Bedside Selection of Positive End Expiratory Pressure by Electrical Impedance Tomography in Patients Undergoing Veno-Venous Extracorporeal Membrane Oxygenation Support: A Comparison between COVID-19 ARDS and ARDS from Other Etiologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. EIT Examination Protocol
2.3. Statistical Analysis
3. Results
3.1. PEEP Trial
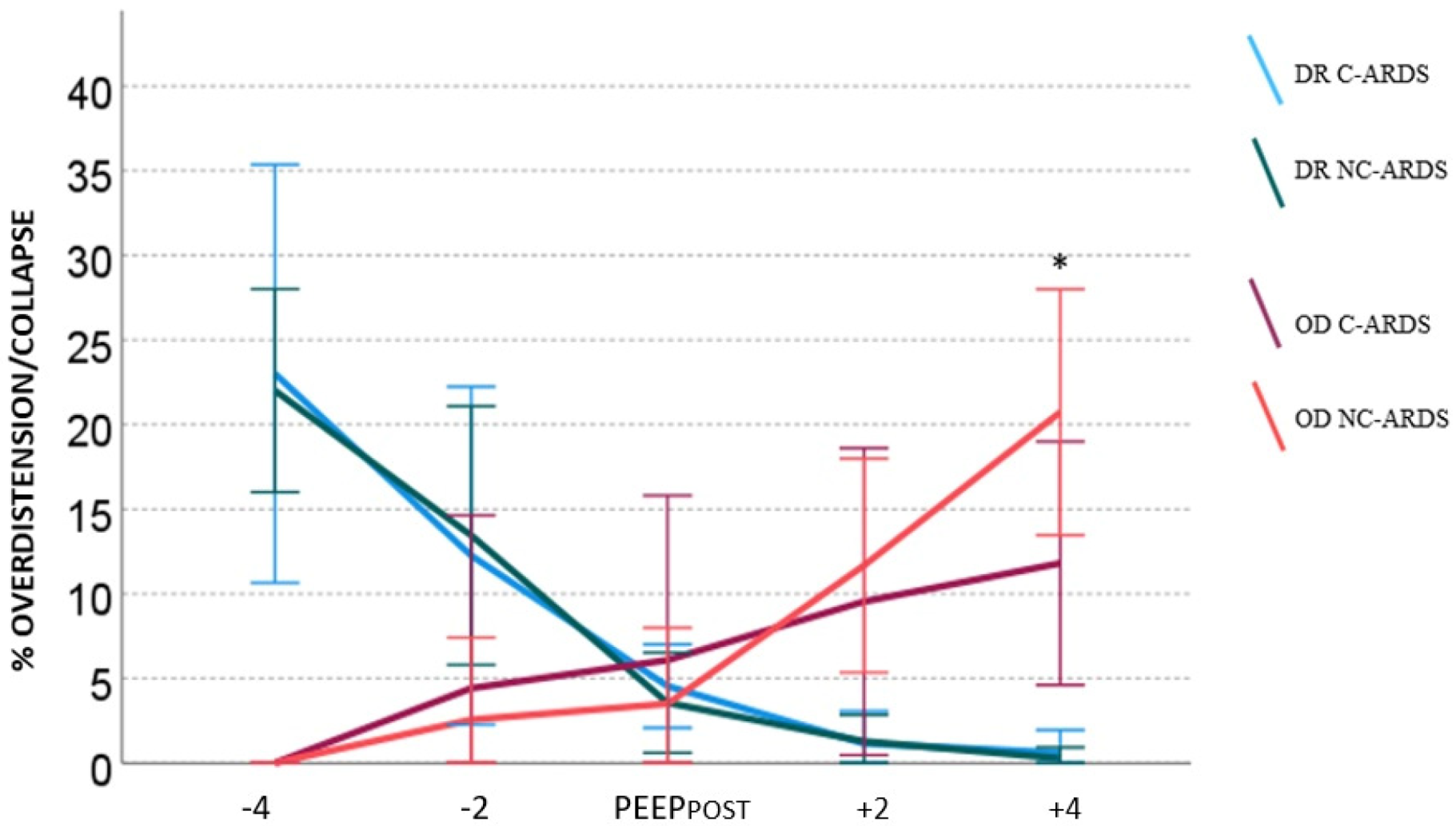
3.2. Overdistension and Derecruitment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Combes, A.; Agerstrand, C.; Annich, G.; Diaz, R.; Fan, E.; Hryniewicz, K.; Lorusso, R.; et al. Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet 2021, 398, 1230–1238. [Google Scholar] [CrossRef]
- Broman, L.M.; Eksborg, S.; Coco, V.L.; De Piero, M.E.; Belohlavek, J.; Lorusso, R. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir. Med. 2021, 9, e80–e81. [Google Scholar] [CrossRef]
- Lorusso, R.; Combes, A.; Coco, V.L.; De Piero, M.E.; Belohlavek, J. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021, 47, 344–348. [Google Scholar] [CrossRef]
- Quintel, M.; Bartlett, R.H.; Grocott, M.P.; Combes, A.; Ranieri, M.V.; Baiocchi, M.; Nava, S.; Brodie, D.; Camporota, L.; Vasques, F.; et al. Extracorporeal Membrane Oxygenation for Respiratory Failure. Anesthesiology 2020, 132, 1257–1276. [Google Scholar] [CrossRef] [PubMed]
- Constantin, J.-M.; Jabaudon, M.; Lefrant, J.-Y.; Jaber, S.; Quenot, J.-P.; Langeron, O.; Ferrandière, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Bachmann, M.C.; Morais, C.; Bugedo, G.; Bruhn, A.; Morales, A.; Borges, J.B.; Costa, E.; Retamal, J. Electrical impedance tomography in acute respiratory distress syndrome. Crit. Care 2018, 22, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inany, H.S.; Rettig, J.S.; Smallwood, C.D.; Arnold, J.H.; Walsh, B.K. Distribution of Ventilation Measured by Electrical Impedance Tomography in Critically Ill Children. Respir. Care 2020, 65, 590–595. [Google Scholar] [CrossRef]
- Perier, F.; Tuffet, S.; Maraffi, T.; Alcala, G.; Victor, M.; Haudebourg, A.-F.; Razazi, K.; De Prost, N.; Amato, M.; Carteaux, G.; et al. Electrical impedance tomography to titrate positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome. Crit. Care 2020, 24, 678. [Google Scholar] [CrossRef]
- Sella, N.; Zarantonello, F.; Andreatta, G.; Gagliardi, V.; Boscolo, A.; Navalesi, P. Positive end-expiratory pressure titration in COVID-19 acute respiratory failure: Electrical impedance tomography vs. PEEP/FiO(2) tables. Crit. Care 2020, 24, 540. [Google Scholar] [CrossRef] [PubMed]
- Franchineau, G.; Bréchot, N.; Hekimian, G.; Lebreton, G.; Bourcier, S.; Demondion, P.; Le Guennec, L.; Nieszkowska, A.; Luyt, C.-E.; Combes, A.; et al. Prone positioning monitored by electrical impedance tomography in patients with severe acute respiratory distress syndrome on veno-venous ECMO. Ann. Intensive Care 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Tonetti, T.; Quintel, M. How best to set the ventilator on extracorporeal membrane lung oxygenation. Curr. Opin. Crit. Care 2017, 23, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Eronia, N.; Mauri, T.; Maffezzini, E.; Gatti, S.; Bronco, A.; Alban, L.; Binda, F.; Sasso, T.; Marenghi, C.; Grasselli, G.; et al. Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: A feasibility study. Ann. Intensive Care 2017, 7, 76. [Google Scholar] [CrossRef]
- Coppadoro, A.; Eronia, N.; Foti, G.; Bellani, G. Event-triggered averaging of electrical impedance tomography (EIT) respiratory waveforms as compared to low-pass filtering for removal of cardiac related impedance changes. J. Clin. Monit. Comput. 2019, 34, 553–558. [Google Scholar] [CrossRef]
- Corte, F.D.; Mauri, T.; Spinelli, E.; Lazzeri, M.; Turrini, C.; Albanese, M.; Abbruzzese, C.; Lissoni, A.; Galazzi, A.; Eronia, N.; et al. Dynamic bedside assessment of the physiologic effects of prone position in acute respiratory distress syndrome patients by electrical impedance tomography. Minerva Anestesiol. 2020, 86, 1057–1064. [Google Scholar]
- Mauri, T.; Eronia, N.; Turrini, C.; Battistini, M.; Grasselli, G.; Rona, R.; Volta, C.; Bellani, G.; Pesenti, A. Bedside assessment of the effects of positive end-expiratory pressure on lung inflation and recruitment by the helium dilution technique and electrical impedance tomography. Intensive Care Med. 2016, 42, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Bellani, G.; Confalonieri, A.; Tagliabue, P.; Turella, M.; Coppadoro, A.; Citerio, G.; Patroniti, N.; Pesenti, A. Topographic distribution of tidal ventilation in acute respiratory distress syndrome: Effects of positive end-expiratory pressure and pressure support. Crit. Care Med. 2013, 41, 1664–1673. [Google Scholar] [CrossRef]
- Sosio, S.; Bellani, G.; Villa, S.; Lupieri, E.; Mauri, T.; Foti, G. A Calibration Technique for the Estimation of Lung Volumes in Nonintubated Subjects by Electrical Impedance Tomography. Respiration 2019, 98, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bronco, A.; Grassi, A.; Meroni, V.; Giovannoni, C.; Rabboni, F.; Rezoagli, E.; Teggia-Droghi, M.; Foti, G.; Bellani, G. Clinical value of electrical impedance tomography (EIT) in the management of patients with acute respiratory failure: A single centre experience. Physiol. Meas. 2021, 42, 074003. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.L.; Cournand, A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J. Appl. Physiol. 1949, 1, 825–847. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen, C.; Bohm, S.H.; Amato, M.B.P. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a ‘Typical’ Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gattinoni, L.; Gattarello, S.; Steinberg, I.; Busana, M.; Palermo, P.; Lazzari, S.; Romitti, F.; Quintel, M.; Meissner, K.; Marini, J.J.; et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 2021, 30, 210138. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Sjoding, M.W.; Admon, A.J.; Saha, A.K.; Kay, S.G.; Brown, C.A.; Co, I.; Claar, D.; McSparron, J.I.; Dickson, R.P. Comparing Clinical Features and Outcomes in Mechanically Ventilated Patients with COVID-19 and Acute Respiratory Distress Syndrome. Ann. Am. Thorac. Soc. 2021, 18, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, E.; Motos, A.; Torres, A.; Ceccato, A.; Ferrer, M.; Cilloniz, C.; Bueno, L.; Badia, J.R.; Castro, P.; Ferrando, C.; et al. SARS-CoV-2-induced Acute Respiratory Distress Syndrome: Pulmonary Mechanics and Gas-Exchange Abnormalities. Ann. Am. Thorac. Soc. 2020, 17, 1164–1168. [Google Scholar] [CrossRef]
- Grasselli, G.; Tonetti, T.; Protti, A.; Langer, T.; Girardis, M.; Bellani, G.; Laffey, J.; Carrafiello, G.; Carsana, L.; Rizzuto, C.; et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir. Med. 2020, 8, 1201–1208. [Google Scholar] [CrossRef]
- Haudebourg, A.F.; Perier, F.; Tuffet, S.; De Prost, N.; Razazi, K.; Mekontso Dessap, A.; Carteaux, G. Respiratory Mechanics of COVID-19- versus Non-COVID-19-associated Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 287–290. [Google Scholar] [CrossRef]
- Dueck, R. Alveolar recruitment versus hyperinflation: A balancing act. Curr. Opin. Anaesthesiol. 2006, 19, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; Rocker, G. Alveolar overdistension is an important mechanism of persistent lung damage following severe protracted ARDS. Anaesth. Intensive Care 1996, 24, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasino, S.; Sassanelli, R.; Marescalco, C.; Meroi, F.; Vetrugno, L.; Bove, T. Electrical Impedance Tomography and Prone Position during Ventilation in COVID-19 Pneumonia: Case Reports and a Brief Literature Review. Semin. Cardiothorac. Vasc. Anesth. 2020, 24, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Martucci, G.; Madotto, F.; Belliato, M.; Fanelli, V.; Garofalo, E.; Forlini, C.; Lucchini, A.; Panarello, G.; Bottino, N.; et al. Prone Positioning during Venovenous Extracorporeal Membrane Oxygenation in Acute Respiratory Distress Syndrome. A Multicenter Cohort Study and Propensity-matched Analysis. Ann. Am. Thorac. Soc. 2021, 18, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.; Fetita, C.; Gaudemer, A.; Treluyer, L.; Lebreton, G.; Franchineau, G.; Hekimian, G.; Chommeloux, J.; de Chambrun, M.P.; Brechot, N.; et al. Prone-Positioning for Severe Acute Respiratory Distress Syndrome Requiring Extracorporeal Membrane Oxygenation. Crit. Care Med. 2021, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Rezoagli, E.; Guervilly, C.; Rilinger, J.; Duburcq, T.; Petit, M.; Textoris, L.; Garcia, B.; Wengenmayer, T.; Grasselli, G.; et al. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A pooled individual patient data analysis. Crit. Care 2022, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.N.G.; Fontes, M.S.; Szmuszkowicz, T.; Isola, A.M.; Maciel, A.T. Electrical impedance tomography monitoring during spontaneous breathing trial: Physiological description and potential clinical utility. Acta Anaesthesiol. Scand. 2019, 63, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
| Population | C-ARDS | NC-ARDS |
|---|---|---|
| (n = 12) | (n = 12) | |
| Age, years | 55 [43.5–60] | 41 [31–59] |
| Sex (male) | 7 (58%) | 7 (58%) |
| BMI, kg/m2 | 33 [31–35] | 28 [21–30] * |
| Total Invasive ventilation duration, days | 25 [17–39] | 42 [18–51] |
| Total ECMO duration, days | 14 [9–25] | 18 [13–32] |
| ICU length of stay, days | 27 [17–35] | 42 [23–53] |
| ICU mortality | 1 (8%) | 3 (25%) * |
| Hospital mortality | 1 (8%) | 3 (25%) * |
| C-ARDS (n = 13) | NC-ARDS (n = 18) | |
|---|---|---|
| Pulmonary shunt fraction, % | 48.5 [35–57] | 53 [45–65] |
| Compliance of the respiratory system, mL/cm H2O | 25 [22–33] | 22 [15–29] |
| PEEP, cm H2O | 14 [12–15.5] | 15 [12–16.5] |
| Respiratory rate, breaths per minute | 10 [10–10] | 10 [10–10] |
| Tidal Volume, mL | 250 [200–345] | 205 [170–270] |
| Tidal Volume, mL/kg | 4 [3.7–5] | 3 [2.5–5] |
| Driving Pressure, cm H2O | 10 [8–10] | 9 [8–11] |
| C-ARDS | NC-ARDS | |||
|---|---|---|---|---|
| (n = 13) | (n = 18) | |||
| Arterial Blood Gas | Before EIT | After EIT | Before EIT | After EIT |
| pH | 7.41 [7.36–7.44] | 7.41 [7.39–7.43] | 7.42 [7.39–7.44] | 7.41 [7.37–7.43] |
| PaO2 | 86 [72–96] | 86.6 [80–95] | 76 [66–79] | 79 [70–94] |
| PaCO2 | 53 [47–56] | 52 [48–57] | 46 [41–54.5] | 45.5 [44–57] |
| Respiratory parameters | Before EIT | After EIT | Before EIT | After EIT |
| PEEP | 14 [12–15.5] | 14 [12.5–16] | 15 [12–16.5] | 15 [12–16] |
| Cpl, mL/cm H2O | 25 [22–33] | 27 [21.5–34.5] | 22 [15–29] | 25 [17–41] |
| Driving Pressure, cm H2O | 10 [8–10] | 10 [8–10.5] | 9 [8–11] | 9 [8–10] |
| Plateau Pressure, cm H2O | 23 [22–25] | 23 [22–25] | 24 [22–27] | 24 [22–25] |
| Tidal Volume, mL | 250 [200–345] | 250 [200–345] | 205 [170–270] | 205 [170–270] |
| Regional ventilation | Before EIT | After EIT | Before EIT | After EIT |
| TV % ROI 1 ventral | 8 [5.5–14] | 8 [5–13.5] | 15 [11–24.5] * | 15 [10–21.5] * |
| TV % ROI 2 middle-ventral | 46 [39.5–57.5] | 47 [38–57.5] | 43 [39–46] | 44 [41–46] |
| TV % ROI 3 middle-dorsal | 37 [27.5–49.5] | 42 [25–49] | 33 [23–41] | 33 [23–41] |
| TV % ROI 4 dorsal | 4 [3–6] | 4 [3–6] | 6 [4–9] * | 5.5 [4–8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pierro, M.; Giani, M.; Bronco, A.; Lembo, F.M.; Rona, R.; Bellani, G.; Foti, G. Bedside Selection of Positive End Expiratory Pressure by Electrical Impedance Tomography in Patients Undergoing Veno-Venous Extracorporeal Membrane Oxygenation Support: A Comparison between COVID-19 ARDS and ARDS from Other Etiologies. J. Clin. Med. 2022, 11, 1639. https://doi.org/10.3390/jcm11061639
Di Pierro M, Giani M, Bronco A, Lembo FM, Rona R, Bellani G, Foti G. Bedside Selection of Positive End Expiratory Pressure by Electrical Impedance Tomography in Patients Undergoing Veno-Venous Extracorporeal Membrane Oxygenation Support: A Comparison between COVID-19 ARDS and ARDS from Other Etiologies. Journal of Clinical Medicine. 2022; 11(6):1639. https://doi.org/10.3390/jcm11061639
Chicago/Turabian StyleDi Pierro, Michela, Marco Giani, Alfio Bronco, Francesca Maria Lembo, Roberto Rona, Giacomo Bellani, and Giuseppe Foti. 2022. "Bedside Selection of Positive End Expiratory Pressure by Electrical Impedance Tomography in Patients Undergoing Veno-Venous Extracorporeal Membrane Oxygenation Support: A Comparison between COVID-19 ARDS and ARDS from Other Etiologies" Journal of Clinical Medicine 11, no. 6: 1639. https://doi.org/10.3390/jcm11061639
APA StyleDi Pierro, M., Giani, M., Bronco, A., Lembo, F. M., Rona, R., Bellani, G., & Foti, G. (2022). Bedside Selection of Positive End Expiratory Pressure by Electrical Impedance Tomography in Patients Undergoing Veno-Venous Extracorporeal Membrane Oxygenation Support: A Comparison between COVID-19 ARDS and ARDS from Other Etiologies. Journal of Clinical Medicine, 11(6), 1639. https://doi.org/10.3390/jcm11061639






