A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Examination
- ABSI = 1000 × WC × Wt −2/3 × Ht5/6 [37];
- TMI = weight (kg)/height (m)3 [26];
- ConI = WC/(0.109 × (Wt/Ht)0.5) [38];
- VAI [39]Male = [WC/(39.68 + (1.88 × BMI))] × (TG/1.03) × (1.31/HDL-C);Female = [WC/(36.58 + (1.89 × BMI))] × (TG/0.81) × (1.52/HDL-C);
- Fat Mass (eFM) = weight − exp(0.3073 × height2 −10.0155 × d-growth-standards/standards/body-mass-index-for-age-bmi-for-age weight − 1 + 0.004571 × weight − 0.9180 × ln(age) + 0.6488 × age0.5 + 0.04723 × male + 2.8055) [40] (exp = exponential function, ln = natural logarithmic transformation, male = 1, female = 0).
2.3. Biochemical Evaluation
2.4. Statistical Analysis
3. Results
3.1. Univariate Analysis
3.2. Multivariate Analysis
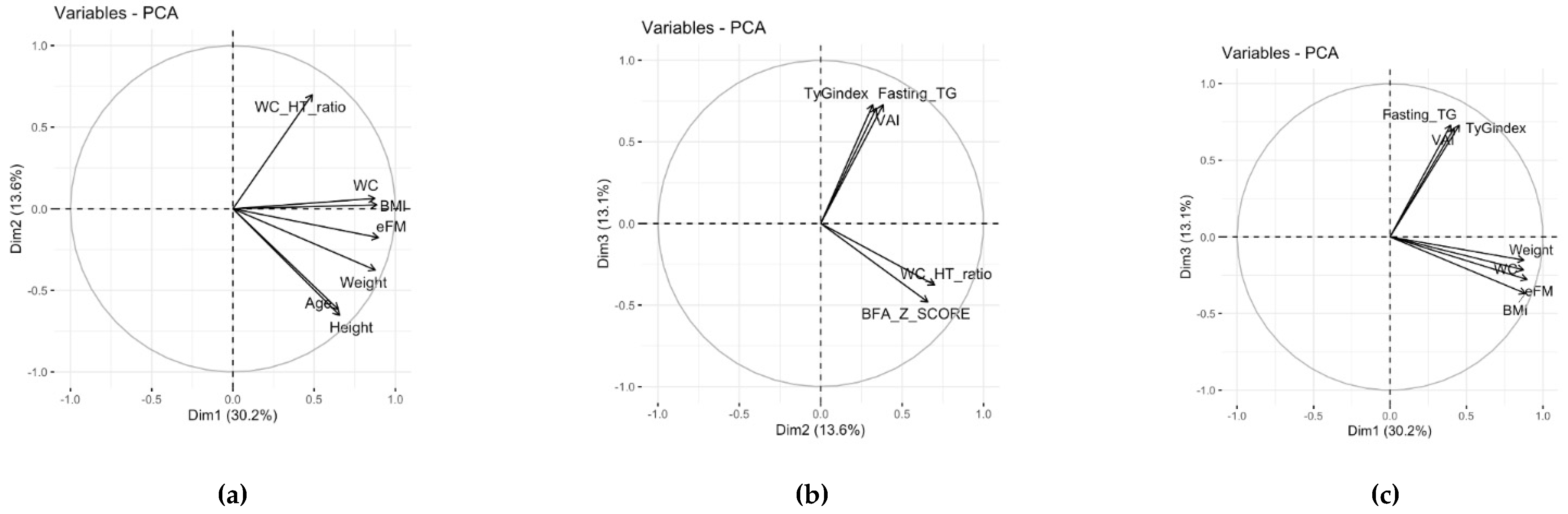
3.2.1. Principal Component Analysis
3.2.2. Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skinner, A.C.; Ravanbakht, S.N.; Skelton, J.A.; Perrin, E.M.; Armstrong, S.C. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics 2018, 141, e20173459. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 February 2022).
- WHO. Levels and Trends in Child Malnutrition: UNICEF/WHO/The World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2020 Edition. Available online: https://www.who.int/publications/i/item/jme-2020-edition (accessed on 2 February 2022).
- Morales Camacho, W.J.; Molina Díaz, J.M.; Plata Ortiz, S.; Plata Ortiz, J.E.; Morales Camacho, M.A.; Calderón, B.P. Childhood Obesity: Aetiology, Comorbidities, and Treatment. Diabetes Metab. Res. Rev. 2019, 35, e3203. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Klersy, C.; Muratori, T.; Telli, S.; Caramagna, C.; Scaglia, F.; Cisternino, M.; Larizza, D. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clin. Endocrinol. 2008, 68, 868–872. [Google Scholar] [CrossRef]
- Reisinger, C.; Nkeh-Chungag, B.N.; Fredriksen, P.M.; Goswami, N. The Prevalence of Pediatric Metabolic Syndrome-a Critical Look on the Discrepancies between Definitions and Its Clinical Importance. Int. J. Obes. 2021, 45, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome: Clinical and Policy Implications of the New Silent Killer. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 365–367. [Google Scholar] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the Metabolic Syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, V. Metabolic Syndrome: What Are the Risks for Humans? Biosci. Trends 2010, 4, 204–212. [Google Scholar] [PubMed]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2016, 15, 30–39. [Google Scholar] [CrossRef]
- Wittcopp, C.; Conroy, R. Metabolic Syndrome in Children and Adolescents. Pediatr. Rev. 2016, 37, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal Programming of the Metabolic Syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Bussler, S.; Penke, M.; Flemming, G.; Elhassan, Y.S.; Kratzsch, J.; Sergeyev, E.; Lipek, T.; Vogel, M.; Spielau, U.; Körner, A.; et al. Novel Insights in the Metabolic Syndrome in Childhood and Adolescence. Horm. Res. Paediatr. 2017, 88, 181–193. [Google Scholar] [CrossRef]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamad, D.; Raman, V. Metabolic Syndrome in Children and Adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Cota, B.C.; Priore, S.E.; Ribeiro, S.A.V.; Juvanhol, L.L.; de Faria, E.R.; de Faria, F.R.; Pereira, P.F. Cardiometabolic Risk in Adolescents with Normal Weight Obesity. Eur. J. Clin. Nutr. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Kahn, H.S.; Mei, Z.; Grummer-Strawn, L.M.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. Relation of Body Mass Index and Waist-to-Height Ratio to Cardiovascular Disease Risk Factors in Children and Adolescents: The Bogalusa Heart Study. Am. J. Clin. Nutr. 2007, 86, 33–40. [Google Scholar] [CrossRef]
- Tee, J.Y.H.; Gan, W.Y.; Lim, P.Y. Comparisons of Body Mass Index, Waist Circumference, Waist-to-Height Ratio and a Body Shape Index (ABSI) in Predicting High Blood Pressure among Malaysian Adolescents: A Cross-Sectional Study. BMJ Open 2020, 10, e032874. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Wormser, D.; Kaptoge, S.; Di Angelantonio, E.; Wood, A.M.; Pennells, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; et al. Separate and Combined Associations of Body-Mass Index and Abdominal Adiposity with Cardiovascular Disease: Collaborative Analysis of 58 Prospective Studies. Lancet 2011, 377, 1085–1095. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.; Chang, Y.; Sun, G.; Sun, Y. New Anthropometric Indices or Old Ones: Which Perform Better in Estimating Cardiovascular Risks in Chinese Adults. BMC Cardiovasc. Disord. 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmanesh, M.; Sardarinia, M.; Hajsheikholeslami, F.; Azizi, F.; Hadaegh, F. CVD-Predictive Performances of “a Body Shape Index” versus Simple Anthropometric Measures: Tehran Lipid and Glucose Study. Eur. J. Nutr. 2016, 55, 147–157. [Google Scholar] [CrossRef]
- Mameli, C.; Krakauer, N.Y.; Krakauer, J.C.; Bosetti, A.; Ferrari, C.M.; Moiana, N.; Schneider, L.; Borsani, B.; Genoni, T.; Zuccotti, G. The Association between a Body Shape Index and Cardiovascular Risk in Overweight and Obese Children and Adolescents. PLoS ONE 2018, 13, e0190426. [Google Scholar] [CrossRef]
- Peterson, C.M.; Su, H.; Thomas, D.M.; Heo, M.; Golnabi, A.H.; Pietrobelli, A.; Heymsfield, S.B. Tri-Ponderal Mass Index vs Body Mass Index in Estimating Body Fat During Adolescence. JAMA Pediatr. 2017, 171, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Correa-Bautista, J.E.; Carrillo, H.A.; González-Jiménez, E.; Schmidt-RioValle, J.; Correa-Rodríguez, M.; García-Hermoso, A.; González-Ruíz, K. Tri-Ponderal Mass Index vs. Fat Mass/Height3 as a Screening Tool for Metabolic Syndrome Prediction in Colombian Children and Young People. Nutrients 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.G.; Guedes, D.P. Performance of Anthropometric Indicators as Predictors of Metabolic Syndrome in Brazilian Adolescents. BMC Pediatr. 2018, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Vizzuso, S.; Brambilla, P.; Mameli, C.; Ravella, S.; De Amicis, R.; Battezzati, A.; Zuccotti, G.; Bertoli, S.; Verduci, E. Evaluation of Different Adiposity Indices and Association with Metabolic Syndrome Risk in Obese Children: Is There a Winner? Int. J. Mol. Sci. 2020, 21, 4083. [Google Scholar] [CrossRef]
- Damanhoury, S.; Morrison, K.M.; Mian, R.; McPhee, P.G.; Kozyrskyj, A.L.; Newton, A.S.; Buchholz, A.; Chanoine, J.-P.; Hamilton, J.; Ho, J.; et al. Metabolically Healthy Obesity in Children Enrolled in the CANadian Pediatric Weight Management Registry (CANPWR): An Exploratory Secondary Analysis of Baseline Data. Clin. Obes. 2021, 12, e12490. [Google Scholar] [CrossRef] [PubMed]
- Chashmniam, S.; Hashemi Madani, N.; Nobakht Mothlagh Ghoochani, B.F.; Safari-Alighiarloo, N.; Khamseh, M.E. The Metabolome Profiling of Obese and Non-Obese Individuals: Metabolically Healthy Obese and Unhealthy Non-Obese Paradox. Iran. J. Basic Med. Sci. 2020, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Rajalahti, T.; Kroksveen, A.C.; Arneberg, R.; Berven, F.S.; Vedeler, C.A.; Myhr, K.-M.; Kvalheim, O.M. A Multivariate Approach to Reveal Biomarker Signatures for Disease Classification: Application to Mass Spectral Profiles of Cerebrospinal Fluid from Patients with Multiple Sclerosis. J. Proteome Res. 2010, 9, 3608–3620. [Google Scholar] [CrossRef]
- Rajalahti, T.; Kvalheim, O.M. Multivariate Data Analysis in Pharmaceutics: A Tutorial Review. Int. J. Pharm. 2011, 417, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; De Silvestri, A.; Magenes, V.C.; Siccardo, F.; Schneider, L.; Vizzuso, S.; Bosetti, A.; Zuccotti, G. Predictive Ability of the Estimate of Fat Mass to Detect Early-Onset Metabolic Syndrome in Prepubertal Children with Obesity. Children 2021, 8, 966. [Google Scholar] [CrossRef]
- Calcaterra, V.; Winickoff, J.P.; Klersy, C.; Schiano, L.M.; Bazzano, R.; Montalbano, C.; Musella, V.; Regalbuto, C.; Larizza, D.; Cena, H. Smoke exposure and cardio-metabolic profile in youth with type 1 diabetes. Diabetol. Metab. Syndr. 2018, 10, 53. [Google Scholar] [CrossRef]
- Calcaterra, V.; De Giuseppe, R.; Biino, G.; Mantelli, M.; Marchini, S.; Bendotti, G.; Madè, A.; Avanzini, M.A.; Montalbano, C.; Cossellu, G.; et al. Relation between circulating oxidized-LDL and metabolic syndrome in children with obesity: The role of hypertriglyceridemic waist phenotype. J. Pediatr. Endocrinol. Metab. 2017, 30, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 2 February 2022).
- Krakauer, N.Y.; Krakauer, J.C. A New Body Shape Index Predicts Mortality Hazard Independently of Body Mass Index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef] [PubMed]
- Mangla, A.G.; Dhamija, N.; Gupta, U.; Dhall, M. Anthropometric Markers as a Paradigm for Obesity Risk Assessment. J. Biosci. Med. 2020, 8, 1–16. [Google Scholar] [CrossRef][Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Licenziati, M.R.; Iannuzzo, G.; Morlino, D.; Campana, G.; Renis, M.; Iannuzzi, A.; Valerio, G. Fat Mass and Vascular Health in Overweight/Obese Children. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1317–1323. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Banzato, C.; Talamini, G.; Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-Height Ratio, a Useful Index to Identify High Metabolic Risk in Overweight Children. J. Pediatr. 2008, 152, 207–213. [Google Scholar] [CrossRef]
- d’Annunzio, G.; Vanelli, M.; Pistorio, A.; Minuto, N.; Bergamino, L.; Iafusco, D.; Lorini, R.; Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes. Insulin Resistance and Secretion in Healthy Italian Children and Adolescents: A Multicentre Study. Acta Bio-Med. Atenei Parm. 2009, 80, 21–28. [Google Scholar]
- Videira-Silva, A.; Fonseca, H. The effect of a physical activity consultation on body mass index z-score of overweight adolescents: Results from a pediatric outpatient obesity clinic. Eur. J. Pediatrics 2017, 176, 655–660. [Google Scholar] [CrossRef]
- Pratley, R.E.; Weyer, C. The Role of Impaired Early Insulin Secretion in the Pathogenesis of Type II Diabetes Mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Hadjiyannakis, S. The Metabolic Syndrome in Children and Adolescents. Paediatr. Child Health 2005, 10, 41–47. [Google Scholar] [CrossRef]
- Valaiyapathi, B.; Gower, B.; Ashraf, A.P. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.L.; Reinehr, T. Roles of Gastrointestinal and Adipose Tissue Peptides in Childhood Obesity and Changes after Weight Loss Due to Lifestyle Intervention. Arch. Pediatr. Adolesc. Med. 2010, 164, 131–138. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel Subgroups of Adult-Onset Diabetes and Their Association with Outcomes: A Data-Driven Cluster Analysis of Six Variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- Friend, A.; Craig, L.; Turner, S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, G.M.; Bedoya, G.; Estrada, A.; Patiño, F.A.; Muñoz, A.M.; Velásquez, C.M. Variations in the Prevalence of Metabolic Syndrome in Adolescents According to Different Criteria Used for Diagnosis: Which Definition Should Be Chosen for This Age Group? Metab. Syndr. Relat. Disord. 2014, 12, 202–209. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; García, E.G.; Robles, L.; Riaño, D.; Ruiz-Gomez, D.G.; García-Ulloa, A.C.; Melgarejo, M.A.; Zamora, M.; Guillen-Pineda, L.E.; Mehta, R.; et al. High Adiponectin Concentrations Are Associated with the Metabolically Healthy Obese Phenotype. J. Clin. Endocrinol. Metab. 2008, 93, 4075–4079. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Faraj, M.; Bastard, J.-P.; St-Pierre, D.H.; Brochu, M.; Prud’homme, D.; Rabasa-Lhoret, R. The Metabolically Healthy but Obese Individual Presents a Favorable Inflammation Profile. J. Clin. Endocrinol. Metab. 2005, 90, 4145–4150. [Google Scholar] [CrossRef]
- Brochu, M.; Tchernof, A.; Dionne, I.J.; Sites, C.K.; Eltabbakh, G.H.; Sims, E.A.; Poehlman, E.T. What Are the Physical Characteristics Associated with a Normal Metabolic Profile despite a High Level of Obesity in Postmenopausal Women? J. Clin. Endocrinol. Metab. 2001, 86, 1020–1025. [Google Scholar] [CrossRef]
- Kwon, B.-J.; Kim, D.-W.; Her, S.-H.; Kim, D.-B.; Jang, S.-W.; Cho, E.-J.; Ihm, S.-H.; Kim, H.-Y.; Youn, H.-J.; Seung, K.-B.; et al. Metabolically Obese Status with Normal Weight Is Associated with Both the Prevalence and Severity of Angiographic Coronary Artery Disease. Metabolism 2013, 62, 952–960. [Google Scholar] [CrossRef]
- Navarro-González, D.; Sánchez-Íñigo, L.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martinez, J.A. Triglyceride-Glucose Index (TyG Index) in Comparison with Fasting Plasma Glucose Improved Diabetes Prediction in Patients with Normal Fasting Glucose: The Vascular-Metabolic CUN Cohort. Prev. Med. 2016, 86, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, M.; Niwa, T.; Nakajima, K.; Kobayashi, M.; Hanyu, N.; Nakayama, T. Serum Triglyceride Levels Correlated with the Rate of Change in Insulin Secretion over Two Years in Prediabetic Subjects. Ann. Nutr. Metab. 2014, 64, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Srinivasan, S.R.; Harsha, D.W.; Webber, L.S.; Berenson, G.S. Relation of Body Fat Patterning to Lipid and Lipoprotein Concentrations in Children and Adolescents: The Bogalusa Heart Study. Am. J. Clin. Nutr. 1989, 50, 930–939. [Google Scholar] [CrossRef]
- Calcaterra, V.; Biganzoli, G.; Pelizzo, G.; Cena, H.; Rizzuto, A.; Penagini, F.; Verduci, E.; Bosetti, A.; Lucini, D.; Biganzoli, E.; et al. A Multivariate Pattern Analysis of Metabolic Profile in Neurologically Impaired Children and Adolescents. Children 2021, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, B.; Liu, Y.; Sun, X.; Luo, X.; Wang, C.; Li, L.; Zhang, L.; Ren, Y.; Zhao, Y.; et al. Cumulative Increased Risk of Incident Type 2 Diabetes Mellitus with Increasing Triglyceride Glucose Index in Normal-Weight People: The Rural Chinese Cohort Study. Cardiovasc. Diabetol. 2017, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Íñigo, L.; Navarro-González, D.; Pastrana-Delgado, J.; Fernández-Montero, A.; Martínez, J.A. Association of Triglycerides and New Lipid Markers with the Incidence of Hypertension in a Spanish Cohort. J. Hypertens. 2016, 34, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Du, T.; Zhang, J.; Lu, H.; Lin, X.; Xie, J.; Yang, Y.; Yu, X. The Triglyceride and Glucose Index (TyG) Is an Effective Biomarker to Identify Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2017, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Íñigo, L.; Navarro-González, D.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martínez, J.A. The TyG Index May Predict the Development of Cardiovascular Events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Hou, C.-Y.; Hsu, W.-H.; Tain, Y.-L. Early-Life Origins of Metabolic Syndrome: Mechanisms and Preventive Aspects. Int. J. Mol. Sci. 2021, 22, 11872. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M. Insulin Resistance and Hypertension: New Insights. Kidney Int. 2015, 87, 497–499. [Google Scholar] [CrossRef]
- Raitakari, O.T.; Juonala, M.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Mäki-Torkko, N.; Järvisalo, M.J.; Uhari, M.; Jokinen, E.; Rönnemaa, T.; et al. Cardiovascular Risk Factors in Childhood and Carotid Artery Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Nittari, G.; Scuri, S.; Petrelli, F.; Pirillo, I.; di Luca, N.M.; Grappasonni, I. Fighting Obesity in Children from European World Health Organization Member States. Epidemiological Data, Medical-Social Aspects, and Prevention Programs. Clin. Ter. 2019, 170, e223–e230. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garach, A.; Cornejo-Pareja, I.; Tinahones, F.J. Does Metabolically Healthy Obesity Exist? Nutrients 2016, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Bell, J.A.; Hamer, M.; Batty, G.D.; Singh-Manoux, A.; Sabia, S.; Kivimäki, M. Incidence of Metabolic Risk Factors among Healthy Obese Adults: 20-Year Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The Metabolic Syndrome—A New Worldwide Definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could Excessive Fructose Intake and Uric Acid Cause Type 2 Diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Hübers, M.; Geisler, C.; Plachta-Danielzik, S.; Müller, M.J. Association between Individual Fat Depots and Cardio-Metabolic Traits in Normal- and Overweight Children, Adolescents and Adults. Nutr. Diabetes 2017, 7, e267. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Poirier, P.; Bergeron, J.; Alméras, N.; Lamarche, B.; Cantin, B.; Dagenais, G.R.; Després, J.-P. Hypertriglyceridemic Waist: A Useful Screening Phenotype in Preventive Cardiology? Can. J. Cardiol. 2007, 23, 23B–31B. [Google Scholar] [CrossRef]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically Healthy Obesity: Facts and Fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef] [PubMed]


| Children with Obesity | Children with Normal Weight | |||||
|---|---|---|---|---|---|---|
| Level | F | M | Level | F | M | |
| n | 274 | 254 | 60 | 59 | ||
| Age (median (IQR)) (years) | 10.00 (9.00, 12.00) | 11.00 (9.25, 13.00) | 10.00 (9.00, 11.25) | 12.00 (11.00, 13.00) | ||
| Weight (median (IQR)) (kg) | 58.25 (45.65, 73.50) | 60.70 (49.78, 72.95) | 35.55 (32.20, 39.90) | 37.50 (29.75, 49.00) | ||
| Height (median (IQR)) (cm) | 146.00 (136.00, 155.30) | 149.00 (140.00, 157.78) | 143.55 (135.70, 152.50) | 146.80 (136.80, 154.30) | ||
| BMI (median (IQR)) (kg/m2) | 27.01 (24.43, 30.25) | 27.26 (25.03, 29.68) | 17.50 (16.17, 18.94) | 17.33 (15.77, 20.07) | ||
| WC (median (IQR)) (cm) | 84.00 (79.00, 92.50) | 88.20 (81.50, 94.88) | 62.75 (60.00, 66.25) | 61.00 (57.50, 73.00) | ||
| WC/Ht (median (IQR)) | 0.59 (0.55, 0.63) | 0.59 (0.57, 0.63) | 0.43 (0.42, 0.47) | 0.44 (0.41, 0.47) | ||
| Total Cholesterol (median (IQR)) (mg/dL) | 149.00 (135.00, 168.75) | 154.00 (138.00, 175.75) | 156.00 (130.00, 166.50) | 154.00 (144.00, 174.00) | ||
| HDL Cholesterol (median (IQR)) (mg/dL) | 45.00 (39.00, 52.00) | 46.00 (39.00, 53.00) | 56.00 (46.75, 60.00) | 54.00 (49.00, 65.00) | ||
| LDL Cholesterol (median (IQR)) (mg/dL) | 86.20 (72.45, 101.15) | 90.20 (75.40, 106.05) | 85.90 (68.65, 101.80) | 87.80 (81.60, 107.80) | ||
| TG (median (IQR)) (mg/dL) | 81.00 (62.00, 108.00) | 72.50 (58.00, 107.00) | 43.50 (34.00, 63.75) | 49.00 (40.00, 57.00) | ||
| Fasting Glucose (median (IQR)) (mg/dL) | 80.00 (75.00, 86.00) | 84.00 (78.25, 88.00) | 75.00 (67.00, 78.25) | 76.00 (71.50, 82.00) | ||
| Insulin (median (IQR)) (µU/mL) | 14.20 (10.43, 21.25) | 13.70 (8.72, 19.70) | 5.60 (4.00, 7.28) | 5.00 (2.88, 8.50) | ||
| HOMA_IR (median (IQR)) | 2.85 (1.96, 4.24) | 2.71 (1.76, 4.11) | 0.99 (0.73, 1.35) | 0.94 (0.51, 1.60) | ||
| Pubertal_stage (%) | 1 | 81 (29.6) | 51 (20.1) | 1 | 14 (23.3) | 12 (20.3) |
| 2 | 138 (50.4) | 163 (64.2) | 2 | 34 (56.7) | 41 (69.5) | |
| 3 | 55 (20.1) | 40 (15.7) | 3 | 12 (20.0) | 6 (10.2) | |
| SBP (median (IQR)) (mmHg) | 110.00 (105.00, 120.00) | 111.00 (105.00, 120.00) | 100.00 (95.00, 106.25) | 105.00 (100.00, 110.00) | ||
| DBP (median (IQR)) (mmHg) | 62.00 (57.00, 70.00) | 61.00 (58.00, 70.00) | 60.00 (60.00, 70.00) | 65.00 (60.00, 70.00) | ||
| VAI (median (IQR)) | 1.38 (0.99, 2.03) | 1.96 (1.37, 3.01) | 0.53 (0.47, 0.97) | 0.86 (0.81, 1.13) | ||
| ABSI (median (IQR)) | 0.05 (0.04, 0.05) | 0.05 (0.04, 0.05) | 0.05 (0.05, 0.06) | 0.05 (0.05, 0.06) | ||
| TMI (median (IQR)) | 18.76 (17.48, 20.26) | 18.51 (17.12, 20.00) | 12.04 (11.46, 13.74) | 12.21 (11.36, 13.01) | ||
| ConI (median (IQR)) | 3.94 (3.75, 4.12) | 4.05 (3.85, 4.20) | 3.64 (3.54, 3.74) | 3.66 (3.52, 3.79) | ||
| TyG Index (median (IQR)) | 8.09 (7.81, 8.40) | 8.04 (7.72, 8.42) | 7.36 (7.21, 7.67) | 7.51 (7.19, 7.75) | ||
| eFM (median (IQR)) (kg) | 24.42 (18.13, 31.23) | 23.45 (19.13, 29.09) | 9.75 (7.56, 11.95) | 7.45 (5.35, 11.97) | ||
| BMI z-score (median (IQR)) | 2.74 (2.42, 3.11) | 2.97 (2.57, 3.48) | 0.40 (−0.42, 0.96) | 0.12 (−0.77, 0.64) | ||
| MS (%) | 0 | 197 (71.9) | 136 (53.5) | 0 | 59 (98.3) | 59 (100.0) |
| 1 | 77 (28.1) | 118 (46.5) | 1 | 1 (1.7) | 0 (0.0) | |
| Level | Children in Subgroup 1 (S1) | Children in Subgroup 2 (S2) | Children in Subgroup 3 (S3) | Children in Subgroup 2 with MS | |
|---|---|---|---|---|---|
| n | 61 | 230 | 237 | 44 | |
| Weight (median (IQR)) (kg) | 82.50 (70.50, 95.60) | 46.00 (40.15, 52.25) | 69.90 (62.42, 77.00) | 46.00 (42.50, 52.70) | |
| Height (median (IQR)) (cm) | 156.00 (149.50, 163.50) | 136.50 (128.50, 142.00) | 155.00 (150.40, 161.85) | 139.00 (131.00, 145.70) | |
| BMI (median (IQR)) (kg/m2) | 33.50 (31.05, 36.47) | 24.69 (23.45, 26.13) | 28.76 (27.08, 30.55) | 24.87 (23.97, 25.71) | |
| WC (median (IQR)) (cm) | 102.00 (97.00, 109.00] | 80.00 (75.00, 83.88] | 90.00 (86.00, 96.00] | 81.50 (78.75, 83.25] | |
| WC/Ht (median (IQR)) | 0.66 (0.63, 0.70) | 0.59 (0.56, 0.62) | 0.58 (0.54, 0.62) | 0.59 (0.56, 0.63) | |
| Total Cholesterol (median (IQR]) (mg/dL) | 160.00 (139.00, 186.00] | 155.50 (140.00, 177.00] | 148.00 (132.00, 162.00) | 152.00 (143.75, 181.00) | |
| HDL Cholesterol (median (IQR)) (mg/dL) | 39.00 (35.00, 45.00) | 48.00 (41.00, 55.00) | 45.00 (40.00, 51.00) | 39.50 (35.00, 46.00) | |
| LDL Cholesterol (median (IQR)) (mg/dL) | 85.60 (71.40, 103.80) | 91.60 (75.45, 108.00) | 84.20 (73.40, 97.40) | 92.20 (77.50, 109.15) | |
| TG (median (IQR)) (mg/dL) | 128.00 (102.00, 193.00) | 71.00 (56.00, 94.00) | 76.00 (59.00, 99.00) | 124.00 (77.00, 156.25) | |
| Fasting Glucose (median (IQR)) (mg/dL) | 87.00 (80.00, 92.00) | 80.00 (76.00, 85.00) | 82.00 (76.00, 89.00) | 82.00 (79.00, 85.00) | |
| Insulin (median (IQR)) (µU/mL) | 30.30 (22.10, 38.10) | 9.95 (7.00, 14.20) | 16.10 (12.20, 20.80) | 13.90 (9.78, 18.10) | |
| HOMA-IR (median (IQR)) | 6.30 (4.70, 7.88) | 2.01 (1.40, 2.86) | 3.18 (2.20, 4.31) | 2.88 (2.07, 3.75) | |
| SBP (median (IQR)) (mmHg) | 123.00 (115.00, 130.00) | 106.00 (100.00, 111.00) | 115.00 (110.00, 122.00) | 105.00 (98.00, 111.50) | |
| DBP (median (IQR)) (mmHg) | 68.00 (60.00, 74.00) | 60.00 (55.00, 65.00) | 65.00 (60.00, 70.00) | 60.00 (53.75, 65.00) | |
| VAI (median (IQR)) | 3.82 (2.20, 5.13) | 1.43 (0.95, 2.04) | 1.64 (1.18, 2.43) | 2.97 (1.90, 4.20) | |
| ABSI (median (IQR)) | 0.05 (0.04, 0.05) | 0.04 (0.04, 0.05) | 0.05 (0.05, 0.05) | 0.05 (0.04, 0.05) | |
| TMI (median (IQR)) (kg/m3) | 21.56 (20.02, 24.04) | 18.26 (17.12, 19.90) | 18.43 (17.32, 19.80) | 17.79 (16.64, 19.19) | |
| ConI (median (IQR)) (cm) | 4.16 (3.99, 4.28) | 4.00 (3.82, 4.15) | 3.94 (3.74, 4.11) | 4.08 (3.87, 4.23) | |
| TyG Index (median (IQR)) | 8.59 (8.36, 9.02) | 7.97 (7.68, 8.24) | 8.06 (7.78, 8.33) | 8.52 (8.15, 8.77) | |
| eFM (median (IQR)) (kg) | 36.52 (32.26, 40.76) | 17.84 (15.32, 20.75) | 27.91 (24.50, 30.91) | 17.78 (16.03, 20.17) | |
| BMI z-score (median (IQR)) | 3.45 (2.98, 3.95) | 2.92 (2.58, 3.55) | 2.68 (2.39, 2.97) | 2.83 (2.55, 3.18) | |
| MS (%) | 0 | 4 (6.6) | 186 (80.9) | 143 (60.3) | 44 (100.0) |
| 1 | 57 (93.4) | 44 (19.1) | 94 (39.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Biganzoli, G.; Ferraro, S.; Verduci, E.; Rossi, V.; Vizzuso, S.; Bosetti, A.; Borsani, B.; Biganzoli, E.; Zuccotti, G. A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome. J. Clin. Med. 2022, 11, 1856. https://doi.org/10.3390/jcm11071856
Calcaterra V, Biganzoli G, Ferraro S, Verduci E, Rossi V, Vizzuso S, Bosetti A, Borsani B, Biganzoli E, Zuccotti G. A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome. Journal of Clinical Medicine. 2022; 11(7):1856. https://doi.org/10.3390/jcm11071856
Chicago/Turabian StyleCalcaterra, Valeria, Giacomo Biganzoli, Simona Ferraro, Elvira Verduci, Virginia Rossi, Sara Vizzuso, Alessandra Bosetti, Barbara Borsani, Elia Biganzoli, and Gianvincenzo Zuccotti. 2022. "A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome" Journal of Clinical Medicine 11, no. 7: 1856. https://doi.org/10.3390/jcm11071856
APA StyleCalcaterra, V., Biganzoli, G., Ferraro, S., Verduci, E., Rossi, V., Vizzuso, S., Bosetti, A., Borsani, B., Biganzoli, E., & Zuccotti, G. (2022). A Multivariate Analysis of “Metabolic Phenotype” Patterns in Children and Adolescents with Obesity for the Early Stratification of Patients at Risk of Metabolic Syndrome. Journal of Clinical Medicine, 11(7), 1856. https://doi.org/10.3390/jcm11071856









