayes-Jordan, A.; Lazar, A.J.; Patel, S.; Wang, W.-L.; Barahmani, N.; Okcu, M.F.; Hicks, J.; et al. Liposarcoma in children and young adults: A multi-institutional experience. Pediatr. Blood Cancer 2011, 57, 1142–1146. [Google Scholar] [CrossRef]
- Alaggio, R.; Coffin, C.M.; Weiss, S.W.; Bridge, J.A.; Issakov, J.; Oliveira, A.M.; Folpe, A.L. Liposarcomas in young patients: A study of 82 cases occurring in patients younger than 22 years of age. Am. J. Surg. Pathol. 2009, 33, 645–658. [Google Scholar] [CrossRef]
- Baday, Y.I.; Navai, S.A.; Hicks, M.J.; Venkatramani, R.; Whittle, S.B. Pediatric liposarcoma: A case series and literature review. Pediatr. Blood Cancer 2021, 68, e29327. [Google Scholar] [CrossRef]
- La Quaglia, M.P.; Spiro, S.A.; Ghavimi, F.; Hajdu, S.I.; Meyers, P.; Exelby, P.R. Liposarcoma in patients younger than or equal to 22 years of age. Cancer 1993, 72, 3114–3119. [Google Scholar] [CrossRef]
- Stanelle, E.J.; Christison-Lagay, E.R.; Sidebotham, E.L.; Singer, S.; Antonescu, C.R.; Meyers, P.A.; La Quaglia, M.P. Prognostic Factors and Survival in Pediatric and Adolescent Liposarcoma. Sarcoma 2012, 2012, 870910. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3, p. 368. [Google Scholar]
- Peng, R.; Chen, H.; Yang, X.; Zhang, X.; Zhang, Z.; He, X.; Zhang, H. A novel sclerosing atypical lipomatous tumor/well-differentiated liposarcoma in a 7-year-old girl: Report of a case with molecular confirmation. Hum. Pathol. 2018, 71, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Debelenko, L.V.; Perez-Atayde, A.R.; Dubois, S.G.; Grier, H.E.; Pai, S.Y.; Shamberger, R.C.; Kozakewich, H.P. p53+/mdm2- atypical lipomatous tumor/well-differentiated liposarcoma in young children: An early expression of Li-Fraumeni syndrome. Pediatric Dev. Pathol. 2010, 13, 218–224. [Google Scholar] [CrossRef]
- Dadone-Montaudié, B.; Burel-Vandenbos, F.; Soler, C.; Rosello, O.; Boyer, C.; Fabas, T.; Bianchini, L.; Pedeutour, F. Double minute chromosomes harboring MDM2 amplification in a pediatric atypical lipomatous tumor. Genes Chromosom. Cancer 2019, 58, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: Liposarcoma. Cancer Genet. Cytogenet. 2004, 155, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Horvai, A.; Greipp, P.; John, I.; Demicco, E.G.; Dickson, B.C.; Tanas, M.R.; Larsen, B.T.; Din, N.U.; Creytens, D.H. Atypical lipomatous tumour/well-differentiated liposarcoma and de-differentiated liposarcoma in patients aged. Histopathology 2019, 75, 833–842. [Google Scholar] [CrossRef]
- Italiano, A.; Bianchini, L.; Gjernes, E.; Keslair, F.; Ranchere-Vince, D.; Dumollard, J.-M.; Haudebourg, J.; Leroux, A.; Mainguené, C.; Terrier, P.; et al. Clinical and Biological Significance of CDK4 Amplification in Well-Differentiated and Dedifferentiated Liposarcomas. Clin. Cancer Res. 2009, 15, 5696–5703. [Google Scholar] [CrossRef]
- Sirvent, N.; Coindre, J.-M.; Maire, G.; Hostein, I.; Keslair, F.; Guillou, L.; Ranchere-Vince, D.; Terrier, P.; Pedeutour, F. Detection of MDM2-CDK4 Amplification by Fluorescence In Situ Hybridization in 200 Paraffin-embedded Tumor Samples: Utility in Diagnosing Adipocytic Lesions and Comparison With Immunohistochemistry and Real-time PCR. Am. J. Surg. Pathol. 2007, 31, 1476–1489. [Google Scholar] [CrossRef]
- Francom, C.R.; Leoniak, S.M.; Lovell, M.A.; Herrmann, B.W. Head and neck pleomorphic myxoid liposarcoma in a child with Li-Fraumeni syndrome. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 191–194. [Google Scholar] [CrossRef]
- Binh, M.B.N.; Sastre-Garau, X.; Guillou, L.; Pinieux, G.d.; Terrier, P.; Lagacé, R.; Aurias, A.; Hostein, I.; Coindre, J.M. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: A comparative analysis of 559 soft tissue neoplasms with genetic data. Am. J. Surg. Pathol. 2005, 29, 1340–1347. [Google Scholar] [CrossRef]
- Sinclair, T.J.; Thorson, C.M.; Alvarez, E.; Tan, S.; Spunt, S.L.; Chao, S.D. Pleomorphic myxoid liposarcoma in an adolescent with Li–Fraumeni syndrome. Pediatr. Surg. Int. 2017, 33, 631–635. [Google Scholar] [CrossRef]
- Zare, S.Y.; Leivo, M.; Fadare, O. Recurrent Pleomorphic Myxoid Liposarcoma in a Patient With Li-Fraumeni Syndrome. Int. J. Surg. Pathol. 2019, 28, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Argani, P.; Facchetti, F.; Inghirami, G.; Rosai, J. Lymphocyte-rich well-differentiated liposarcoma: Report of nine cases. Am. J. Surg. Pathol. 1997, 21, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Kraus, M.D.; Guillou, L.; Fletcher, C.D.M. Well-Differentiated Inflammatory Liposarcoma: An Uncommon and Easily Overlooked Variant of a Common Sarcoma. Am. J. Surg. Pathol. 1997, 21, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.R.; Paulsen, E.B.; Noordhuis, P.; Pedeutour, F.; Saeter, G.; Myklebost, O. Potential for treatment of liposarcomas with the MDM2 antagonist Nutlin-3A. Int. J. Cancer 2007, 121, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bill, K.L.J.; Garnett, J.; Meaux, I.; Ma, X.; Creighton, C.J.; Bolshakov, S.; Barriere, C.; Debussche, L.; Lazar, A.; Prudner, B.C.; et al. SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma. Clin. Cancer Res. 2016, 22, 1150–1160. [Google Scholar] [CrossRef]
- Saâda-Bouzid, E.; Burel-Vandenbos, F.; Ranchère-Vince, D.; Birtwisle-Peyrottes, I.; Chetaille, B.; Bouvier, C.; Château, M.-C.; Peoc’H, M.; Battistella, M.; Bazin, A.; et al. Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod. Pathol. 2015, 28, 1404–1414. [Google Scholar] [CrossRef]
- McCormick, D.; Mentzel, T.; Beham, A.; Fletcher, C.D. Dedifferentiated Liposarcoma Clinicopathologic Analysis of 32 Cases Suggesting a Better Prognostic Subgroup Among Pleomorphic Sarcomas. Am. J. Surg. Pathol. 1994, 18, 1213–1223. [Google Scholar] [CrossRef]
- Weiss, S.W.; Rao, V.K. Well-Differentiated Liposarcoma (Atypical Lipoma) of Deep Soft Tissue of the Extremities, Retroperitoneum, and Miscellaneous Sites. Am. J. Surg. Pathol. 1992, 16, 1051–1058. [Google Scholar] [CrossRef]
- Demicco, E.G. Molecular updates in adipocytic neoplasms. Semin. Diagn. Pathol. 2019, 36, 85–94. [Google Scholar] [CrossRef]
- Thway, K. Well-differentiated liposarcoma and dedifferentiated liposarcoma: An updated review. Semin. Diagn. Pathol. 2019, 36, 112–121. [Google Scholar] [CrossRef]
- Hornick, J.L.; Bosenberg, M.W.; Mentzel, T.; McMenamin, M.E.; Oliveira, A.M.; Fletcher, C.D. Pleomorphic liposarcoma: Clinicopathologic analysis of 57 cases. Am. J. Surg. Pathol. 2004, 28, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Creytens, D. A contemporary review of myxoid adipocytic tumors. Semin. Diagn. Pathol. 2019, 36, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Creytens, D.; van Gorp, J.; Ferdinande, L.; Van Roy, N.; Libbrecht, L. Array-based comparative genomic hybridization analysis of a pleomorphic myxoid liposarcoma. J. Clin. Pathol. 2014, 67, 834–835. [Google Scholar] [CrossRef] [PubMed]
- Creytens, D.; Folpe, A.L.; Koelsche, C.; Mentzel, T.; Ferdinande, L.; van Gorp, J.M.; Van der Linden, M.; Raman, L.; Menten, B.; Fritchie, K.; et al. Myxoid pleomorphic liposarcoma—a clinicopathologic, immunohistochemical, molecular genetic and epigenetic study of 12 cases, suggesting a possible relationship with conventional pleomorphic liposarcoma. Mod. Pathol. 2021, 34, 2043–2049. [Google Scholar] [CrossRef]
- Barretina, J.; Taylor, B.S.; Banerji, S.; Ramos, A.H.; Lagos-Quintana, M.; DeCarolis, P.L.; Shah, K.; Socci, N.D.; Weir, B.A.; Ho, A.; et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat. Genet. 2010, 42, 715–721. [Google Scholar] [CrossRef]
- Creytens, D. What’s new in adipocytic neoplasia? Virchows Arch. 2020, 476, 29–39. [Google Scholar] [CrossRef]
- Hofvander, J.; Jo, V.Y.; Ghanei, I.; Gisselsson, D.; Mårtensson, E.; Mertens, F. Comprehensive genetic analysis of a paediatric pleomorphic myxoid liposarcoma reveals near-haploidization and loss of theRB1gene. Histopathology 2016, 69, 141–147. [Google Scholar] [CrossRef]


| WHO Classification of Pediatric Adipocytic Neoplasms | Most Frequently Associated Cytogenetic/Molecular Characteristics |
|---|---|
| Lipoblastoma/lipoblastomatosis | Structural alteration of chromosome 8q leading to PLAG1 rearrangements |
| Lipomatosis | Germline, mosaic or somatic mutations of PTEN, PIK3CA and TSC |
| Well differentiated liposarcoma/Atypical lipomatous tumor | Amplification of MDM2 and/or CDK4 Except in Li-Fraumeni-associated cases: TP53 germline mutation |
| Dedifferentiated liposarcoma | |
| Myxoid liposarcoma | t(12;16)(q13;p11) translocation, generating FUS-DDIT3 fusion transcripts |
| Pleomorphic liposarcoma | Complex karyotype with multiple (whole chromosomal) gains and losses, most common mutations in TP53 and NF1 |
| Myxoid pleomorphic liposarcoma | Inactivation of RB1 and a complex chromosomal profile with gains and losses of chromosomes with deletions/mutations of TP53, and deletions of KMT2D or NF1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameloot, E.; Cordier, F.; Van Dorpe, J.; Creytens, D. Update of Pediatric Lipomatous Lesions: A Clinicopathological, Immunohistochemical and Molecular Overview. J. Clin. Med. 2022, 11, 1938. https://doi.org/10.3390/jcm11071938
Ameloot E, Cordier F, Van Dorpe J, Creytens D. Update of Pediatric Lipomatous Lesions: A Clinicopathological, Immunohistochemical and Molecular Overview. Journal of Clinical Medicine. 2022; 11(7):1938. https://doi.org/10.3390/jcm11071938
Chicago/Turabian StyleAmeloot, Eline, Fleur Cordier, Jo Van Dorpe, and David Creytens. 2022. "Update of Pediatric Lipomatous Lesions: A Clinicopathological, Immunohistochemical and Molecular Overview" Journal of Clinical Medicine 11, no. 7: 1938. https://doi.org/10.3390/jcm11071938
APA StyleAmeloot, E., Cordier, F., Van Dorpe, J., & Creytens, D. (2022). Update of Pediatric Lipomatous Lesions: A Clinicopathological, Immunohistochemical and Molecular Overview. Journal of Clinical Medicine, 11(7), 1938. https://doi.org/10.3390/jcm11071938






