Optimal Anticoagulant Strategy for Periprocedural Management of Atrial Fibrillation Ablation: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Outcomes
2.5. Data Extraction and Synthesis
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Identification and Study Population Characteristics
3.2. Risk of Bias Assessment
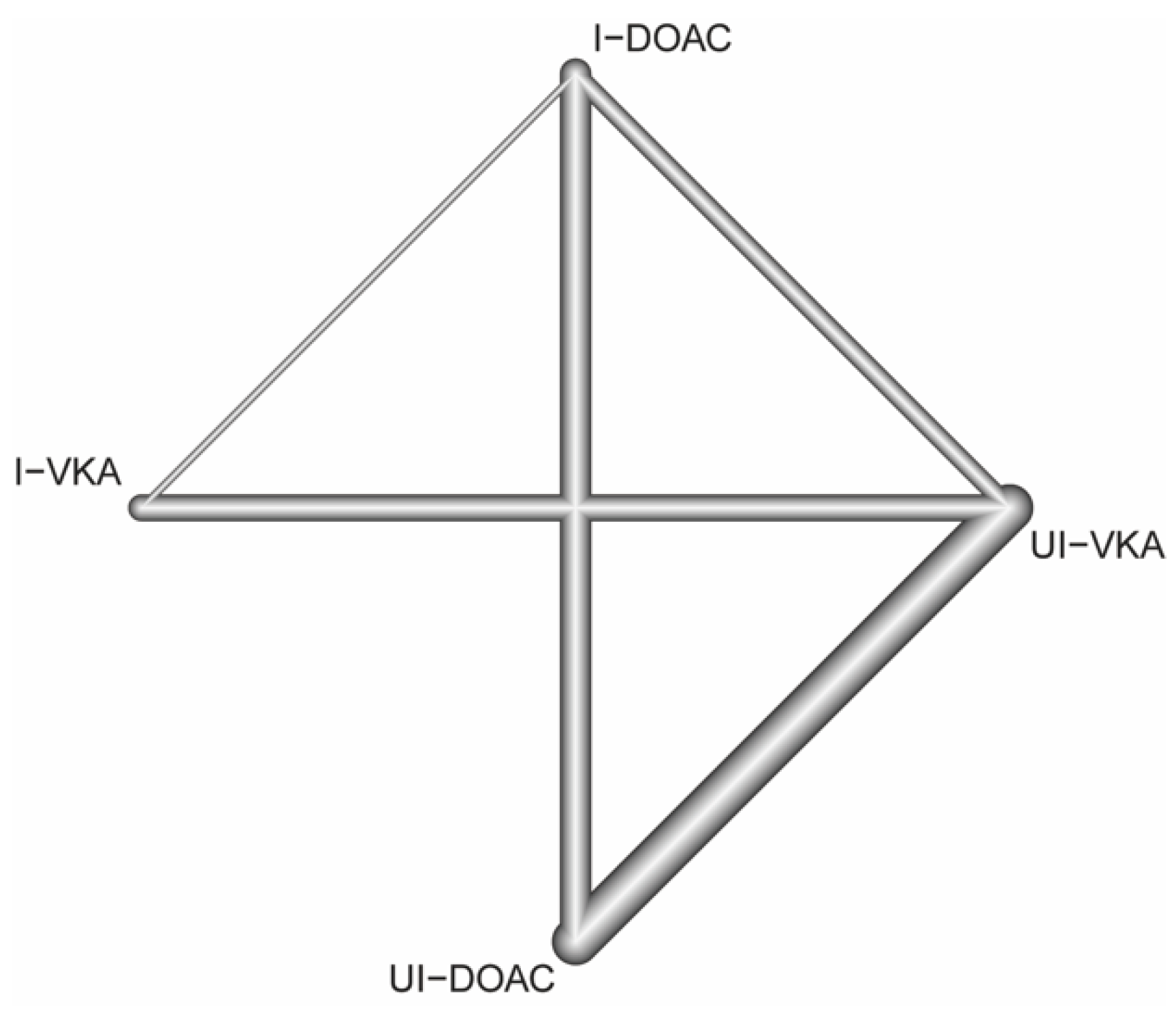
3.3. Structure of the Network
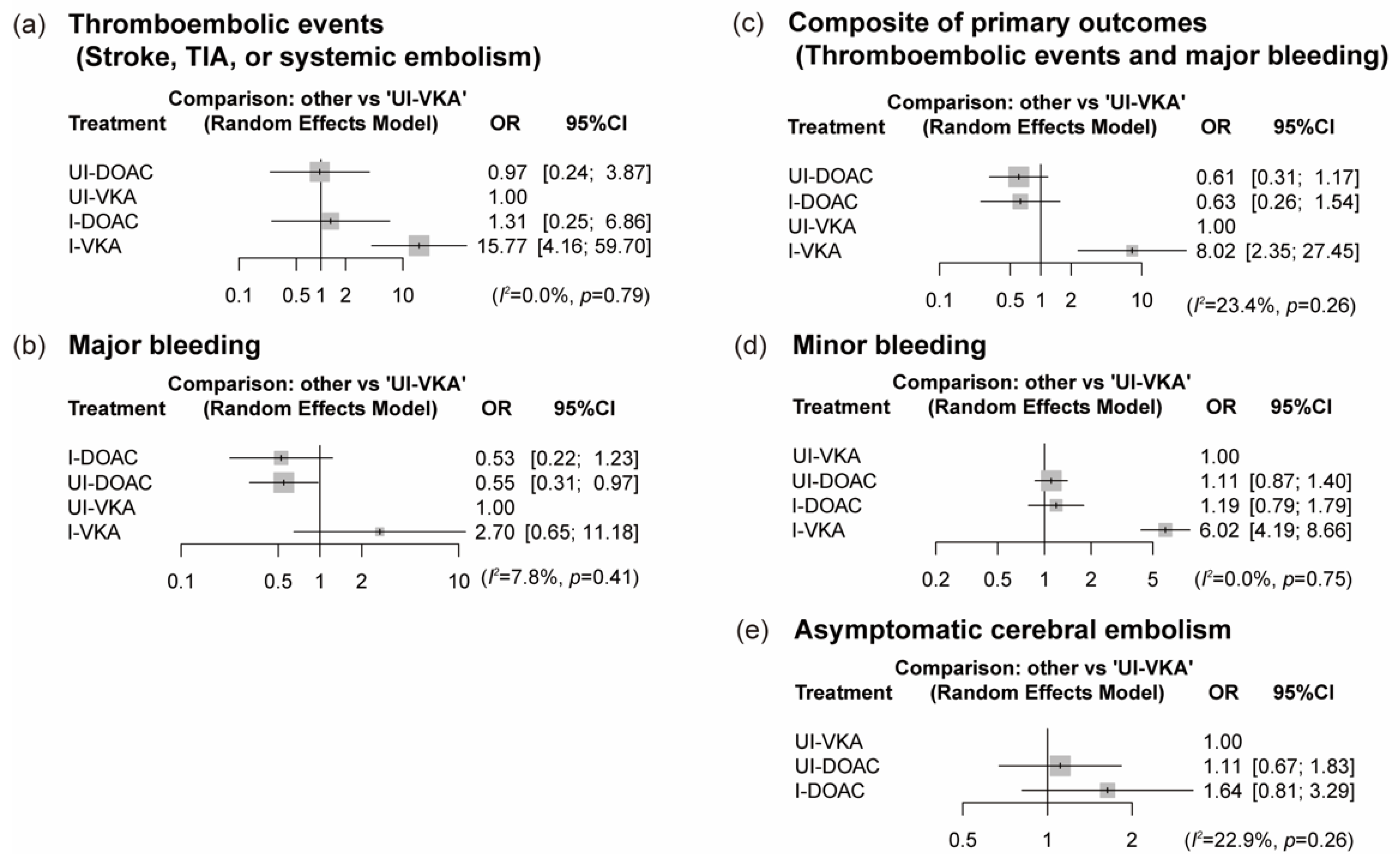
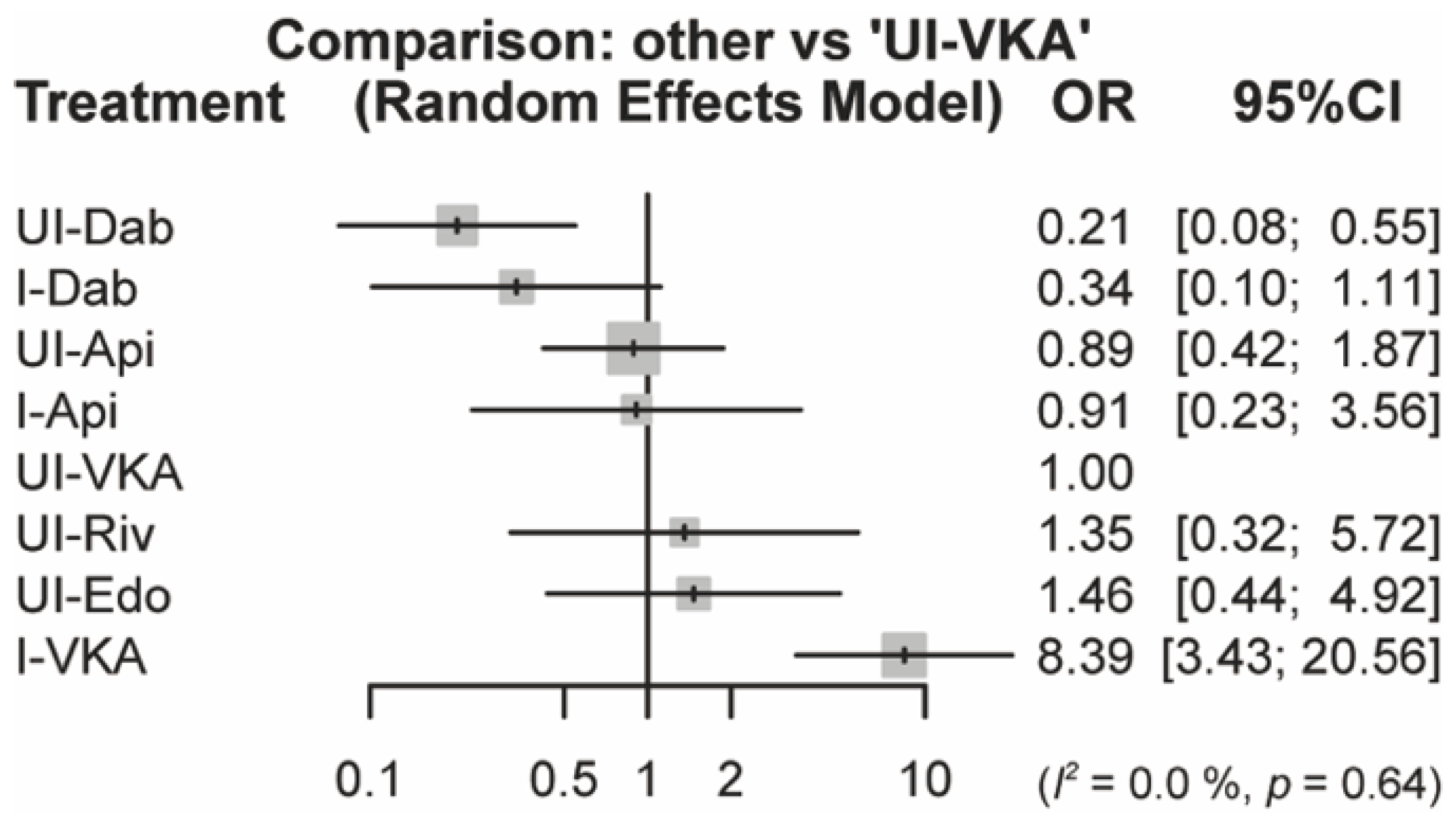
3.4. NMA Results for the Primary and Secondary Outcomes
3.5. Sensitivity Analyses
3.6. Assessment of Inconsistency and Publication Bias
3.7. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Brugada, J.; Hindricks, G.; Maggioni, A.; Tavazzi, L.; Vardas, P.; Anselme, F.; Inama, G.; Jais, P.; Kalarus, Z.; et al. ESC-EURObservational Research Programme: The Atrial Fibrillation Ablation Pilot Study, conducted by the European Heart Rhythm Association. Europace 2012, 14, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Natale, A.; Albenque, J.P.; Kautzner, J.; Shah, D.; Michaud, G.; Wharton, M.; Harari, D.; et al. Randomized, Controlled Trial of the Safety and Effectiveness of a Contact Force-Sensing Irrigated Catheter for Ablation of Paroxysmal Atrial Fibrillation: Results of the TactiCath Contact Force Ablation Catheter Study for Atrial Fibrillation (TOCCASTAR) Study. Circulation 2015, 132, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Stabile, G.; Scaglione, M.; del Greco, M.; De Ponti, R.; Bongiorni, M.G.; Zoppo, F.; Soldati, E.; Marazzi, R.; Marini, M.; Gaita, F.; et al. Reduced fluoroscopy exposure during ablation of atrial fibrillation using a novel electroanatomical navigation system: A multicentre experience. Europace 2012, 14, 60–65. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.J.; Ling, L.H.; Azzopardi, S.; Lee, G.A.; Lee, G.; Kumar, S.; Wong, M.C.; Walters, T.E.; Lee, J.M.; Looi, K.L.; et al. A minimal or maximal ablation strategy to achieve pulmonary vein isolation for paroxysmal atrial fibrillation: A prospective multi-centre randomized controlled trial (the Minimax study). Eur. Heart J. 2015, 36, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.Y.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Nery, P.B.; Belliveau, D.; Nair, G.M.; Bernick, J.; Redpath, C.J.; Szczotka, A.; Sadek, M.M.; Green, M.S.; Wells, G.; Birnie, D.H. Relationship Between Pulmonary Vein Reconnection and Atrial Fibrillation Recurrence: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2016, 2, 474–483. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef] [Green Version]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Bassiouny, M.; Saliba, W.; Rickard, J.; Shao, M.; Sey, A.; Diab, M.; Martin, D.O.; Hussein, A.; Khoury, M.; Abi-Saleh, B.; et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ. Arrhyth. Electrophysiol. 2013, 6, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Bin Abdulhak, A.A.; Khan, A.R.; Tleyjeh, I.M.; Spertus, J.A.; Sanders, S.U.; Steigerwalt, K.E.; Garbati, M.A.; Bahmaid, R.A.; Wimmer, A.P. Safety and efficacy of interrupted dabigatran for peri-procedural anticoagulation in catheter ablation of atrial fibrillation: A systematic review and meta-analysis. Europace 2013, 15, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Camm, A.J. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: A meta-analysis of the literature. Europace 2013, 15, 1407–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Providência, R.; Marijon, E.; Albenque, J.P.; Combes, S.; Combes, N.; Jourda, F.; Hireche, H.; Morais, J.; Boveda, S. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014, 16, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, H.L.; Lindsley, J.P.; Moranville, M.P.; Habibi, M.; Khurram, I.M.; Spragg, D.D.; Berger, R.D.; Calkins, H.; Marine, J.E. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann. Pharm. 2015, 49, 278–284. [Google Scholar] [CrossRef]
- Rahman, H.; Khan, S.U.; DePersis, M.; Hammad, T.; Nasir, F.; Kaluski, E. Meta-analysis of safety and efficacy of oral anticoagulants in patients requiring catheter ablation for atrial fibrillation. Cardiovasc. Revasc. Med. 2019, 20, 147–152. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Y.; Qin, Y. A meta-analysis of randomized controlled trials of uninterrupted periprocedural anticoagulation strategy in patients undergoing atrial fibrillation catheter ablation. Int. J. Cardiol. 2018, 270, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, M.; Lin, Y.; Parco, C.; Karathanos, A.; Krieger, T.; Schulze, V.; Heinen, Y.; Bejinariu, A.; Müller, P.; Makimoto, H.; et al. Uninterrupted anticoagulation during catheter ablation for atrial fibrillation: No difference in major bleeding and stroke between direct oral anticoagulants and vitamin K antagonists in an updated meta-analysis of randomised controlled trials. Acta Cardiol. 2021, 76, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Bejinariu, A.G.; Makimoto, H.; Wakili, R.; Mathew, S.; Kosiuk, J.; Linz, D.; Steinfurt, J.; Dechering, D.G.; Meyer, C.; Veltmann, C.; et al. One-Year Course of Periprocedural Anticoagulation in Atrial Fibrillation Ablation: Results of a German Nationwide Survey. Cardiology 2020, 145, 676–681. [Google Scholar] [CrossRef]
- Ottóffy, M.; Mátrai, P.; Farkas, N.; Hegyi, P.; Czopf, L.; Márta, K.; Garami, A.; Balaskó, M.; Pótóné-Oláh, E.; Mikó, A.; et al. Uninterrupted or Minimally Interrupted Direct Oral Anticoagulant Therapy is a Safe Alternative to Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Atrial Fibrillation: An Updated Meta-Analysis. J. Clin. Med. 2020, 9, 3073. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, S.; Westra, S.W.; Volleberg, R.; Hannink, G.; Nakamura, R.; de Asmundis, C.; Chierchia, G.B.; Navarese, E.P.; Brouwer, M.A. Meta-analysis of controlled studies on minimally interrupted vs. continuous use of non-vitamin K antagonist oral anticoagulants in catheter ablation for atrial fibrillation. Europace 2021, 23, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.; Chaimani, A.; Li, T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017, 12, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rücker, G.; Schwarzer, G. Resolve conflicting rankings of outcomes in network meta-analysis: Partial ordering of treatments. Res. Synth. Methods 2017, 8, 526–536. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef] [Green Version]
- Krahn, U.; Binder, H.; König, J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med. Res. Methodol. 2013, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veroniki, A.A.; Vasiliadis, H.S.; Higgins, J.P.; Salanti, G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013, 42, 332–345. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Barrett, J.K.; Jackson, D.; Higgins, J.P. Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res. Synth. Methods 2012, 3, 111–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Santangeli, P.; Mohanty, P.; Sanchez, J.E.; Horton, R.; Gallinghouse, G.J.; Themistoclakis, S.; Rossillo, A.; Lakkireddy, D.; et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: Results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014, 129, 2638–2644. [Google Scholar] [CrossRef] [Green Version]
- Nin, T.; Sairaku, A.; Yoshida, Y.; Kamiya, H.; Tatematsu, Y.; Nanasato, M.; Inden, Y.; Hirayama, H.; Murohara, T. A randomized controlled trial of dabigatran versus warfarin for periablation anticoagulation in patients undergoing ablation of atrial fibrillation. Pacing Clin. Electrophysiol. 2013, 36, 172–179. [Google Scholar] [CrossRef]
- Nogami, A.; Harada, T.; Sekiguchi, Y.; Otani, R.; Yoshida, Y.; Yoshida, K.; Nakano, Y.; Nuruki, N.; Nakahara, S.; Goya, M.; et al. Safety and Efficacy of Minimally Interrupted Dabigatran vs Uninterrupted Warfarin Therapy in Adults Undergoing Atrial Fibrillation Catheter Ablation: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e191994. [Google Scholar] [CrossRef]
- Cappato, R.; Marchlinski, F.E.; Hohnloser, S.H.; Naccarelli, G.V.; Xiang, J.; Wilber, D.J.; Ma, C.S.; Hess, S.; Wells, D.S.; Juang, G.; et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur. Heart J. 2015, 36, 1805–1811. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, T.; Abe, M.; Yamaki, M.; Fujieda, H.; Abe, Y.; Hashimoto, K.; Ishiba, M.; Sakai, H.; Hishikari, K.; Takigawa, M.; et al. Apixaban versus Warfarin for the Prevention of Periprocedural Cerebral Thromboembolism in Atrial Fibrillation Ablation: Multicenter Prospective Randomized Study. J. Cardiovasc. Electrophysiol. 2016, 27, 549–554. [Google Scholar] [CrossRef]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; Okumura, K.; Serota, H.; Nordaby, M.; Guiver, K.; et al. Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation. N. Engl. J. Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Kashimura, S.; Nishiyama, T.; Katsumata, Y.; Inagawa, K.; Ikegami, Y.; Nishiyama, N.; Fukumoto, K.; Tanimoto, Y.; Aizawa, Y.; et al. Asymptomatic Cerebral Infarction During Catheter Ablation for Atrial Fibrillation: Comparing Uninterrupted Rivaroxaban and Warfarin (ASCERTAIN). JACC Clin. Electrophysiol. 2018, 4, 1598–1609. [Google Scholar] [CrossRef]
- Kirchhof, P.; Haeusler, K.G.; Blank, B.; De Bono, J.; Callans, D.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Gentlesk, P.; Grimaldi, M.; et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur. Heart J. 2018, 39, 2942–2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohnloser, S.H.; Camm, J.; Cappato, R.; Diener, H.C.; Heidbüchel, H.; Mont, L.; Morillo, C.A.; Abozguia, K.; Grimaldi, M.; Rauer, H.; et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: The ELIMINATE-AF trial. Eur. Heart J. 2019, 40, 3013–3021. [Google Scholar] [CrossRef]
- Yoshimura, A.; Iriki, Y.; Ichiki, H.; Oketani, N.; Okui, H.; Maenosono, R.; Namino, F.; Miyata, M.; Ohishi, M. Evaluation of safety and efficacy of periprocedural use of rivaroxaban and apixaban in catheter ablation for atrial fibrillation. J. Cardiol. 2017, 69, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, M.R.; Allison, J.S.; Natale, A.; Weisberg, I.L.; Ellenbogen, K.A.; Richards, M.; Hsieh, W.H.; Sutherland, J.; Cannon, C.P. A Prospective Randomized Trial of Apixaban Dosing During Atrial Fibrillation Ablation: The AEIOU Trial. JACC Clin. Electrophysiol. 2018, 4, 580–588. [Google Scholar] [CrossRef]
- Yu, H.T.; Shim, J.; Park, J.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Joung, B.; Lee, M.H.; Kim, Y.H.; Pak, H.N. When is it appropriate to stop non-vitamin K antagonist oral anticoagulants before catheter ablation of atrial fibrillation? A multicentre prospective randomized study. Eur. Heart J. 2019, 40, 1531–1537. [Google Scholar] [CrossRef]
- Nakamura, K.; Naito, S.; Sasaki, T.; Take, Y.; Minami, K.; Kitagawa, Y.; Motoda, H.; Inoue, M.; Otsuka, Y.; Niijima, K.; et al. Uninterrupted vs. interrupted periprocedural direct oral anticoagulants for catheter ablation of atrial fibrillation: A prospective randomized single-centre study on post-ablation thrombo-embolic and haemorrhagic events. Europace 2019, 21, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Suzuki, H.; Matsunaga, S.; Nishikawa, Y.; Harada, K.; Mamiya, K.; Shinoda, N.; Harada, K.; Kato, M.; Marui, N.; et al. Impact of periprocedural anticoagulation therapy on the incidence of silent stroke after atrial fibrillation ablation in patients receiving direct oral anticoagulants: Uninterrupted vs. interrupted by one dose strategy. Europace 2019, 21, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Inden, Y.; Yoshida, Y.; Sairaku, A.; Yanagisawa, S.; Suzuki, H.; Watanabe, R.; Takenaka, M.; Maeda, M.; Murohara, T. Differences in prothrombotic response between the uninterrupted and interrupted apixaban therapies in patients undergoing cryoballoon ablation for paroxysmal atrial fibrillation: A randomized controlled study. Heart Vessels 2019, 34, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, H.; Murakami, T.; Hina, K.; Higashiya, S.; Kawamura, H.; Murakami, M.; Kamikawa, S.; Hirohata, S.; Kusachi, S. Activated clotting time on the day of atrial fibrillation ablation for minimally interrupted and uninterrupted direct oral anticoagulation therapy: Sequential changes, differences among direct oral anticoagulants, and ablation safety outcomes. J. Cardiovasc. Electrophysiol. 2019, 30, 2823–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, I.; Iriki, Y.; Oketani, N.; Okui, H.; Ichiki, H.; Maenosono, R.; Namino, F.; Miyata, M.; Ohishi, M. A randomized comparison of two direct oral anticoagulants for patients undergoing cardiac ablation with a contemporary warfarin control arm. J. Interv. Card. Electrophysiol. 2021, 60, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Halbfass, P.; Szöllösi, A.; Dietrich, J.W.; Fochler, F.; Nentwich, K.; Roos, M.; Krug, J.; Schmitt, R.; Mügge, A.; et al. Impact of periprocedural anticoagulation strategy on the incidence of new-onset silent cerebral events after radiofrequency catheter ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2016, 46, 203–211. [Google Scholar] [CrossRef]
- Di Biase, L.; Lakkireddy, D.; Trivedi, C.; Deneke, T.; Martinek, M.; Mohanty, S.; Mohanty, P.; Prakash, S.; Bai, R.; Reddy, M.; et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: Results from a multicenter study. Heart Rhythm 2015, 12, 1162–1168. [Google Scholar] [CrossRef]
- Nakamura, K.; Naito, S.; Sasaki, T.; Minami, K.; Take, Y.; Goto, E.; Shimizu, S.; Yamaguchi, Y.; Suzuki, N.; Yano, T.; et al. Silent Cerebral Ischemic Lesions After Catheter Ablation of Atrial Fibrillation in Patients on 5 Types of Periprocedural Oral Anticoagulation—Predictors of Diffusion-Weighted Imaging-Positive Lesions and Follow-up Magnetic Resonance Imaging. Circ. J. 2016, 80, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.J.; Wang, H.; Huang, P.F. Peri-procedural novel oral anticoagulants dosing strategy during atrial fibrillation ablation: A meta-analysis. Pacing Clin. Electrophysiol. 2020, 43, 1104–1114. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Prins, N.D.; den Heijer, T.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 2003, 348, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Giambrone, A.E.; Gialdini, G.; Finn, C.; Delgado, D.; Gutierrez, J.; Wright, C.; Beiser, A.S.; Seshadri, S.; Pandya, A.; et al. Silent Brain Infarction and Risk of Future Stroke: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Haines, D.E.; Stewart, M.T.; Barka, N.D.; Kirchhof, N.; Lentz, L.R.; Reinking, N.M.; Urban, J.F.; Halimi, F.; Deneke, T.; Kanal, E. Microembolism and catheter ablation II: Effects of cerebral microemboli injection in a canine model. Circ. Arrhythmia Electrophysiol. 2013, 6, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takami, M.; Lehmann, H.I.; Parker, K.D.; Welker, K.M.; Johnson, S.B.; Packer, D.L. Effect of Left Atrial Ablation Process and Strategy on Microemboli Formation During Irrigated Radiofrequency Catheter Ablation in an In Vivo Model. Circ. Arrhythmia Electrophysiol. 2016, 9, e003226. [Google Scholar] [CrossRef] [PubMed]
- Maury, P.; Belaid, S.; Ribes, A.; Voglimacci-Stephanopoli, Q.; Mondoly, P.; Blaye, M.; Mandel, F.; Monteil, B.; Carrié, D.; Galinier, M.; et al. Coagulation and heparin requirements during ablation in patients under oral anticoagulant drugs. J. Arrhythmia 2020, 36, 644–651. [Google Scholar] [CrossRef]
- Martin, A.C.; Kyheng, M.; Foissaud, V.; Duhamel, A.; Marijon, E.; Susen, S.; Godier, A. Activated Clotting Time Monitoring during Atrial Fibrillation Catheter Ablation: Does the Anticoagulant Matter? J. Clin. Med. 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.C.; Godier, A.; Narayanan, K.; Smadja, D.M.; Marijon, E. Management of Intraprocedural Anticoagulation in Patients on Non-Vitamin K Antagonist Oral Anticoagulants Undergoing Catheter Ablation for Atrial Fibrillation: Understanding the Gaps in Evidence. Circulation 2018, 138, 627–633. [Google Scholar] [CrossRef]
- Pollack, C.V.; Reilly, P.A., Jr.; Eikelboom, J.; Glund, S.; Verhamme, P.; Bernstein, R.A.; Dubiel, R.; Huisman, M.V.; Hylek, E.M.; Kamphuisen, P.W.; et al. Idarucizumab for Dabigatran Reversal. N. Engl. J. Med. 2015, 373, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, S.J.; Milling, T.J., Jr.; Eikelboom, J.W.; Gibson, C.M.; Curnutte, J.T.; Gold, A.; Bronson, M.D.; Lu, G.; Conley, P.B.; Verhamme, P.; et al. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N. Engl. J. Med. 2016, 375, 1131–1141. [Google Scholar] [CrossRef] [Green Version]

| Study | Year | Regimen | n | Age (years) | Male Sex | Paroxysmal AF | CHA2DS2-VASc | HAS-BLED | Mean ACT | Target ACT | Total UFH Dose | Protamine | ICE | Ablation Technology | Follow-Up Period |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMPARE [36] (International) | 2014 | UI-Warfarin | 794 | 59 | 230 (73%) | 200 (63%) | NR | NR | NR | >300 | NR | Used † | 794 (100%) | RF | 48 h |
| I-Warfarin | 790 | 59 | 245 (77%) | 229 (72%) | >350 | 790 (100%) | |||||||||
| Nin [37] (Japan) | 2013 | I-Dabigatran 110 mg BID | 45 | 61 | 38 (84%) | 34 (76%) | NR | NR | NR | 300–400 | NR | Used † | NR | RF | 14 days |
| I-Warfarin | 45 | 61 | 36 (80%) | 32 (71%) | |||||||||||
| ABRIDGE-J [38] (Japan) | 2019 | I-Dabigatran 150/110 mg BID | 220 | 65 | 171 (78%) | 138 (63%) | 2.0 * | 1.0 * | NR | 300–400 | 14,000 | Used † | 52 (24%) | RF/Cryo | 3 months |
| UI-Warfarin | 222 | 66 | 160 (72%) | 138 (62%) | 2.0 * | 1.0 * | 9000 | 58 (26%) | |||||||
| VENTURE-AF [39] (International) | 2015 | UI-Rivaroxaban 20 mg OD | 114 | 59 | 86 (75%) | 95 (83%) | 1.5 | NR | 302 | 300–400 | 13,871 | 32 (28%) | Used † | Unclear | 30 days |
| UI-Warfarin | 107 | 61 | 90 (84%) | 87 (81%) | 1.7 | 332 | 10,964 | 27 (25%) | |||||||
| Kuwahara [40] (Japan) | 2016 | UI-Apixaban 5/2.5 mg BID | 100 | 65 | 75 (75%) | 59 (59%) | 2.1 | NR | 322 | >300 | 14,000 | Used † | NR | RF | 7 days |
| UI-Warfarin | 100 | 66 | 72 (72%) | 60 (60%) | 2.4 | 357 | 9000 | ||||||||
| RE-CIRCUIT [41] (International) | 2017 | UI-Dabigatran 150 mg BID | 317 | 59 | 230 (73%) | 213 (67%) | 2.0 | NR | 330 | >300 | 12,402 | Used † | NR | Mixed | 56 days |
| UI-Warfarin | 318 | 59 | 245 (77%) | 219 (69%) | 2.2 | 342 | 11,910 | ||||||||
| ASCERTAIN [42] (Japan) | 2018 | UI-Rivaroxaban 15/10 mg OD | 64 | 59 | 53 (83%) | 40 (63%) | NR | NR | 299 | 300–350 | 12,500 | Used † | NR | RF | 30 days |
| UI-Warfarin | 63 | 62 | 53 (84%) | 42 (67%) | 341 | 9000 | |||||||||
| AXAFA-AFNET 5 [43] (International) | 2018 | UI-Apixaban 5/2.5 mg BID | 318 | 64 | 218 (69%) | 189 (59%) | 2.4 | NR | 310 | >300 | NR | Used † | Used † | Mixed | 3 months |
| UI-Warfarin | 315 | 64 | 206 (65%) | 178 (57%) | 2.4 | 349 | |||||||||
| ELIMINATE-AF [44] (International) | 2019 | UI-Edoxaban 60 mg OD | 375 | 60 | 290 (77%) | 284 (76%) | 1.8 | NR | 303 | 300–400 | 14,261 | NR | 92 (25%) | RF/Cryo | 90 days |
| UI-Warfarin | 178 | 61 | 149 (84%) | 131 (74%) | 1.7 | 338 | 11,473 | 42 (24%) | |||||||
| Yoshimura [45] (Japan) | 2017 | UI-Rivaroxaban 15/10 mg OD | 55 | 59 | 45 (82%) | 33 (60%) | 1.7 | NR | 275 | >300 | 15,745 | NR | NR | RF | Unclear |
| I-Apixaban 5/2.5 mg BID | 50 | 59 | 41 (82%) | 31 (62%) | 1.7 | 286 | 14,240 | ||||||||
| AEIOU [46] (USA) | 2018 | UI-Apixaban 5 mg BID | 150 | 63 | 101 (67%) | 100 (67%) | 2.2 | 1.0 | NR | >300 | 17,800 | 137 (91%) | NR | RF/Cryo | 30 days |
| I-Apixaban 5/2.5 mg BID | 145 | 64 | 97 (67%) | 91 (63%) | 2.4 | 1.1 | 19,700 | 128 (88%) | |||||||
| Yu [47] (Korea) | 2019 | UI-DOAC (Api/Dab/Riv) | 106 | 59 | 81 (76%) | 67 (63%) | 1.6 | NR | 352 | 350–400 | 18,740 | NR | Used † | RF | 1 month |
| I-DOAC (Api/Dab/Riv) | 110 | 58 | 79 (72%) | 74 (67%) | 1.7 | 348 | 20,136 | ||||||||
| Nakamura [48] (Japan) | 2019 | UI-DOAC (Api/Dab/Edo/Riv) | 421 | 65 | 298 (71%) | 222 (53%) | 2.0 | 1.3 | 358 | 300–400 | 12,936 | 405 (96%) | NR | RF | 30 days |
| I-DOAC (Api/Dab/Edo/Riv) | 423 | 65 | 298 (70%) | 236 (58%) | 2.1 | 1.4 | 330 | 13,830 | 371 (88%) | ||||||
| Nagao [49] (Japan) | 2019 | UI-DOAC (Api/Edo/Riv) | 100 | 70 | 64 (64%) | 57 (57%) | 2.8 | NR | 285 | >300 | 8704 | Used † | Used † | RF | 1 month |
| I-DOAC (Api/Edo/Riv) | 100 | 70 | 62 (62%) | 59 (59%) | 2.6 | 280 | 9945 | ||||||||
| Ando [50] (Japan) | 2019 | UI-Apixaban 5 mg BID | 32 | 67 | 26 (81%) | 32 (100%) | NR | NR | NR | 300–350 | NR | Used † | NR | Cryo | 30 days |
| I-Apixaban 5 mg BID | 65 | 66 | 49 (75%) | 65 (100%) | |||||||||||
| Yamaji [51] (Japan) | 2019 | UI-DOAC (Api/Dab/Edo/Riv) | 277 | 66 | 211 (76%) | 171 (62%) | 1.9 | 1.4 | NR | 300–400 | NR | Used † | NR | RF | 90 days |
| I-DOAC (Api/Dab/Edo/Riv) | 307 | 65 | 212 (69%) | 199 (65%) | 1.9 | 1.4 | |||||||||
| Yoshimoto [52] (Japan) | 2021 | UI-Edoxaban 60/30 mg OD | 61 | 62 | 43 (70%) | 38 (62%) | 1.7 | 1.1 | 300 | >300 | 7333 | NR | NR | RF | Unclear |
| UI-Rivaroxaban 15/10 mg OD | 63 | 62 | 46 (73%) | 45 (71%) | 1.8 | 1.2 | 298 | 7865 |
| Strategy | Thromboembolic Events | Major Bleeding | Composite of Primary Outcomes | Minor Bleeding | Asymptomatic Cerebral Embolism | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-Score | SUCRA | P-Score | SUCRA | P-Score | SUCRA | P-Score | SUCRA | P-Score | SUCRA | |
| UI-DOAC | 0.72 | 0.73 | 0.81 | 0.76 | 0.82 | 0.82 | 0.62 | 0.65 | 0.64 | 0.60 |
| I-DOAC | 0.68 | 0.70 | 0.82 | 0.85 | 0.77 | 0.77 | 0.52 | 0.49 | 0.07 | 0.09 |
| UI-VKA | 0.60 | 0.57 | 0.33 | 0.34 | 0.41 | 0.40 | 0.87 | 0.86 | 0.79 | 0.82 |
| I-VKA | 0.00 | 0.00 | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | - | - |
| Strategy | Composite of Primary Outcomes | |
|---|---|---|
| P-Score | SUCRA | |
| UI-dabigatran | 0.93 | 0.95 |
| I-dabigatran | 0.89 | 0.82 |
| UI-apixaban | 0.52 | 0.53 |
| I-apixaban | 0.51 | 0.52 |
| UI-VKA | 0.46 | 0.47 |
| UI-rivaroxaban | 0.36 | 0.41 |
| UI-edoxaban | 0.33 | 0.30 |
| I-VKA | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kino, T.; Kagimoto, M.; Yamada, T.; Ishii, S.; Asai, M.; Asano, S.; Yano, H.; Ishikawa, T.; Ishigami, T. Optimal Anticoagulant Strategy for Periprocedural Management of Atrial Fibrillation Ablation: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2022, 11, 1872. https://doi.org/10.3390/jcm11071872
Kino T, Kagimoto M, Yamada T, Ishii S, Asai M, Asano S, Yano H, Ishikawa T, Ishigami T. Optimal Anticoagulant Strategy for Periprocedural Management of Atrial Fibrillation Ablation: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine. 2022; 11(7):1872. https://doi.org/10.3390/jcm11071872
Chicago/Turabian StyleKino, Tabito, Minako Kagimoto, Takayuki Yamada, Satoshi Ishii, Masanari Asai, Shunichi Asano, Hideto Yano, Toshiyuki Ishikawa, and Tomoaki Ishigami. 2022. "Optimal Anticoagulant Strategy for Periprocedural Management of Atrial Fibrillation Ablation: A Systematic Review and Network Meta-Analysis" Journal of Clinical Medicine 11, no. 7: 1872. https://doi.org/10.3390/jcm11071872
APA StyleKino, T., Kagimoto, M., Yamada, T., Ishii, S., Asai, M., Asano, S., Yano, H., Ishikawa, T., & Ishigami, T. (2022). Optimal Anticoagulant Strategy for Periprocedural Management of Atrial Fibrillation Ablation: A Systematic Review and Network Meta-Analysis. Journal of Clinical Medicine, 11(7), 1872. https://doi.org/10.3390/jcm11071872







