The Computer Simulation of Therapy with the NMDA Antagonist in Excitotoxic Neurodegeneration in an Alzheimer’s Disease-like Pathology
Abstract
1. Introduction
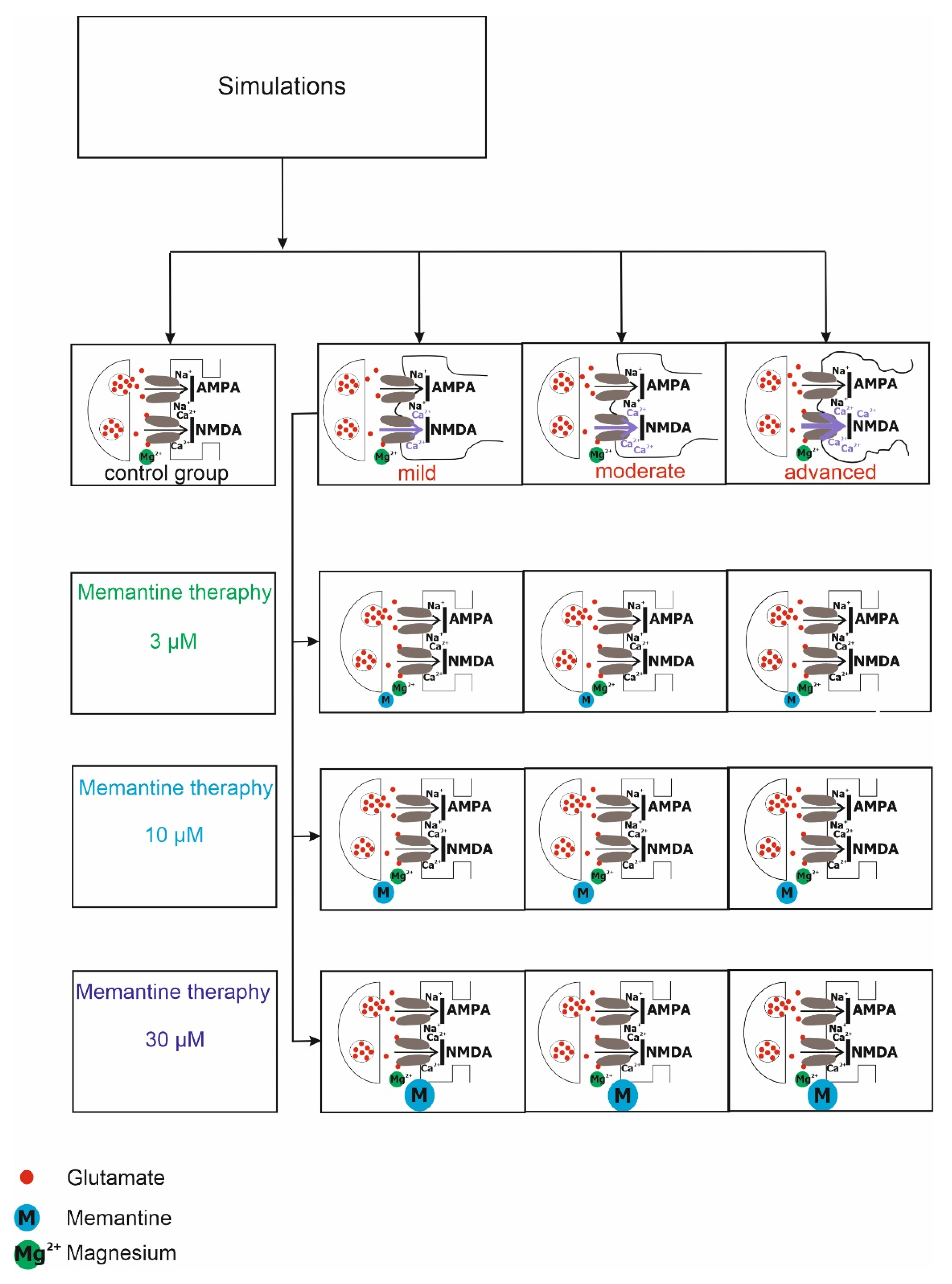
2. Methods
2.1. Synaptic Properties
2.2. Long-Term Potentiation (LTP) and LTP Duration
2.3. Excitotoxicity Model
2.4. Virtual Therapy with the NMDA Antagonist Memantine
2.5. Statistical Analysis
3. Results
3.1. NMDA Receptor Activity under Physiological, Pathological Conditions and Virtual Therapy with Memantine
3.2. Neuronal Parameters
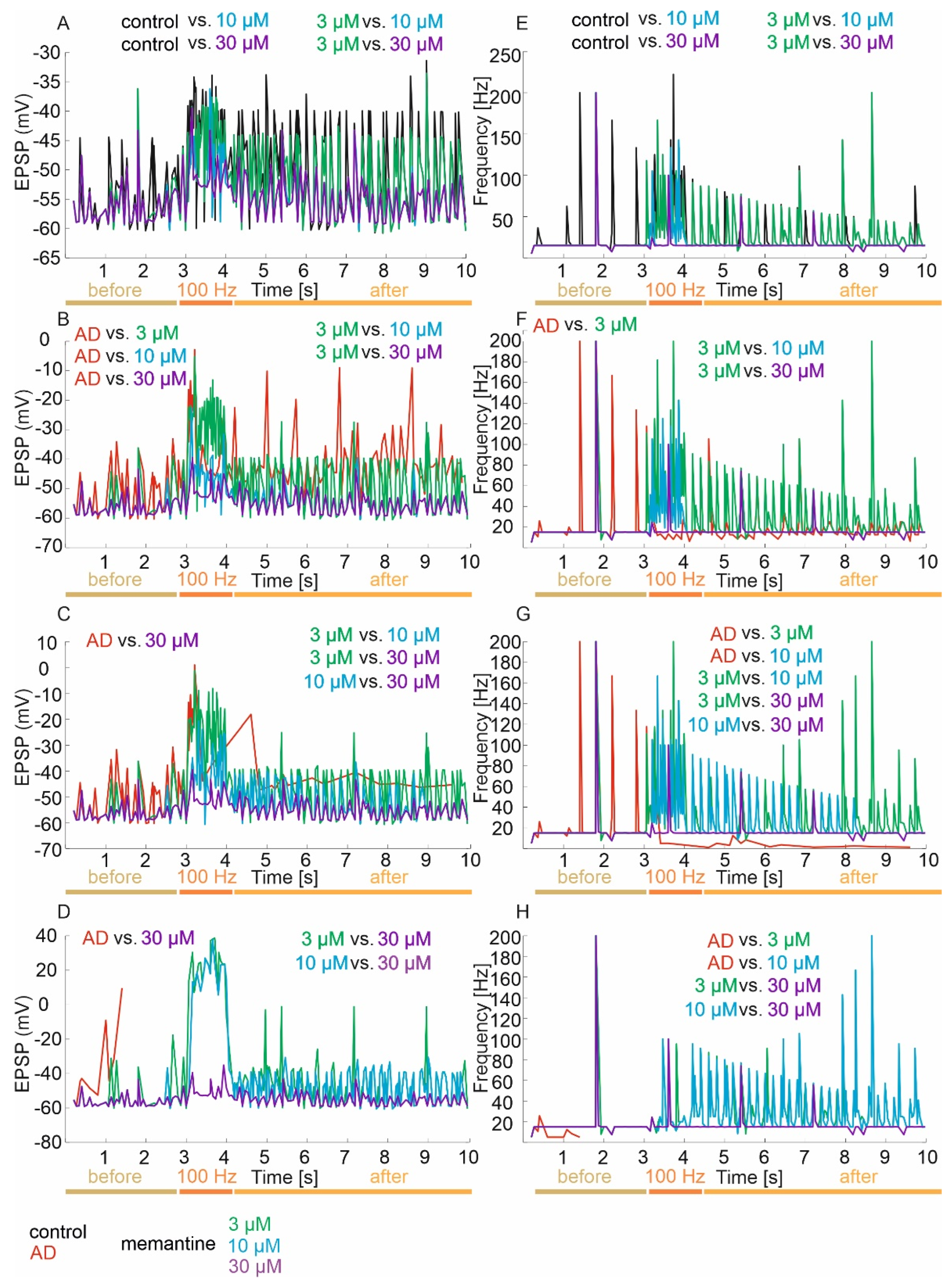
3.2.1. ISI, EPSP and Frequency
Control Group
Mild Degree of Excitotoxicity
Moderate Degree of Excitotoxicity
Advanced Degree of Excitotoxicity
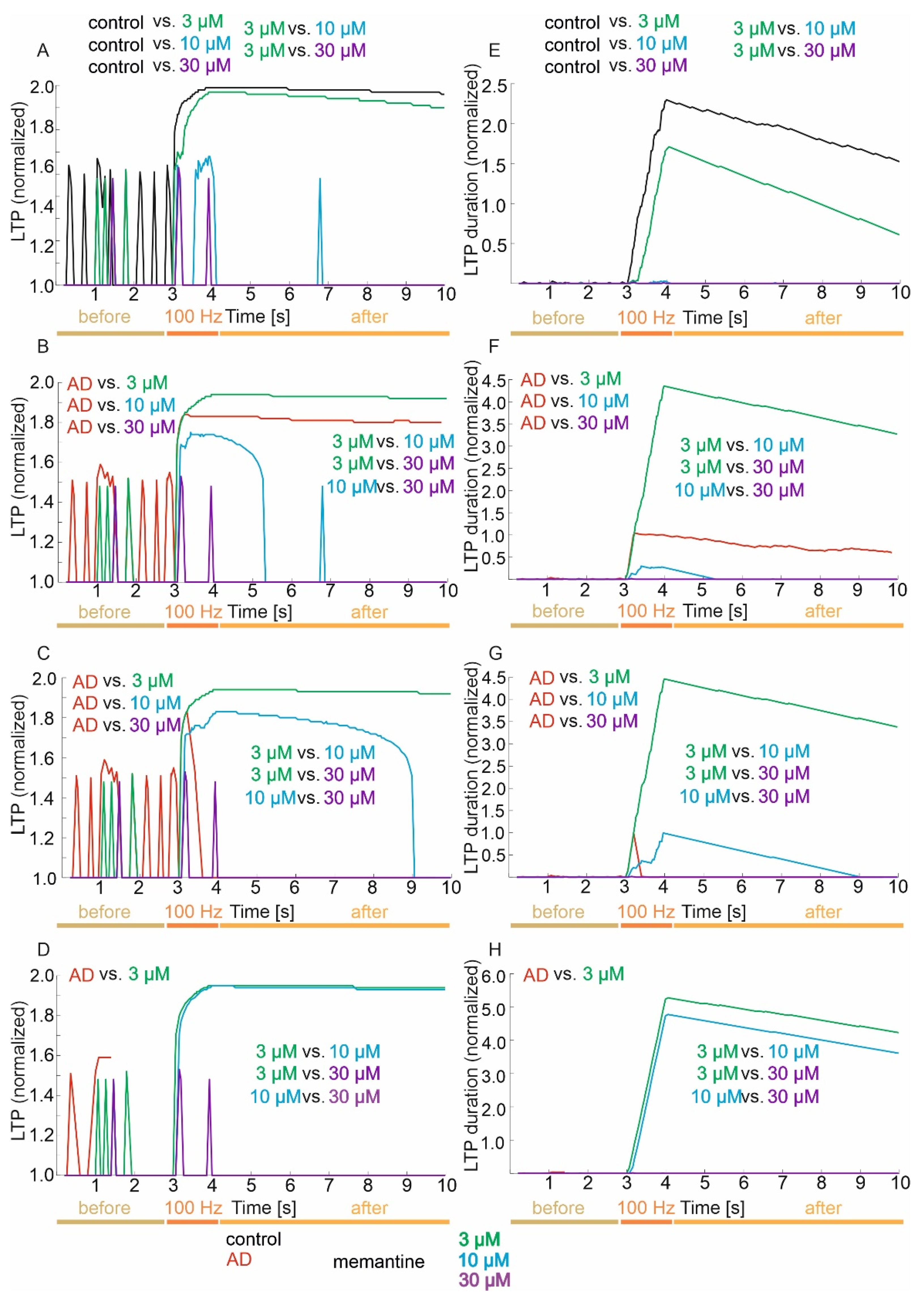
3.3. Memory (LTP) and LTP Duration
3.3.1. Control Group
3.3.2. Mild Degree of Excitotoxicity
3.3.3. Moderate Degree of Excitotoxicity
3.3.4. Advanced Degree of Excitotoxicity
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dementia in Europe Yearbook 2019. Estimating the Prevalence of Dementia in Europe; Rue Dicks: Luxembourg, 2019; pp. 72–74.
- Liu-Seifert, H.; Schumi, J.; Miao, X.; Tian, Y.; Rabbia, M.; Andersen, S.W.; Wilson, S.; Li, W.; Entsuah, R. Disease Modification in Alzheimer’s Disease: Current Thinking. Ther. Innov. Regul. Sci. 2020, 54, 396–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Feng, J.; Wu, M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 2016, 37, 1039–1047. [Google Scholar] [CrossRef]
- Uddin, M.S.; Nasrullah, M.; Hossain, M.S. Evaluation of nootropic activity of persicaria flaccida on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: Implication for the management of Alzheimer’s disease. Am. J. Psychiatry Neurosci. 2016, 4, 26. [Google Scholar] [CrossRef][Green Version]
- Uddin, M.S.; Mamun, A.A.; Kabir, M.T. Neurochemistry of Neurochemicals: Messengers of Brain Functions. J. Intellect. Disabil. Diagnosis Treat. 2018, 5, 137–151. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.R. LTP forms 1, 2 and 3: Different mechanisms for the ‘long’ in long-term potentiation. Trends Neurosci. 2007, 30, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.E. Alzheimer’s disease: Targeting the glutamatergic system. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, E.K. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol. 1998, 54, 369–415. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Mattson, M.P. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron 1990, 4, 105–117. [Google Scholar] [CrossRef]
- PelTy, G.; Nunomura, A.; Cash, A.D.; Tadoko, M.R.; Hirai, K.; Aliev, G.; Avila, J.; Watoya, T.; Shimohama, S.; Atwood, C.S.; et al. Reactive oxygen: Its sources and significance in Alzheimer’s disease. J. Neurol. Transm. Suppl. 2002, 69–75. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G.; Mobius, H.J.; Stoffler, A.; Quack, G. Neuroprotective and symptomatological action of memantine relevant for Alzheimer’s disease–A unified glutamatergic hypothesis of mechanism of action. Neurotox. Res. 2000, 2, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C. Memantine for the treatment of dementia: A review on its current and future applications. J. Alzheimers Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.K.; Kaul, C.L.; Ramarao, P. Dementia of Alzheimer’s disease and other neurodegenerative disorders-memantine, a new hope. Pharmacol. Res. 2005, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Areosa, S.A.; Sherriff, F.; McShane, R. Memantine for dementia. Cochrane Database Syst. Rev. 2005, 3, CD003154. [Google Scholar]
- Johnson, J.W.; Kotermanski, S.E. Mechanism of action of memantine. Curr. Opin. Pharmacol. 2006, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Quack, G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—A review of preclinical data. Neuropharmacology 1999, 38, 735–767. [Google Scholar] [CrossRef]
- Lipton, S.A.; Chen, H.S. Paradigm shift in NMDA receptor drug development. Expert Opin. Ther. Targets 2005, 9, 427–429. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Cichońska, D. Memory and forgetting processes with the firing neuron model. Folia Morphol. 2018, 77, 221–233. [Google Scholar] [CrossRef]
- Świetlik, D. Simulations of Learning, Memory, and Forgetting Processes with Model of CA1 Region of the Hippocampus. Complexity 2018, 1297150. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J.; Kusiak, A.; Cichońska, D. A computational simulation of long-term synaptic potentiation inducing protocol processes with model of CA3 hippocampal microcircuit. Folia Morphol. 2018, 77, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D.; Białowąs, J.; Moryś, J.; Kusiak, A. Computer Model of Synapse Loss During an Alzheimer’s Disease-like Pathology in Hippocampal Subregions DG, CA3 and CA1—the Way to Chaos and Information Transfer. Entropy 2019, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D.; Białowąs, J.; Moryś, J.; Klejbor, I.; Kusiak, A. Effects of Inducing Gamma Oscillations in Hippocampal Subregions DG, CA3, and CA1 on the Potential Alleviation of Alzheimer’s Disease-Related Pathology: Computer Modeling and Simulations. Entropy 2019, 21, 587. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J. Application of Artificial Neural Networks to Identify Alzheimer’s Disease Using Cerebral Perfusion SPECT Data. Int. J. Environ. Res. Public Health 2019, 16, 1303. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, D.; Bandurski, T.; Lass, P. Artificial neural networks in nuclear medicine. Nucl. Med. Rev. Cent East Eur. 2004, 7, 59–67. [Google Scholar] [PubMed]
- Rajendran, V.; Purohit, R.; Sethumadhavan, R. In silico investigation of molecular mechanism of laminopathy caused by a point mutation (R482W) in lamin A/C protein. Amino Acids 2012, 43, 603–615. [Google Scholar] [CrossRef]
- Rajendran, V.; Sethumadhavan, R. Drug resistance mechanism of PncA in Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2014, 32, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.; Gopalakrishnan, C.; Sethumadhavan, R. Pathological role of a point mutation (T315I) in BCR-ABL1 protein-A computational insight. J. Cell. Biochem. 2018, 119, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V.; Gopalakrishnan, C.; Purohit, R. Impact of point mutation P29S in RAC1 on tumorigenesis. Tumour. Biol. 2016, 37, 15293–15304. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhardwaj, V.K.; Sharma, J.; Das, P.; Purohit, R. Discovery and in silico evaluation of aminoarylbenzosuberene molecules as novel checkpoint kinase 1 inhibitor determinants. Genomics 2021, 113, 707–715. [Google Scholar] [CrossRef]
- Singh, R.; Bhardwaj, V.K.; Sharma, J.; Purohit, R. Identification of novel and selective agonists for ABA receptor PYL3. Plant Physiol. Biochem. 2020, 154, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, V. Structural analysis of oncogenic mutation of isocitrate dehydrogenase 1. Mol. Biosyst. 2016, 12, 2276–2287. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Chen, H.S.; Wang, Y.F.; Rayudu, P.V.; Edgecomb, P.; Neill, J.C.; Segall, M.M.; Lipton, S.A.; Jensen, F.E. Neuroprotective concentrations of N-methylD-asparate open-chane1 blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or lon g-term potentiation. Neuroscience 1998, 86, 1121–1132. [Google Scholar] [CrossRef]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Masuko, T.; Nguyen, C.D. Channel blockers acting at N-methyl-D-aspartate receptors: Differential effects of mutations in the vestibule and ion channel pore. Mol. Pharmacol. 2002, 61, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Gruner, R.; Rozental, J.; Millar, J.; Lodge, D. Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan). Neuropharmacology 1993, 32, 1337–1350. [Google Scholar] [CrossRef]
- Sobolevsky, A.I.; Koshelev, S.G.; Khodorov, B.I. Interaction of memantine and amantadine with agonist-unbound NMDA-receptor channels in acutely isolated rat hippocampal neurons. J. Physiol. 1998, 512, 47–60. [Google Scholar] [CrossRef]
- Bresink, I.; Benke, T.A.; Collett, V.J. Effects of memantine on recombinant rat NMDA receptors expressed in HEK 293 cells. Br. J. Pharmacol. 1996, 119, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Danysz, W.; Bartmann, A. Amino-alkyl-cyclohexanes are novel uncompetitive NMDA receptor antagonists with strong voltage-dependency and fast blocking kinetics: In vitro and in vivo characterization. Neuropharmacology 1999, 38, 85–108. [Google Scholar] [CrossRef]
- Statistical Software. 2017. Available online: https://www.tibco.com/ (accessed on 1 September 2017).
- Bames, C.A.; Danysz, W.; Parsons, C.G. Effects of the uncompetitive NMDA receptor antagonist memantine on hippocampallong-term potentiation, short-term exploratory modulation and spatial memory in awake, fee1y moving rats. Eur. J. Neurosci. 1996, 8, 565–567I. [Google Scholar]
- Mondadori, C.; Weiskrantz, L.; Buerki, H.; Petschke, F.; Fagg, G.E. NMDA receptor antagonists can enhance or impair leaming performance in animals. Exp. Brain Res. 1989, 75, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowski, W.; Quack, G.; Danysz, W. Infusion of (+) MK-80l and memantine–Contrasting effects on radial maze learning in rats with entorhinal cortex lesion. Eur. J. Pharmacol. 1996, 296, 239–346. [Google Scholar] [CrossRef]
- Wenk, G.L.; Zajączkowski, W.; Danysz, W. Neuroprotection of acetylocholinergic basal forebrain neurons by memantine and neurokinin B. Behav. Brain Res. 1997, 83, 129–133. [Google Scholar] [CrossRef]
- Winblad, B.; Poritis, N. Memantine in severe dementia: Results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine). Int. J. Geriatr. Psychiatry 1999, 14, 135–146. [Google Scholar] [CrossRef]
- Doody, R.S.; Tariot, P.N.; Pfeiffer, E.; Olin, J.T.; Graham, S.M.; Bell, J.M.; for the Memantine Study Group. Meta-analysis of 6- -month memantine clinical trials in Alzheimer’s disease. In Proceedings of the Conference Proceeding: New Clinical Drug Evaluation Unit 45 Annual Meeting, Boca Raton, FL, USA, 6–9 June 2005. [Google Scholar]
- Schneider, L.S.; Dagerman, K.S.; Higgins, J.P.; McShane, R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch. Neurol. 2011, 68, 991–998. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świetlik, D.; Kusiak, A.; Krasny, M.; Białowąs, J. The Computer Simulation of Therapy with the NMDA Antagonist in Excitotoxic Neurodegeneration in an Alzheimer’s Disease-like Pathology. J. Clin. Med. 2022, 11, 1858. https://doi.org/10.3390/jcm11071858
Świetlik D, Kusiak A, Krasny M, Białowąs J. The Computer Simulation of Therapy with the NMDA Antagonist in Excitotoxic Neurodegeneration in an Alzheimer’s Disease-like Pathology. Journal of Clinical Medicine. 2022; 11(7):1858. https://doi.org/10.3390/jcm11071858
Chicago/Turabian StyleŚwietlik, Dariusz, Aida Kusiak, Marta Krasny, and Jacek Białowąs. 2022. "The Computer Simulation of Therapy with the NMDA Antagonist in Excitotoxic Neurodegeneration in an Alzheimer’s Disease-like Pathology" Journal of Clinical Medicine 11, no. 7: 1858. https://doi.org/10.3390/jcm11071858
APA StyleŚwietlik, D., Kusiak, A., Krasny, M., & Białowąs, J. (2022). The Computer Simulation of Therapy with the NMDA Antagonist in Excitotoxic Neurodegeneration in an Alzheimer’s Disease-like Pathology. Journal of Clinical Medicine, 11(7), 1858. https://doi.org/10.3390/jcm11071858





