Factors Influencing Immune Restoration in People Living with HIV/AIDS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Immune Recovery Analyses
2.3. DNA Extraction and Genotyping
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
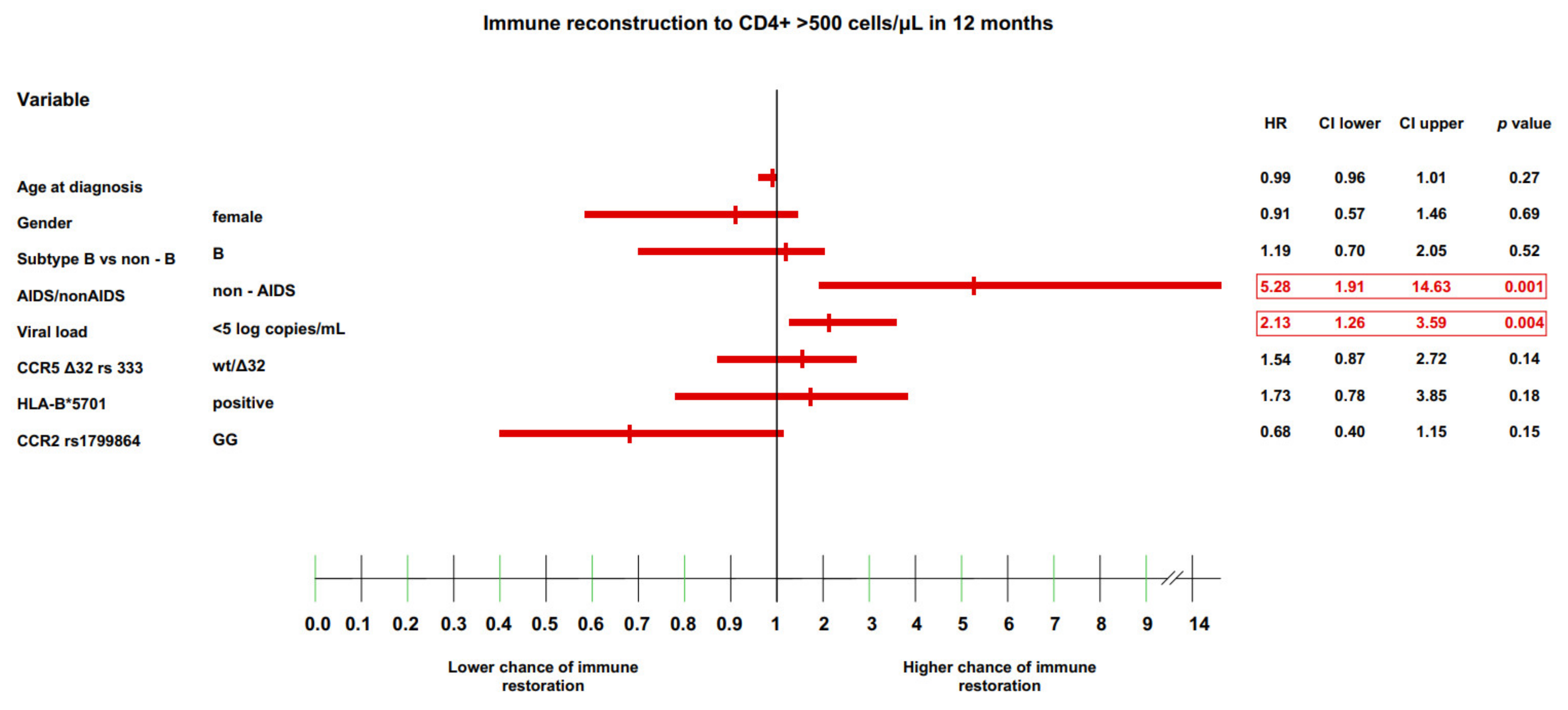
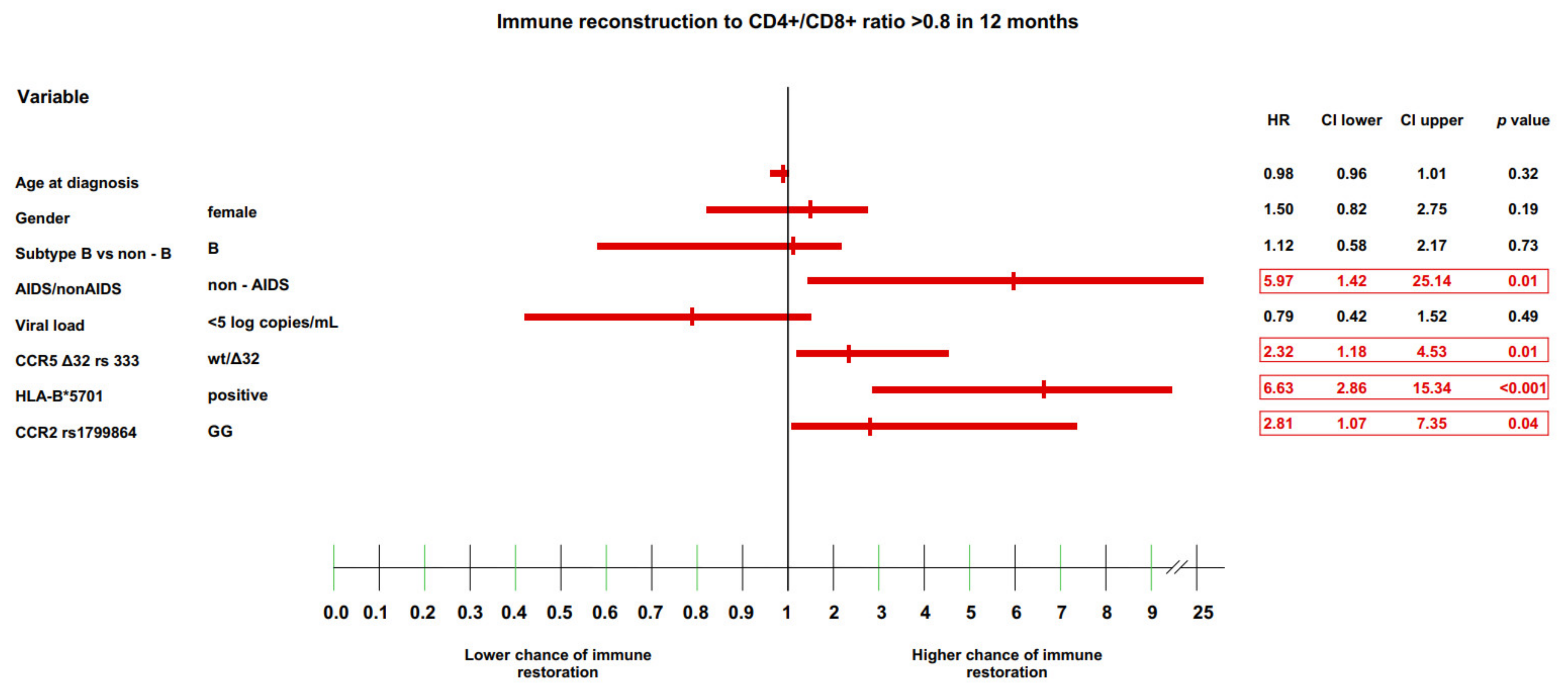
3.2. Genetic Factors and Immune Restoration
3.3. Clinical Factors Associated with Immune Recovery
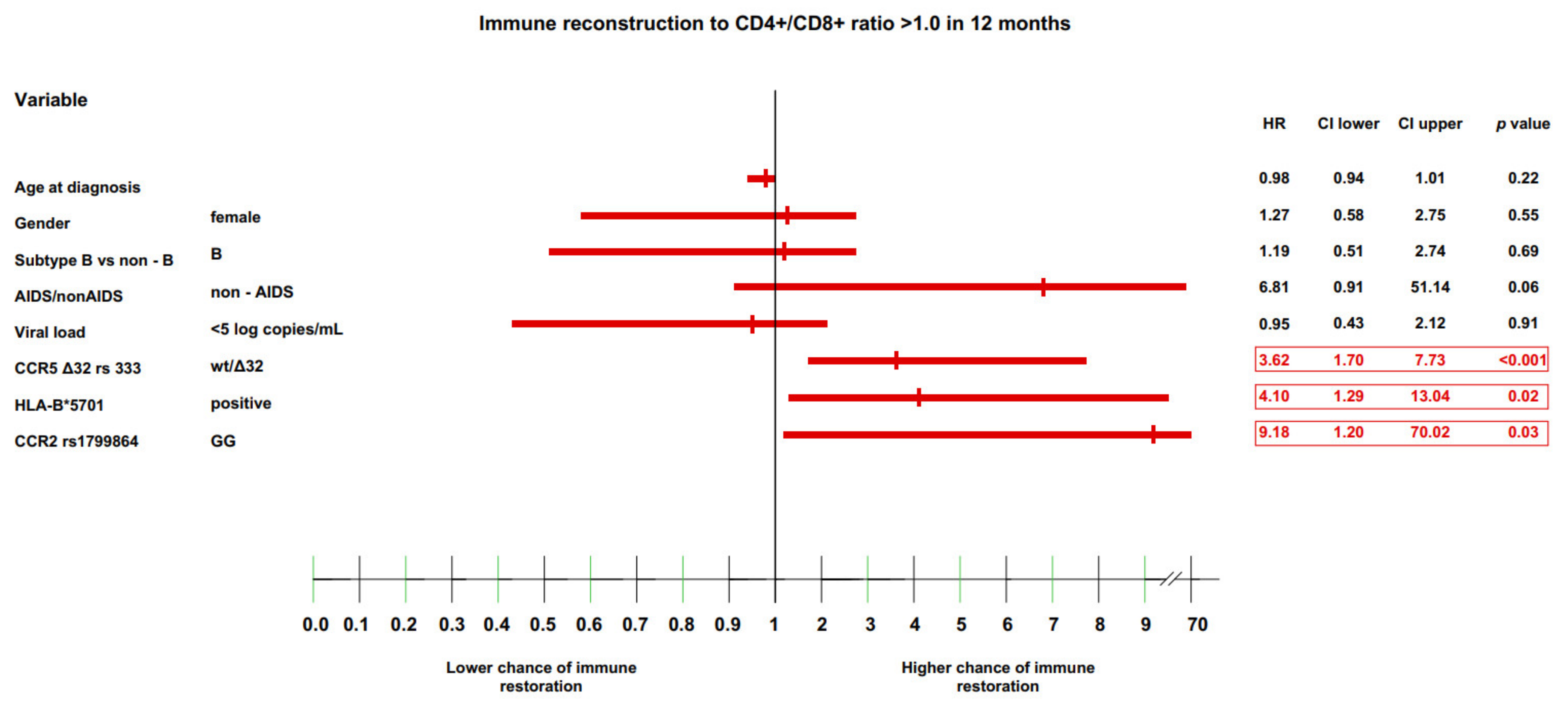
3.4. Multivariate Model of Immune Recovery
4. Discussion
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabin, C.A.; Lundgren, J. The natural history of HIV infection. Curr. Opin. HIV AIDS 2013, 8, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Okulicz, J.F.; Marconi, V.C.; Landrum, M.L.; Wegner, S.; Weintrob, A.; Ganesan, A.; Hale, B.; Crum-Cianflone, N.; Delmar, J.; Barthel, V.; et al. Clinical Outcomes of Elite Controllers, Viremic Controllers, and Long-Term Nonprogressors in the US Department of Defense HIV Natural History Study. J. Infect. Dis. 2009, 200, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Sereti, I. Immune restoration after antiretroviral therapy: The pitfalls of hasty or incomplete repairs. Immunol. Rev. 2013, 254, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.D.; Keruly, J.C. CD4+ Cell Count 6 Years after Commencement of Highly Active Antiretroviral Therapy in Persons with Sustained Virologic Suppression. Clin. Infect. Dis. 2007, 44, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Valdez, H.; Connick, E.; Smith, K.Y.; Lederman, M.M.; Bosch, R.J.; Kim, R.S.; St. Clair, M.; Kuritzkes, D.R.; Kessler, H.; Fox, L.; et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS 2002, 16, 1859–1866. [Google Scholar] [CrossRef]
- Robbins, G.K.; Spritzler, J.G.; Chan, E.S.; Asmuth, D.M.; Gandhi, R.T.; Rodriguez, B.A.; Skowron, G.; Skolnik, P.R.; Shafer, R.W.; Pollard, R.B.; et al. Incomplete Reconstitution of T Cell Subsets on Combination Antiretroviral Therapy in the AIDS Clinical Trials Group Protocol 384. Clin. Infect. Dis. 2009, 48, 350–361. [Google Scholar] [CrossRef]
- McLaren, P.J.; Carrington, M. The impact of host genetic variation on infection with HIV-1. Nat. Immunol. 2015, 16, 577. [Google Scholar] [CrossRef]
- Mulherin, S.A.; O’Brien, T.R.; Ioannidis, J.P.; Goedert, J.J.; Buchbinder, S.P.; Coutomho, R.A.; Jamieson, B.D.; Meyer, L.; Michael, N.L.; Pantaleo, G.; et al. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: The protection varies with duration of infection. AIDS 2003, 17, 377–387. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Contopoulos-Ioannidis, D.G.; Rosenberg, P.S.; Goedert, J.J.; De Rossi, A.; Espanol, T.; Frenkel, L.; Mayaux, M.J.; Newell, M.L.; Pahwa, S.G.; et al. Effects of CCR5-delta32 and CCR2-64I alleles on disease progression of perinatally HIV-1-infected children: An international meta-analysis. AIDS 2003, 17, 1631–1638. [Google Scholar] [CrossRef]
- Knudsen, T.B.; Kristiansen, T.B.; Katzenstein, T.L.; Eugen-Olsen, J. Adverse effect of the CCR5 promoter− 2459A allele on HIV-1 disease progression. J. Med. Virol. 2001, 65, 441–444. [Google Scholar] [CrossRef]
- Parczewski, M.; Urbañska, A.; Maciejewska, K.; Clark, J.; Leszczyszyn-Pynka, M. Association of chemokine receptor gene variants with HIV-1 genotype predicted tropism. HIV Med. 2014, 15, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Fellay, J.; Ge, D.; Shianna, K.V.; Colombo, S.; Ledergerber, B.; Cirulli, E.T.; Urban, T.J.; Zhang, K.; Gumbs, C.E.; Smith, J.P.; et al. Common Genetic Variation and the Control of HIV-1 in Humans. PLoS Genet. 2009, 5, e1000791. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Apps, R.; Qi, Y.; Gao, X.; Male, V.; O’hUigin, C.; O’Connor, G.; Ge, D.; Fellay, J.; Martin, J.N.; et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat. Genet. 2009, 41, 1290–1294. [Google Scholar] [CrossRef] [Green Version]
- Blais, M.E.; Zhang, Y.; Rostron, T.; Griffin, H.; Taylor, S.; Xu, K.; Yan, H.; Wu, H.; James, I.; John, M.; et al. High Frequency of HIV Mutations Associated with HLA-C Suggests Enhanced HLA-C–Restricted CTL Selective Pressure Associated with an AIDS-Protective Polymorphism. J. Immunol. 2012, 188, 4663–4670. [Google Scholar] [CrossRef] [Green Version]
- Apps, R.; Qi, Y.; Carlson, J.M.; Chen, H.; Gao, X.; Thomas, R.; Yuki, Y.; Del Prete, G.Q.; Goulder, P.; Brumme, Z.L.; et al. Influence of HLA-C Expression Level on HIV Control. Science 2013, 340, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Fellay, J.; Shianna, K.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Catagna, A.; Cossarizza, A.; et al. A Whole-Genome Association Study of Major Determinants for Host Control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Catano, G.; Kulkarni, H.; He, W.; Marconi, V.C.; Agan, B.K.; Landrum, M.; Anderson, S.; Delmar, J.; Telles, V.; Song, L.; et al. HIV-1 Disease-Influencing Effects Associated with ZNRD1, HCP5 and HLA-C Alleles Are Attributable Mainly to Either HLA-A10 or HLA-B*57 Alleles. PLoS ONE 2008, 3, e3636. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.; Savan, R.; Qi, Y.; Gao, X.; Yuki, Y.; Bass, S.E.; Martin, M.P.; Hunt, P.; Deeks, S.G.; Telenti, A.; et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature 2011, 472, 495–498. [Google Scholar] [CrossRef]
- Kulpa, D.A.; Collins, K.L. The emerging role of HLA-C in HIV-1 infection. Immunology 2011, 134, 116–122. [Google Scholar] [CrossRef]
- Parczewski, M.; Leszczyszyn-Pynka, M.; Wnuk, A.; Urbañska, A.; Fuksiñska, K.; Bander, D.; Boroñ-Kaczmarska, A. Introduction of pharmacogenetic screening for the human leucocyte antigen (HLA) B*5701 variant in Polish HIV-infected patients. HIV Med. 2010, 11, 345–348. [Google Scholar] [CrossRef]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomažič, J.; Jägel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 Screening for Hypersensitivity to Abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.D.; Lee, K.C.; Kuo, G.M. HLA-B*5701 testing to predict abacavir hypersensitivity. PLoS Curr. 2010, 2, RRN1203. [Google Scholar] [CrossRef]
- Migueles, S.A.; Sabbaghian, M.S.; Shupert, W.L.; Bettinotti, M.P.; Marincola, F.M.; Martino, L.; Hallahan, C.W.; Selig, S.M.; Schwartz, D.; Sullivan, J.; et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 2000, 97, 2709–2714. [Google Scholar] [CrossRef] [Green Version]
- Leszczyszyn-Pynka, M.; Aksak-Wąs, B.; Urbańska, A.; Parczewski, M. Protective Effect of HLA-B*5701 and HLA-C -35 Genetic Variants in HIV-Positive Caucasians from Northern Poland. PLoS ONE 2015, 10, e0127867. [Google Scholar] [CrossRef]
- 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm (accessed on 1 November 2021).
- Gras, L.; May, M.; Ryder, L.P.; Trickey, A.; Helleberg, M.; Obel, N.; Thiebaut, R.; Guest, J.; Gill, J.; Crane, H.; et al. Determinants of Restoration of CD4 and CD8 Cell Counts and Their Ratio in HIV-1-Positive Individuals with Sustained Virological Suppression on Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2019, 80, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Gras, L.; Kesselring, A.M.; Griffin, J.T.; van Sighem, A.; Fraser, C.; Ghani, A.; Miedema, F.; Reiss, P.; Lange, J.M.A.; de Wolf, F.; et al. CD4 Cell Counts of 800 Cells/mm3 or Greater After 7 Years of Highly Active Antiretroviral Therapy Are Feasible in Most Patients Starting With 350 Cells/mm3 or Greater. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 45, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Mehraj, V.; Vyboh, K.; Cao, W.; Li, T.; Routy, J.-P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J. Int. AIDS Soc. 2015, 18, 20052. [Google Scholar] [CrossRef]
- Rector, A.; Vermeire, S.; Thoelen, I.; Keyaerts, E.; Struyf, F.; Vlietinck, R.; Rutgeerts, P.; Van Ranst, M. Analysis of the CC chemokine receptor 5 (CCR5) delta-32 polymorphism in inflammatory bowel disease. Qual. Life Res. 2001, 108, 190–193. [Google Scholar] [CrossRef]
- Kamps, B.S.; Brodt, H.-R.; Staszewski, S.; Bergmann, L.; Helm, E.B. AIDS-free survival and overall survival in HIV infection: The new CDC classification system (1993) for HIV disease and AIDS. Klin. Wochenschr. 1994, 72, 283–287. [Google Scholar] [CrossRef]
- Menozzi, M.; Zona, S.; Santoro, A.; Carli, F.; Stentarelli, C.; Mussini, C.; Guaraldi, G. CD4/CD8 ratio is not predictive of multi-morbidity prevalence in HIV-infected patients but identify patients with higher CVD risk. J. Int. AIDS Soc. 2014, 17 (Suppl. S3), 19709. [Google Scholar] [CrossRef]
- Lee, S.S.; Wong, N.S.; Wong, B.C.K.; Wong, K.H.; Chan, K.C.W. Combining CD4 recovery and CD4: CD8 ratio restoration as an indicator for evaluating the outcome of continued antiretroviral therapy: An observational cohort study. BMJ Open 2017, 7, e016886. [Google Scholar] [CrossRef] [PubMed]
- Collin, A.; Le Marec, F.; Vandenhende, M.-A.; Lazaro, E.; Duffau, P.; Cazanave, C.; Gérard, Y.; Dabis, F.; Bruyand, M.; Bonnet, F.; et al. Incidence and Risk Factors for Severe Bacterial Infections in People Living with HIV. ANRS CO3 Aquitaine Cohort, 2000–2012. PLoS ONE 2016, 11, e0152970. [Google Scholar] [CrossRef] [PubMed]
- Florence, E.; Lundgren, J.; Dreezen, C.; Fisher, M.; Kirk, O.; Blaxhult, A.; Panos, G.; Katlama, C.; Vella, S.; Phillips, A.; et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003, 4, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Deeks, S.G.; Rodriguez, B.; Hernan, V.; Shade, S.B.; Abrams, D.I.; Kitahata, M.M.; Krone, M.; Neilands, T.B.; Brand, R.J.; et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS 2003, 17, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, G.R.; Perrin, L.; Pantaleo, G.; Opravil, M.; Furrer, H.; Telenti, A.; Hirschel, B.; Ledergerber, B.; Vernazza, P.; Bernasconi, E.; et al. CD4 T-Lymphocyte Recovery in Individuals With Advanced HIV-1 Infection Receiving Potent Antiretroviral Therapy for 4 YearsThe Swiss HIV Cohort Study. Arch. Intern. Med. 2003, 163, 2187–2195. [Google Scholar] [CrossRef]
- Viard, J.P.; Mocroft, A.; Chiesi, A.; Kirk, O.; Røge, B.; Panos, G.; Vetter, N.; Bruun, J.N.; Johnson, M.; Lundgren, J.D. Influence of Age on CD4 Cell Recovery in Human Immunodeficiency Virus–Infected Patients Receiving Highly Active Antiretroviral Therapy: Evidence from the EuroSIDA Study. J. Infect. Dis. 2001, 183, 1290–1294. [Google Scholar] [CrossRef] [Green Version]
- Roul, H.; Mary-Krause, M.; Ghosn, J.; Delaugerre, C.; Pialoux, G.; Cuzin, L.; Launay, O.; Lacombe, J.-M.; Menard, A.; De Truchis, P.; et al. CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS 2018, 32, 2605–2614. [Google Scholar] [CrossRef]
- Nunes, C.C.; Matte, M.C.C.; Dias, C.F.; Luvison Araújo, L.A.; Guimarães, L.S.P.; Almeida, S.; Brígido, L.F.M. A influência dos subtipos C, CRF31_BC e B do vírus HIV-1 na progressão da infecção e resposta virológica inicial a terapia antirretroviral em uma coorte no sul do Brasil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 205–211. [Google Scholar] [CrossRef]
- Geretti, A.M.; Harrison, L.; Green, H.; Sabin, C.; Hill, T.; Fearnhill, E.; Pillay, D.; Dunn, D. Effect of HIV-1 subtype on virologic and immunologic response to starting highly active antiretroviral therapy. Clin. Infect. Dis. 2009, 48, 1296–1305. [Google Scholar] [CrossRef] [Green Version]
- Easterbrook, P.J.; Smith, M.; Mullen, J.; O’Shea, S.; Chrystie, I.; de Ruiter, A.; Tatt, I.D.; Geretti, A.M.; Zuckerman, M. Impact of HIV-1 viral subtype on disease progression and response to antiretroviral therapy. J. Int. AIDS Soc. 2010, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Junqueira, D.M.; de Matos Almeida, S.E. HIV-1 subtype B: Traces of a pandemic. Virology 2016, 495, 173–184. [Google Scholar] [CrossRef]
- Parczewski, M.; Scheibe, K.; Witak-Jędra, M.; Pynka, M.; Aksak-Wąs, B.; Urbańska, A. Infection with HIV-1 subtype D adversely affects the live expectancy independently of antiretroviral drug use. Infect. Genet. Evol. 2021, 90, 104754. [Google Scholar] [CrossRef]
- Parczewski, M.; Leszczyszyn-Pynka, M.; Witak-Jedra, M.; Rymer, W.; Zalewska, M.; Gąsiorowski, J.; Bociąga-Jasik, M.; Kalinowska-Nowak, A.; Garlicki, A.; Grzeszczuk, A.; et al. Distribution and time trends of HIV-1 variants in Poland: Characteristics of non-B clades and recombinant viruses. Infect. Genet. Evol. 2016, 39, 232–240. [Google Scholar] [CrossRef]
- Egger, S.; Petoumenos, K.; Kamarulzaman, A.; Hoy, J.; Sungkanuparph, S.; Chuah, J.; Falster, K.; Zhou, J.; Law, M. Long-Term Patterns in CD4 Response Are Determined by an Interaction Between Baseline CD4 Cell Count, Viral Load, and Time: The Asia Pacific HIV Observational Database (APHOD). JAIDS J. Acquir. Immune Defic. Syndr. 2009, 50, 513–520. [Google Scholar] [CrossRef]
- Vaidya, S.A.; Streeck, H.; Beckwith, N.; Ghebremichael, M.; Pereyra, F.; Kwon, D.S.; Addo, M.M.; Rychert, J.; Routy, J.-P.; Jessen, H.; et al. Temporal effect of HLA-B*57 on viral control during primary HIV-1 infection. Retrovirology 2013, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Jose, M.S. HLA B*5701 status, disease progression, and response to antiretroviral therapy. AIDS 2013, 27, 2587–2592. [Google Scholar] [CrossRef] [Green Version]
- Kamya, P.; Boulet, S.; Tsoukas, C.M.; Routy, J.-P.; Thomas, R.; Côté, P.; Boulassel, M.-R.; Baril, J.-G.; Kovacs, C.; Migueles, S.A.; et al. Receptor-Ligand Requirements for Increased NK Cell Polyfunctional Potential in Slow Progressors Infected with HIV-1 Coexpressing KIR3DL1*h/*y and HLA-B*57. J. Virol. 2011, 85, 5949–5960. [Google Scholar] [CrossRef] [Green Version]
- Kloverpris, H.N.; Stryhn, A.; Harndahl, M.; van der Stok, M.; Payne, R.P.; Matthews, P.C.; Chen, F.; Riddell, L.; Walker, B.D.; Ndung’u, T.; et al. HLA-B*57 Micropolymorphism Shapes HLA Allele-Specific Epitope Immunogenicity, Selection Pressure, and HIV Immune Control. J. Virol. 2012, 86, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, C.; Gutierrez-Rivas, M.; Pacheco, Y.M.; García, M.; Blanco, J.; Medrano, L.M.; Navarrete-Muñoz, M.A.; Gutiérrez, F.; Miralles, P.; Dalmau, D.; et al. Genetic variation in CCR2 and CXCL12 genes impacts on CD4 restoration in patients initiating cART with advanced immunesupression. PLoS ONE 2019, 14, e0214421. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Qiao, Y.; Zhang, X.; Sun, H.; Wang, J.; Sun, D.; Jin, Y.; Yu, Y.; Chen, F.; Bai, J.; et al. CCR2-64I allele is associated with the progression of AIDS in a Han Chinese population. Mol. Biol. Rep. 2009, 37, 311–316. [Google Scholar] [CrossRef]
- Passam, A.M.; Zafiropoulos, A.; Miyakis, S.; Zagoreos, I.; Stavianeas, N.G.; Krambovitis, E.; Spandidos, D.A. CCR2-64I and CXCL12 3′A alleles confer a favorable prognosis to AIDS patients undergoing HAART therapy. J. Clin. Virol. 2005, 34, 302–309. [Google Scholar] [CrossRef]
- Rigato, P.; Hong, M.; Casseb, J.; Ueda, M.; de Castro, I.; Benard, G.; Duarte, A.J.S. Better CD4+ T Cell Recovery in Brazilian HIV-Infected Individuals Under HAART Due to Cumulative Carriage of SDF-1-3A, CCR2-V64I, CCR5- D32 and CCR5-Promoter 59029A/G Polymorphisms. Curr. HIV Res. 2008, 6, 466–473. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Rosenberg, P.S.; Goedert, J.J.; Ashton, L.J.; Benfield, T.L.; Buchbinder, S.P.; Coutinho, R.A.; Eugen-Olsen, J.; Gallart, T.; Katzenstein, T.L.; et al. Effects of CCR5-Δ 32, CCR2-64I, and SDF-1 3′A Alleles on HIV-1 Disease Progression: An International Meta-Analysis of Individual-Patient Data. Ann. Intern. Med. 2001, 135, 782–795. [Google Scholar] [CrossRef]
- Katzenstein, T.L.; Eugen-Olsen, J.; Hofmann, B.; Benfield, T.; Pedersen, C.; Iversen, A.K.; Sørensen, A.M.; Garred, P.; Koppelhus, U.; Svejgaard, A.; et al. HIV-infected individuals with the CCR delta32/CCR5 genotype have lower HIV RNA levels and higher CD4 cell counts in the early years of the infection than do patients with the wild type. Copenhagen AIDS Cohort Study Group. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997, 16, 10–14. [Google Scholar] [CrossRef]

| HR (95% CI) (12 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | HR (95% CI) (18 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | HR (95% CI) (24 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | HR (95% CI) (30 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | HR (95% CI) (36 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | HR (95% CI) (42 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | HR (95% CI) (48 Months) | p (Cox Regression) | Number (%) of Patients Who Reconstructed (Kaplan–Meier Estimator) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-B*5701 immune recovery to CD4/CD8 ratio >0.8 (number of patients 265) | ||||||||||||||||||||||||||||
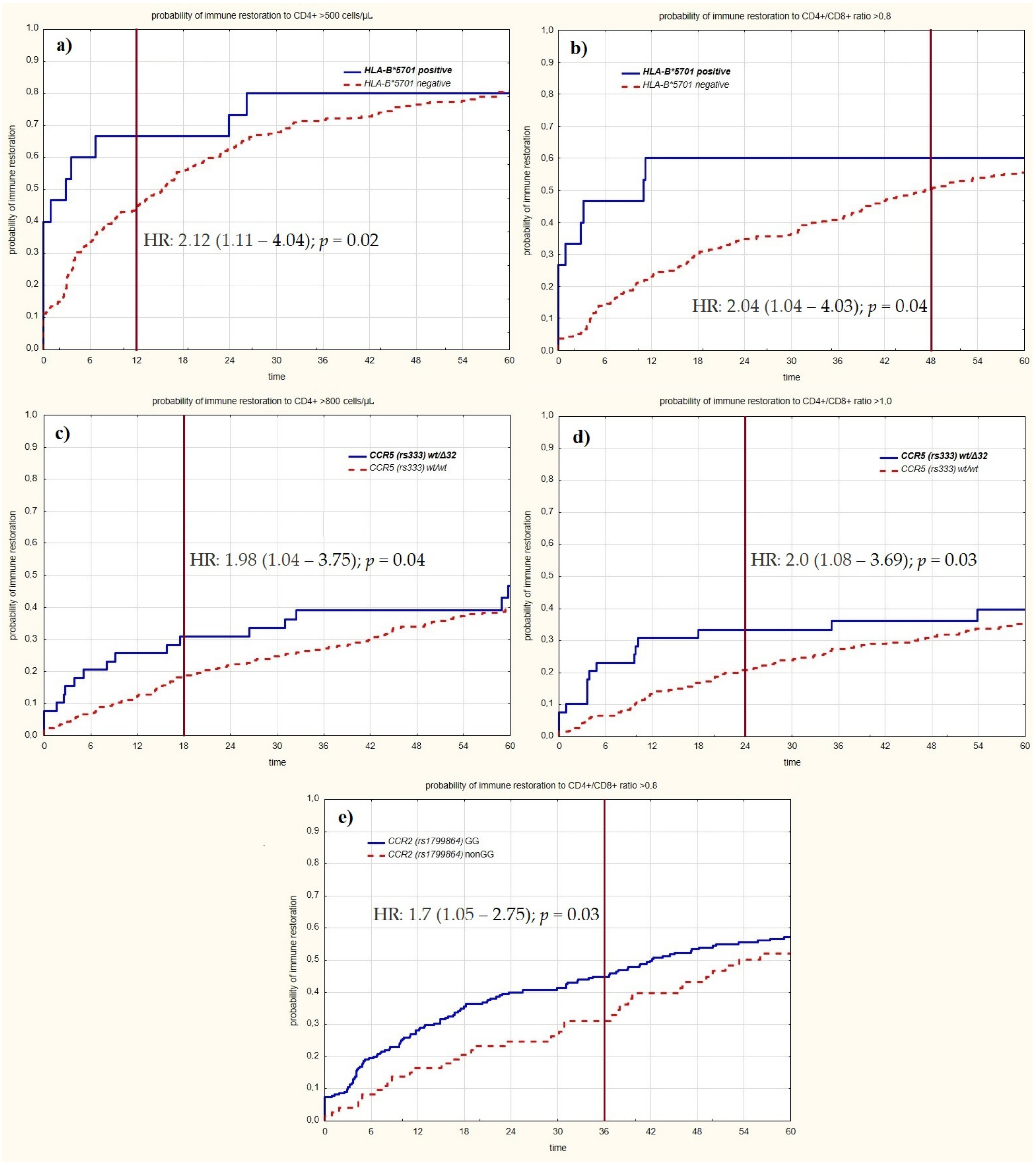
| positive | 3.74 (1.84–7.54) | p < 0.001 | 6 (40.00%) | p < 0.001 | 3.47 (1.73–6.95) | p < 0.001 | 9 (64.29%) | p = 0.002 | 3.02 (1.52–6.0) | p = 0.001 | 9 (64.29%) | p = 0.008 | 2.89 (1.46–5.75) | p = 0.002 | 9 (64.29%) | p = 0.01 | 2.59 (1.31–5.12) | p = 0.006 | 9 (64.29%) | p = 0.02 | 2.24 (1.13–4.41) | p = 0.02 | 9 (64.29%) | p = 0.06 | 2.04 (1.04–4.03) | p = 0.04 | 9 (64.29%) | p = 009 |
| negative | ref. | 63 (23.08%) | ref. | 75 (29.88%) | ref. | 88 (35.06%) | ref. | 92 (36.65%) | ref. | 103 (41.04%) | ref. | 118 (47.01%) | ref. | 127 (50.60%) | ||||||||||||||
| CCR2 (rs1799864) immune recovery to CD4/CD8 ratio >0.8 (number of patients 277) | ||||||||||||||||||||||||||||
| GG | 1.87 (1.01–3.45) | p = 0.05 | 65 (28.14%) | p = 0.04 | 2.0 (1.11–3.61) | p = 0.02 | 76 (35.85%) | p = 0.01 | 1.87 (1.1–3.19) | p = 0.02 | 86 (40.57%) | p = 0.02 | 1.83 (1.09–3.07) | p = 0.02 | 89 (41.98%) | p = 0.02 | 1.7 (1.05–2.75) | p = 0.03 | 97 (45.75%) | p = 0.02 | in this time interval, the data were not statistically significant | |||||||
| nonGG | ref. | 12 (16.44%) | ref. | 13 (20.00%) | ref. | 16 (24.62%) | ref. | 17 (26.15%) | ref. | 20 (30.77%) | ||||||||||||||||||
| CCR5 Δ32 (rs333) immune recovery to CD4/CD8 ratio >1.0 (number of patients 276) | ||||||||||||||||||||||||||||
| wt/Δ32 | 2.62 (1.36–5.05) | p = 0.003 | 12 (30.77%) | p = 0.004 | 2.44 (1.3–4.56) | p = 0.005 | 13 (34.21%) | p = 0.007 | 2.0 (1.08–3.69) | p = 0.03 | 13 (34.21%) | p = 0.03 | in this time interval, the data were not statistically significant | |||||||||||||||
| wt/wt | ref. | 35 (13.41%) | ref. | 39 (16.39%) | ref. | 48 (20.17%) | ||||||||||||||||||||||
| CCR5 Δ32 (rs333) immune recovery to CD4+ >800 cells/µL (number of patients 276) | ||||||||||||||||||||||||||||
| wt/Δ32 | 2.32 (1.14–4.73) | p = 0.02 | 10 (25.64%) | p = 0.02 | 1.98 (1.04–3.75) | p = 0.04 | 12 (31.58%) | p = 0.04 | in this time interval, the data were not statistically significant | |||||||||||||||||||
| wt/wt | ref. | 32 (12.26%) | ref. | 43 (18.07%) | ||||||||||||||||||||||||
| HLA-B*5701 immune recovery to CD4+ >500 cells/µL (number of patients 287) | ||||||||||||||||||||||||||||
| positive | 2.12 (1.11–4.04) | p = 0.02 | 10 (66.67%) | p = 0.04 | in this time interval, the data were not statistically significant | |||||||||||||||||||||||
| negative | ref. | 122 (44.85%) | ||||||||||||||||||||||||||
| Immunological Recovery >500 Cells/µL | Immunological Recovery >800 Cells/µL | Immunological Recovery of CD4+/CD8+ Ratio >0.8 | Immunological Recovery of CD4+/CD8+ Ratio >1.0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | |||||
| Sex | ||||||||||||
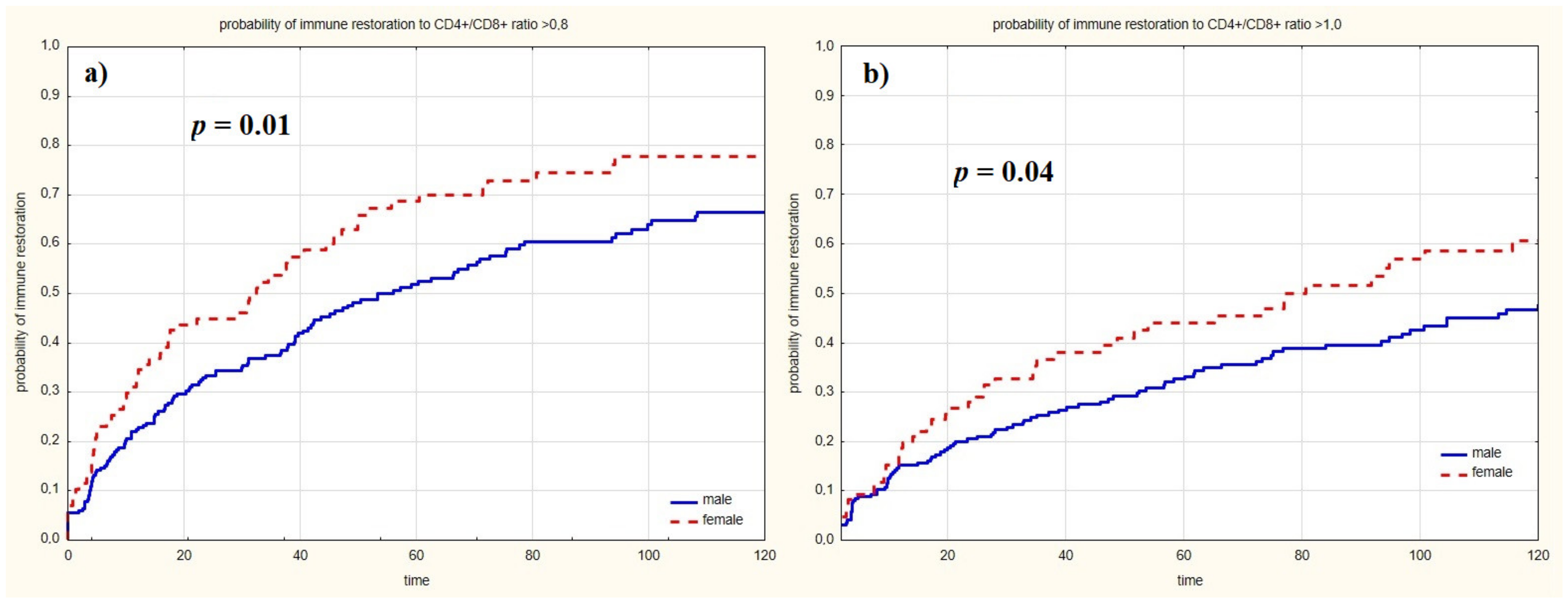
| female, n = 86 (%) | 10 (11.6%) | 76 (88.4%) | p = 0.22 | 48 (55.8%) | 38 (44.2%) | p = 0.57 | 24 (27.9%) | 62 (72.1%) | p = 0.02 | 39 (45.3%) | 47 (54.7%) | p = 0.05 |
| male, n = 226 (%) | 39 (17.3%) | 187 (82.7%) | 118 (52.2%) | 108 (47.8%) | 94 (41.6%) | 132 (58.4%) | 131 (58%) | 95 (42%) | ||||
| median age at diagnosis (IQR) | 37 (32–44) | 31.5 (26–39) | p = 0.001 | 34 (27–41) | 30 (26–39) | p = 0.02 | 34 (27–43) | 31 (26–38) | p = 0.04 | 34 (27–42) | 31 (27–38) | p = 0.08 |
| Likely mode of transmission | ||||||||||||
| MSM, n = 114 (%) | 13 (11.4%) | 101 (88.6%) | p = 0.72 | 51 (44.7%) | 63 (55.3%) | p = 0.46 | 41 (36%) | 73 (64%) | p = 0.93 | 64 (56.1%) | 50 (43.9%) | p = 0.91 |
| HSX, n = 98 (%) | 14 (14.3%) | 84 (85.7%) | 55 (56.1%) | 43 (43.9%) | 37 (37.8%) | 61 (62.2%) | 49 (50%) | 49 (50%) | ||||
| IDU, n = 56 (%) | 11 (19.6%) | 45 (80.4%) | 32 (57.1%) | 24 (42.9%) | 21 (37.5%) | 35 (62.5%) | 28 (50%) | 28 (50%) | ||||
| Clinical category at diagnosis (according to CDC from 1993 y.) | ||||||||||||
| Asymptomatic, n = 112 (%) | 8 (20%) | 104 (47.9%) | p < 0.001 | 44 (33.1%) | 68 (54.8%) | p < 0.001 | 28 (30.1%) | 84 (51.2%) | p < 0.001 | 41 (30.6%) | 71 (57.7%) | p < 0.001 |
| Symptomatic-non-AIDS, n = 95 (%) | 15 (37.5%) | 80 (36.9%) | 53 (39.8%) | 42 (33.9%) | 34 (36.6%) | 61 (37.2%) | 54 (40.3%) | 41 (33.3%) | ||||
| AIDS defining illness, n = 50 (%) | 17 (42.5%) | 33 (15.2%) | 36 (27.1%) | 14 (11.3%) | 31 (33.3%) | 19 (11.6%) | 39 (29.1%) | 11 (8.9%) | ||||
| HIV subtype | ||||||||||||
| B, n = 154 (%) | 23 (14.9%) | 131 (85.1%) | p = 0.06 | 77 (50%) | 77 (50%) | p = 0.003 | 56 (36.4%) | 98 (63.6%) | p = 0.65 | 83 (53.9%) | 71 (46.1%) | p = 0.41 |
| Non-B, n = 53 (%) | 14 (26.4%) | 39 (73.6%) | 39 (73.6%) | 14 (26.4%) | 21 (39.6%) | 32 (60.4%) | 32 (60.4%) | 21 (39.6%) | ||||
| Virological characteristics | ||||||||||||
| Median viral load at diagnosis (IQR) (log copies/mL) | 5.07 (4.81–5.54) | 4.9 (5.52–6.31) | p = 0.13 | 5.04 (4.35–5.65) | 4.78 (4.15–5.32) | p = 0.003 | 5.0 (4.11–5.42) | 4.81 (4.27–5.42) | p = 0.06 | 4.99 (4.2–5.59) | 4.79 (4.18–5.18) | p = 0.07 |
| Viral load <5log copies/mL, n = 154 (%) | 17 (11%) | 137 (89%) | p = 0.06 | 52 (38%) | 85 (62%) | p = 0.03 | 35 (25.5%) | 102 (74.5%) | p = 0.22 | 60 (43.8%) | 77 (56.2%) | p = 0.36 |
| Viral load >5 log copies/mL, n = 144 (%) | 27 (18.8%) | 117 (81.3%) | 60 (51.3%) | 57 (48.7%) | 38 (32.5%) | 79 (67.5%) | 58 (49.6%) | 59 (50.4%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksak-Wąs, B.J.; Urbańska, A.; Scheibe, K.; Serwin, K.; Leszczyszyn-Pynka, M.; Rafalska-Kosior, M.; Gołąb, J.; Chober, D.; Parczewski, M. Factors Influencing Immune Restoration in People Living with HIV/AIDS. J. Clin. Med. 2022, 11, 1887. https://doi.org/10.3390/jcm11071887
Aksak-Wąs BJ, Urbańska A, Scheibe K, Serwin K, Leszczyszyn-Pynka M, Rafalska-Kosior M, Gołąb J, Chober D, Parczewski M. Factors Influencing Immune Restoration in People Living with HIV/AIDS. Journal of Clinical Medicine. 2022; 11(7):1887. https://doi.org/10.3390/jcm11071887
Chicago/Turabian StyleAksak-Wąs, Bogusz Jan, Anna Urbańska, Kaja Scheibe, Karol Serwin, Magdalena Leszczyszyn-Pynka, Milena Rafalska-Kosior, Joanna Gołąb, Daniel Chober, and Miłosz Parczewski. 2022. "Factors Influencing Immune Restoration in People Living with HIV/AIDS" Journal of Clinical Medicine 11, no. 7: 1887. https://doi.org/10.3390/jcm11071887
APA StyleAksak-Wąs, B. J., Urbańska, A., Scheibe, K., Serwin, K., Leszczyszyn-Pynka, M., Rafalska-Kosior, M., Gołąb, J., Chober, D., & Parczewski, M. (2022). Factors Influencing Immune Restoration in People Living with HIV/AIDS. Journal of Clinical Medicine, 11(7), 1887. https://doi.org/10.3390/jcm11071887






