Evaluation of the Existing Electrophysiological Severity Classifications in Carpal Tunnel Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Nerve Conduction Study
2.3. Analysis
3. Results
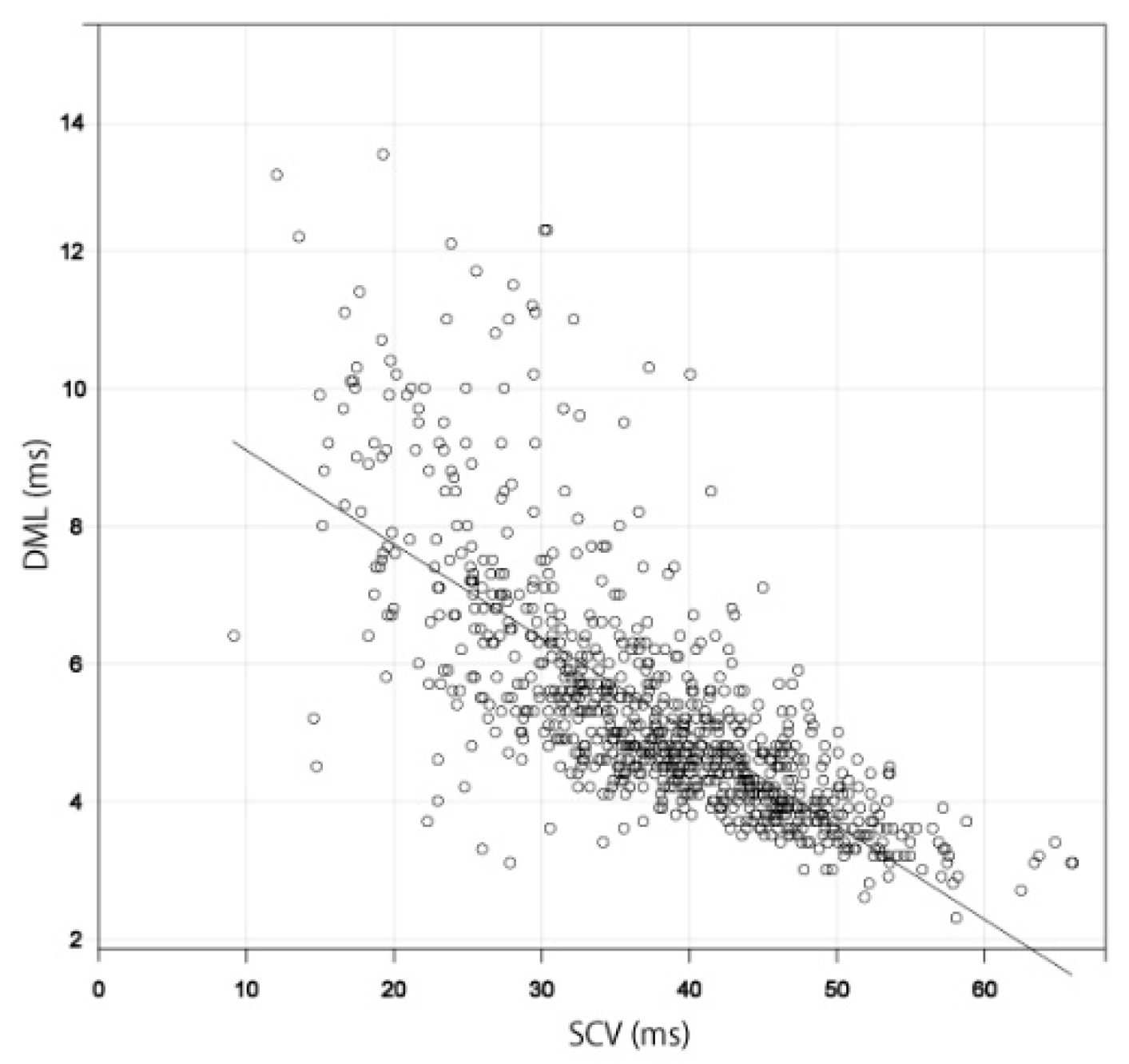
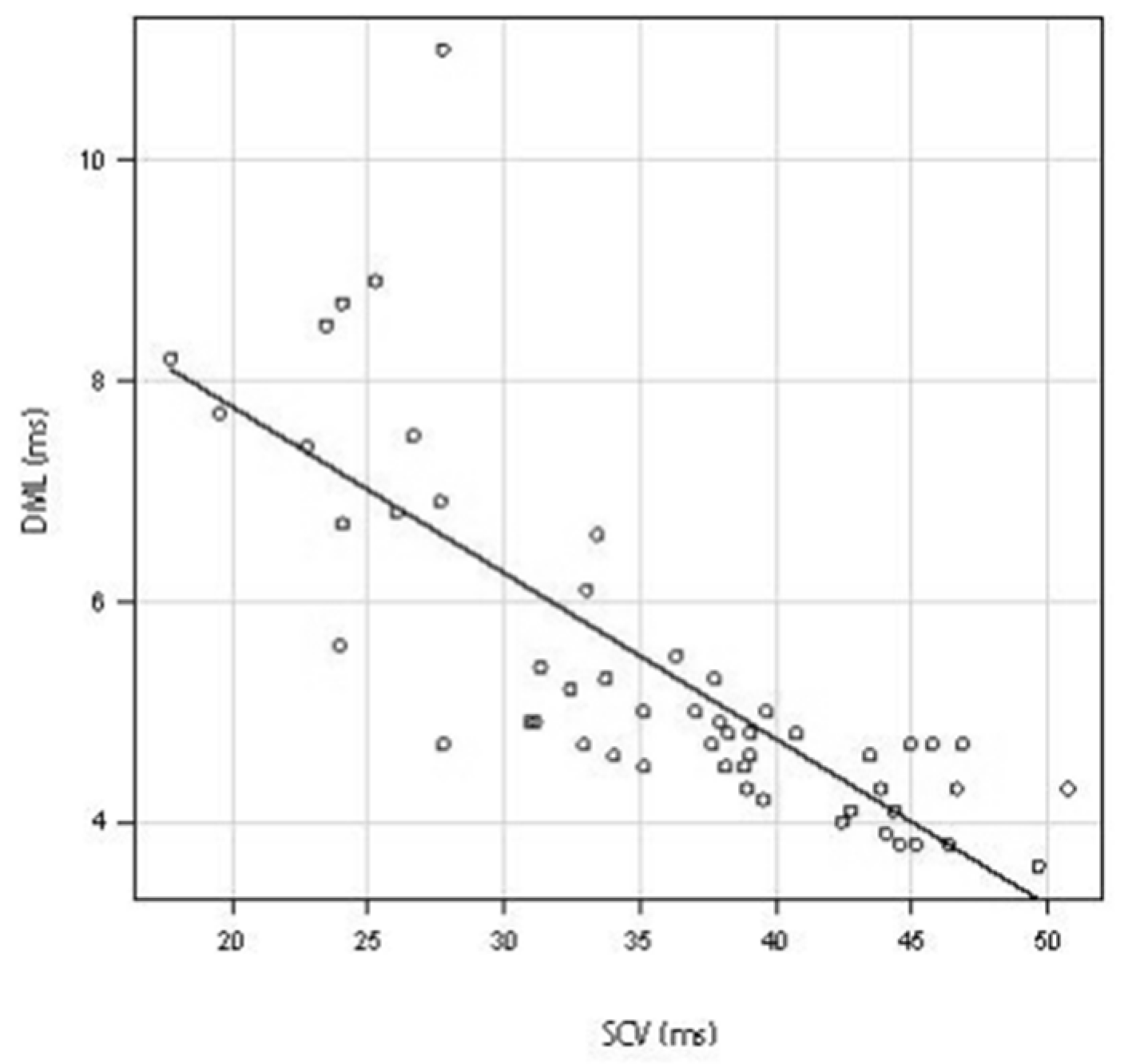
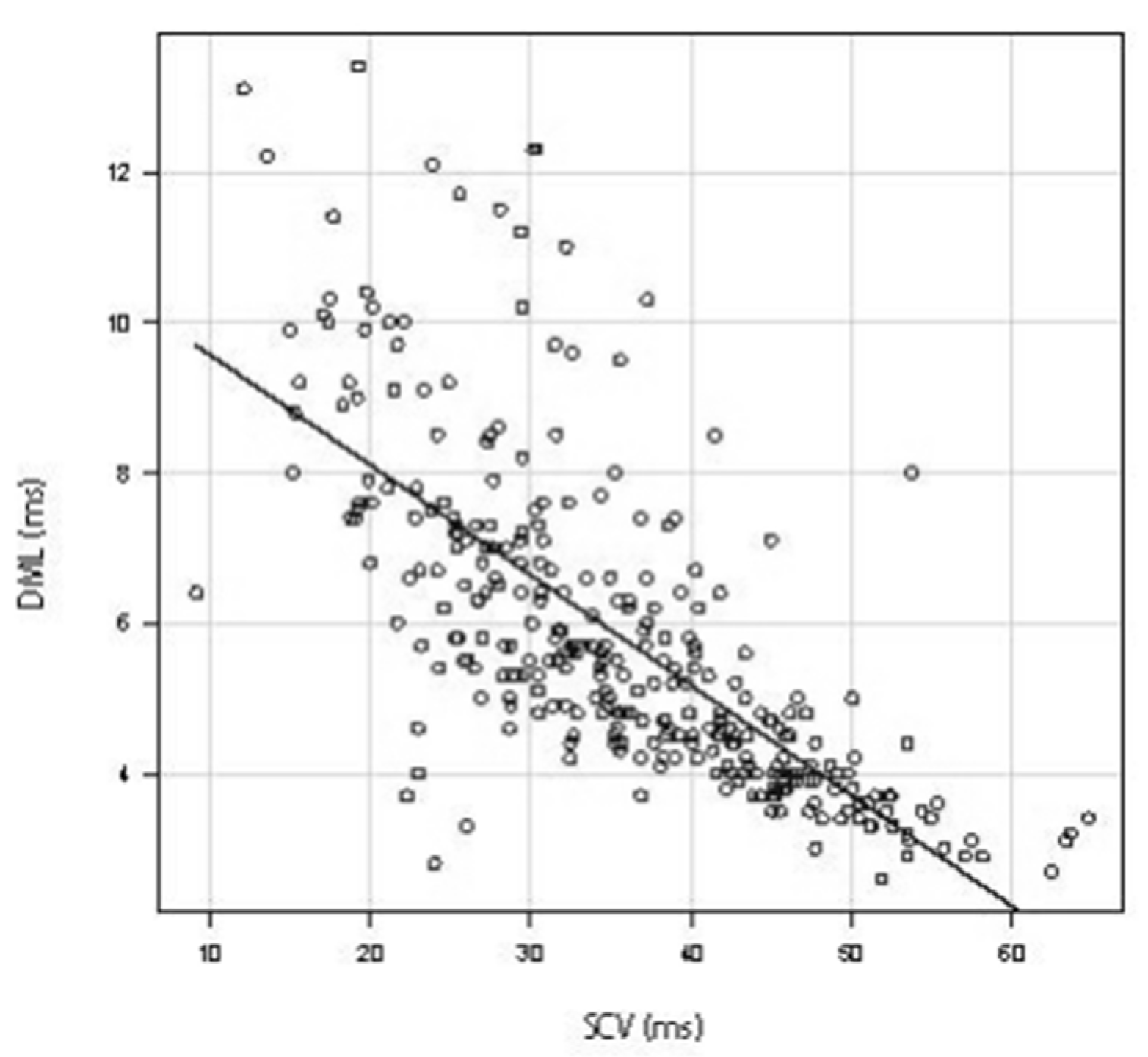
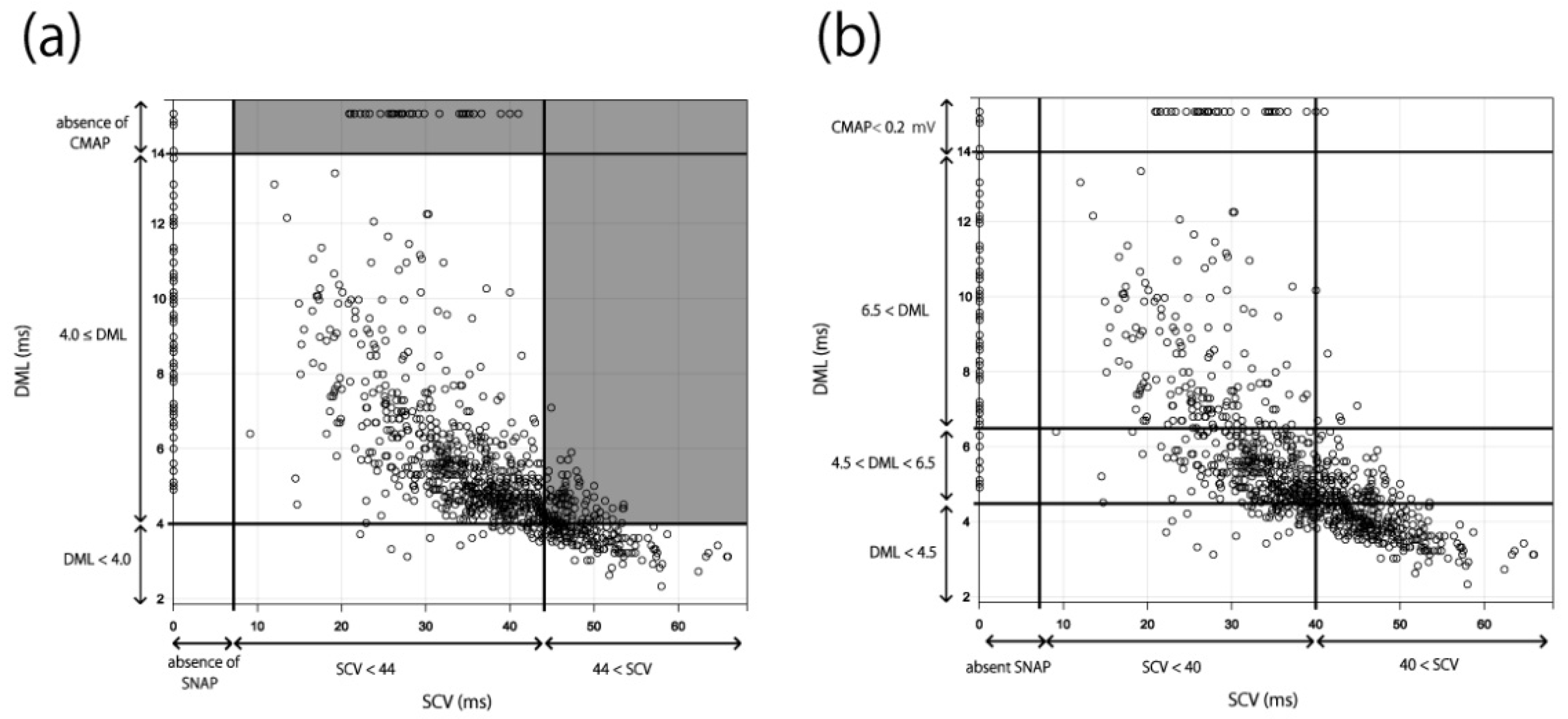
3.1. Relationship between SCV and DML
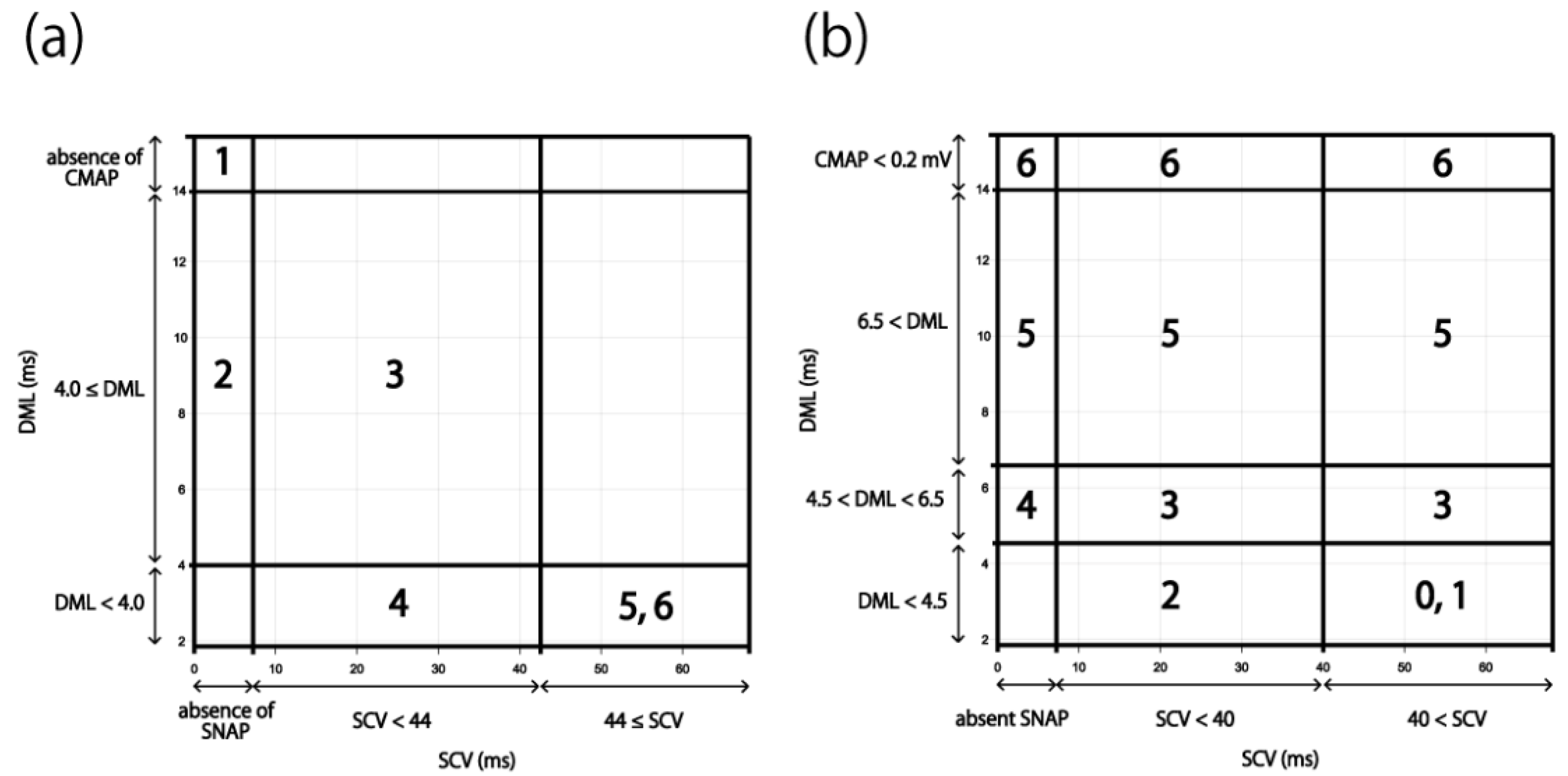
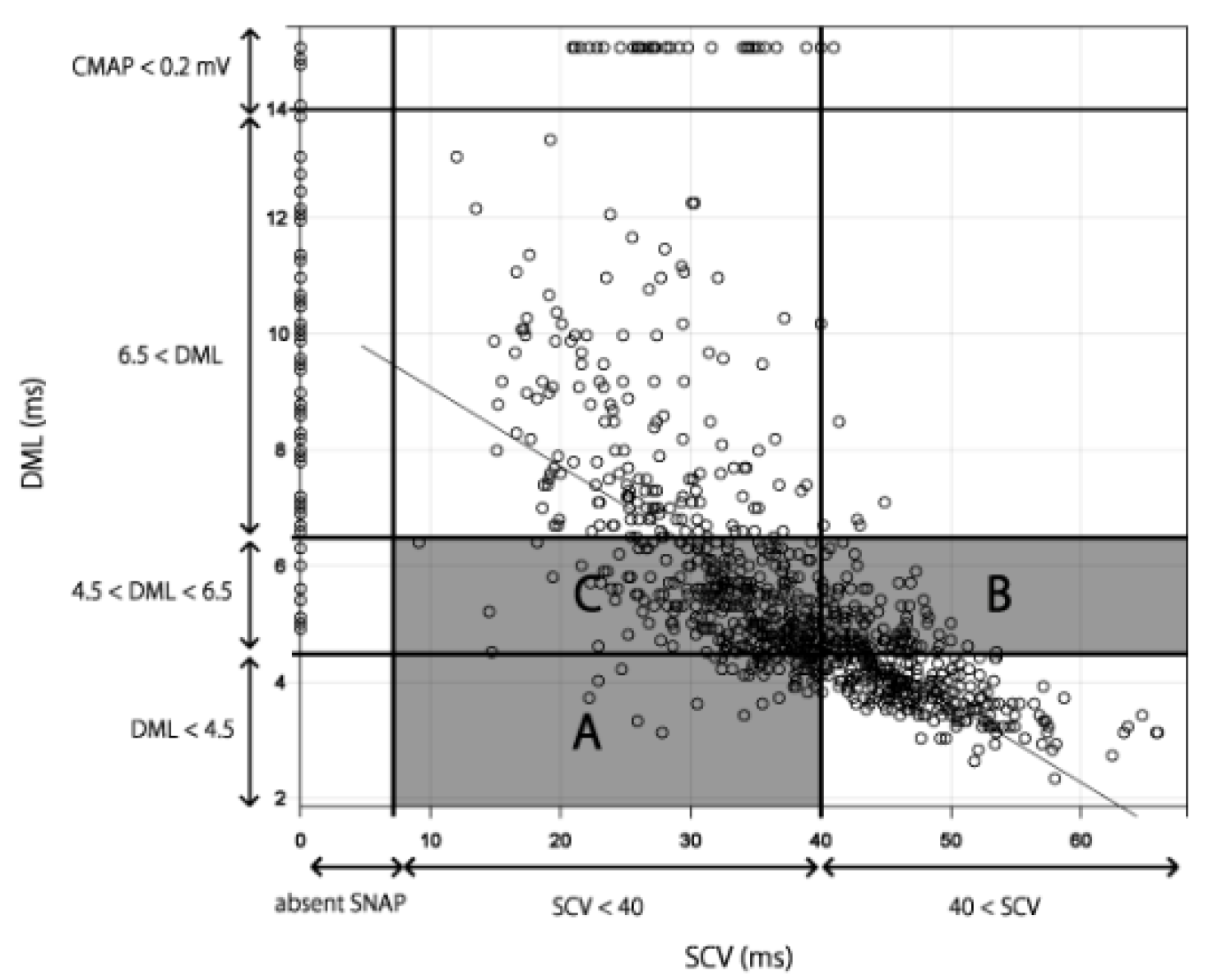
3.2. Applying the Results to the Existing Severity Classifications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujita, K.; Kimori, K.; Nimura, A.; Okawa, A.; Ikuta, Y. MRI analysis of carpal tunnel syndrome in hemodialysis patients versus non-hemodialysis patients: A multicenter case-control study. J. Orthop. Surg. Res. 2019, 14, 91. [Google Scholar] [CrossRef]
- Jablecki, C.K.; Andary, M.T.; Floeter, M.K.; Miller, R.G.; Quartly, C.A.; Vennix, M.J.; Wilson, J.R. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome: Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2002, 58, 1589–1592. [Google Scholar] [CrossRef]
- Kouyoumdjian, J.; Zanetta, D.M.; Morita, M.P. Evaluation of age, body mass index, and wrist index as risk factors for carpal tunnel syndrome severity. Muscle Nerve 2001, 25, 93–97. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Fujita, K.; Nimura, A.; Miyamoto, T.; Sasaki, T.; Okawa, A. A new method of measuring the thumb pronation and palmar abduction angles during opposition movement using a three-axis gyroscope. J. Orthop. Surg. Res. 2018, 13, 288. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Nimura, A.; Suzuki, S.; Sasaki, T.; Okawa, A.; Fujita, K. Measurement of thumb pronation and palmar abduction angles with a small motion sensor: A comparison with Kapandji scores. J. Hand Surg. 2019, 44, 728–733. [Google Scholar] [CrossRef]
- Werner, R.A.; Andary, M. Electrodiagnostic evaluation of carpal tunnel syndrome. Muscle Nerve 2011, 44, 597–607. [Google Scholar] [CrossRef]
- AAEM Quality Assurance Committee; Jablecki, C.K.; Andary, C.M.T.; So, Y.T.; Wilkins, D.E.; Williams, F.H. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve 1993, 16, 1392–1414. [Google Scholar] [CrossRef]
- Nathan, P.A.; Keniston, R.C.; Meadows, K.D.; Lockwood, R.S. Predictive value of nerve conduction measurements at the carpal tunnel. Muscle Nerve 1993, 16, 1377–1382. [Google Scholar] [CrossRef]
- Sonoo, M.; Menkes, D.L.; Bland, J.D.; Burke, D. Nerve conduction studies and EMG in carpal tunnel syndrome: Do they add value? Clin. Neurophysiol. Pract. 2018, 3, 78–88. [Google Scholar] [CrossRef]
- You, H.C.; Simmons, Z.; Freivalds, A.; Kothari, M.J.; Naidu, S.H. Relationships Between Clinical Symptom Severity Scales and Nerve Conduction Measures in Carpal Tunnel Syndrome. Muscle Nerve 1999, 22, 497–501. [Google Scholar] [CrossRef]
- Bland, J.D. A Neurophysiological Grading Scale for Carpal Tunnel Syndrome. Muscle Nerve 2000, 23, 1280–1283. [Google Scholar] [CrossRef]
- Padua, L.; Lomonaco, M.; Gregori, B.; Valente, E.M.; Padua, R.; Tonali, P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol. Scand. 2009, 96, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C. AAEM Minimonograph #26: The Electrodiagnosis of Carpal Tunnel Syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve 1997, 20, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Aulisa, L.; Tamburrelli, F.; Padua, R.; Romanini, E.; Monaco, M.L.; Padua, L. Carpal tunnel syndrome: Indication for surgical treatment based on electrophysiologic study. J. Hand Surg. 1998, 23, 687–691. [Google Scholar] [CrossRef]
- Bland, J.D. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve 2001, 24, 935–940. [Google Scholar] [CrossRef]
- Graham, B. The Value Added by Electrodiagnostic Testing in the Diagnosis of Carpal Tunnel Syndrome. J. Bone Jt. Surg. 2008, 90, 2587–2593. [Google Scholar] [CrossRef]
- Padua, L.; Lomonaco, M.; Aulisa, L.; Tamburrelli, F.; Valente, E.M.; Padua, R.; Gregori, B.; Tonali, P. Surgical prognosis in carpal tunnel syndrome: Usefulness of a preoperative neurophysiological assessment. Acta Neurol. Scand. 1996, 94, 343–346. [Google Scholar] [CrossRef]
- Sunderland, S.; Smith, J.W. Nerves and nerve injuries. Plast. Reconstr. Surg. 1969, 44, 601. [Google Scholar] [CrossRef]
- Cranford, C.S.; Ho, J.Y.; Kalainov, D.M.; Hartigan, B.J. Carpal Tunnel Syndrome. J. Am. Acad. Orthop. Surg. 2007, 15, 537–548. [Google Scholar] [CrossRef]
- Padua, L.; Coraci, D.; Erra, C.; Pazzaglia, C.; Paolasso, I.; Loreti, C.; Caliandro, P.; Hobson-Webb, L.D. Carpal tunnel syndrome: Clinical features, diagnosis, and management. Lancet Neurol. 2016, 15, 1273–1284. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Gagliardo, A.; Barbone, F.; Vitale, M.; Ferri, L.; Lupica, A.; Iacono, S.; Di Muzio, A.; Brighina, F. Median-to-Ulnar Nerve Communication in Carpal Tunnel Syndrome: An Electrophysiological Study. Neurol. Int. 2021, 13, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Ise, M.; Saito, T.; Katayama, Y.; Nakahara, R.; Shimamura, Y.; Hamada, M.; Senda, M.; Ozaki, T. Relationship between clinical outcomes and nerve conduction studies before and after surgery in patients with carpal tunnel syndrome. BMC Musculoskelet. Disord. 2021, 22, 882. [Google Scholar] [CrossRef] [PubMed]
- Nanno, M.; Kodera, N.; Tomori, Y.; Hagiwara, Y.; Takai, S. Electrophysiological Assessment for Splinting in the Treatment of Carpal Tunnel Syndrome. Neurol. Med.-Chir. 2017, 57, 472–480. [Google Scholar] [CrossRef][Green Version]
- Watson, J.C. The Electrodiagnostic Approach to Carpal Tunnel Syndrome. Neurol. Clin. 2012, 30, 457–478. [Google Scholar] [CrossRef]
- Afshar, A.; Tabrizi, A.; Tajbakhsh, M.; Navaeifar, N. Subjective Outcomes of Carpal Tunnel Release in Patients with Diabetes and Patients without Diabetes. J. Hand Microsurg. 2019, 12, 183–188. [Google Scholar] [CrossRef]
- Chen, S.-R.; Ho, T.-Y.; Shen, Y.-P.; Li, T.-Y.; Su, Y.-C.; Lam, K.H.S.; Chen, L.-C.; Wu, Y.-T. Comparison of short- and long-axis nerve hydrodissection for carpal tunnel syndrome: A prospective randomized, single-blind trial. Int. J. Med. Sci. 2021, 18, 3488–3497. [Google Scholar] [CrossRef]
- Martikkala, L.; Mäkelä, K.; Himanen, S.-L. Reduction in median nerve cross-sectional area at the forearm correlates with axon loss in carpal tunnel syndrome. Clin. Neurophysiol. Pract. 2021, 6, 209–214. [Google Scholar] [CrossRef]
- Matesanz, L.; Hausheer, A.C.; Baskozos, G.; Bennett, D.L.; Schmid, A.B. Somatosensory and psychological phenotypes associated with neuropathic pain in entrapment neuropathy. Pain 2021, 162, 1211–1220. [Google Scholar] [CrossRef]
- Moon, H.; Lee, B.J.; Park, D. Change to movement and morphology of the median nerve resulting from steroid injection in patients with mild carpal tunnel syndrome. Sci. Rep. 2020, 10, 15067. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, F.; Negro, F.D.; Bravini, E.; Ferriero, G.; Corna, S.; Invernizzi, M.; Vercelli, S. Relationship between nerve conduction studies and the Functional Dexterity Test in workers with carpal tunnel syndrome. BMC Musculoskelet. Disord. 2020, 21, 15607. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Terao, T.; Saito, E.; Ohara, K.; Michishita, S.; Kato, N.; Tani, S.; Murayama, Y. Clinical predictors of surgical outcomes of severe carpal tunnel syndrome patients: Utility of palmar stimulation in a nerve conduction study. BMC Musculoskelet. Disord. 2020, 21, 725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chu, H.; Wang, H.; Jin, Y.; Zhao, X.; Weng, C.; Lu, Z. Ring finger sensory latency difference in the diagnosis and treatment of carpal tunnel syndrome. BMC Neurol. 2021, 21, 432. [Google Scholar] [CrossRef] [PubMed]
| n = 1120 Hands | |
|---|---|
| Age 1 | 69.5 (60–77) |
| Sex | |
| Male | 132 patients, 264 hands |
| Female | 428 patients, 856 hands |
| Pre-operation | 247 patients, 494 hands |
| Post-operation | 313 patients, 626 hands |
| Measurable DML and measurable SCV | 934 hands |
| Non-measurable DML and non-measurable SCV | 62 hands |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, T.; Koyama, T.; Kuroiwa, T.; Nimura, A.; Okawa, A.; Wakabayashi, Y.; Fujita, K. Evaluation of the Existing Electrophysiological Severity Classifications in Carpal Tunnel Syndrome. J. Clin. Med. 2022, 11, 1685. https://doi.org/10.3390/jcm11061685
Sasaki T, Koyama T, Kuroiwa T, Nimura A, Okawa A, Wakabayashi Y, Fujita K. Evaluation of the Existing Electrophysiological Severity Classifications in Carpal Tunnel Syndrome. Journal of Clinical Medicine. 2022; 11(6):1685. https://doi.org/10.3390/jcm11061685
Chicago/Turabian StyleSasaki, Toru, Takafumi Koyama, Tomoyuki Kuroiwa, Akimoto Nimura, Atsushi Okawa, Yoshiaki Wakabayashi, and Koji Fujita. 2022. "Evaluation of the Existing Electrophysiological Severity Classifications in Carpal Tunnel Syndrome" Journal of Clinical Medicine 11, no. 6: 1685. https://doi.org/10.3390/jcm11061685
APA StyleSasaki, T., Koyama, T., Kuroiwa, T., Nimura, A., Okawa, A., Wakabayashi, Y., & Fujita, K. (2022). Evaluation of the Existing Electrophysiological Severity Classifications in Carpal Tunnel Syndrome. Journal of Clinical Medicine, 11(6), 1685. https://doi.org/10.3390/jcm11061685






