The Serum Profile of Transferrin Isoforms in Pancreatitis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Blood Sampling
2.3. Diagnosis
2.4. Laboratory Testing
2.5. Transferrin Isoforms Testing
2.6. Statistical Analysis
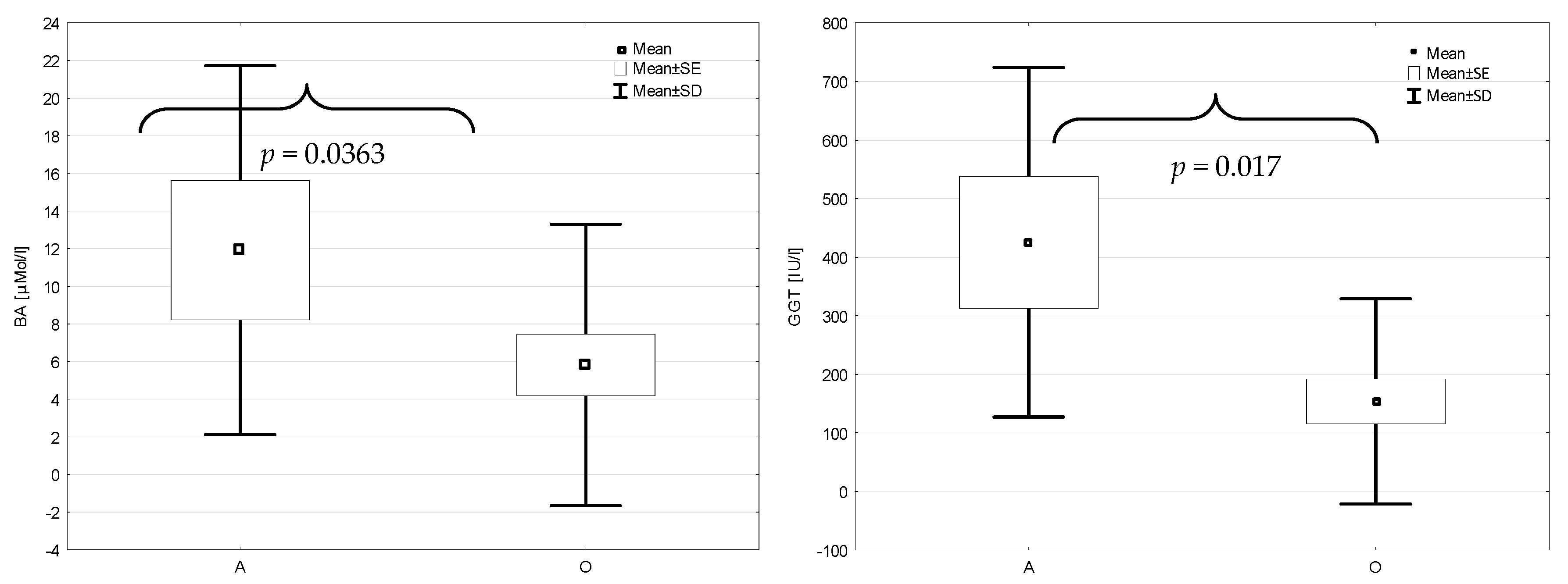
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Axford, J.S. Glycosylation and rheumatic diseases. Biochem. Biophys. Acta 1999, 1455, 219–229. [Google Scholar] [CrossRef]
- Blomme, B.; van Steenkiste, C.; Callewaert, N.; van Vlierberghe, H. Alteration of protein glycosylation in liver diseases. J. Hepatol. 2009, 50, 592–603. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, H.G.; van Noort, W.L.; de Jong, G.; Koster, J.F. Human serum sialo transferrins in diseases. Clin. Chim. Acta 1987, 165, 141–145. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to 17 inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Cylwik, B.; Gruszewska, E.; Gudowska, M.; Lipartowska-Klimiuk, K.; Szmitkowski, M.; Kedra, B.; Chrostek, L. Serum carbohydrate-deficient transferrin in pancreatic diseases of different etiologies. Clin. Lab. 2016, 62, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Gruszewska, E.; Cylwik, B.; Matus, L.; Gudowska, M.; Szmitkowski, M.; Kedra, B.; Chrostek, L. Changes in transferrin isoforms in pancreatic cancer. Ann. Clin. Lab. Sci. 2016, 46, 286–290. [Google Scholar] [PubMed]
- Gornik, O.; Gornik, I.; Gasparovic, V.; Lauc, G. Change in transferrin sialylation is a potential prognostic marker for severity of acute pancreatitis. Clin. Biochem. 2008, 41, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, F.; Wielders, J.P. Evaluation of capillary electrophoresis assay for CDT on SEBIA’s Capillarys System: Intra and inter laboratory precision, reference interval and cut-off. Clin. Chim. Acta 2010, 411, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Gudowska, M.; Gruszewska, E.; Panasiuk, A.; Cylwik, B.; Swiderska, M.; Flisiak, R.; Szmitkowski, M.; Chrostek, L. Changed profile of serum transferrin isoforms in liver diseases. Clin. Lab. 2017, 63, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Grytczuk, A.; Bauer, A.; Gruszewska, E.; Cylwik, B.; Chrostek, L. Changed profile of serum transferrin isoforms in Primary Biliary Cholangitis. J. Clin. Med. 2020, 9, 2894. [Google Scholar] [CrossRef] [PubMed]
- Gudowska, M.; Gruszewska, E.; Wrona, A.; Gindzienska-Sieskiewicz, E.; Domysłowska, I.; Cylwik, B.; Sierakowski, S.; Chrostek, L. The profile of serum transferrin isoforms in rheumatoid arthritis. JCR J. Clin. Rheumatol. 2019, 25, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Chrostek, L.; Gindzieńska-Sieśkiewicz, E.; Gruszewska, E.; Kowal-Bielecka, O.; Cylwik, B. Transferrin isoforms analysis by capillary electrophoresis in systemic lupus erythrematosus and systemic sclerosis. Scand. J. Clin. Lab. Investig. 2020, 80, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Gruszewska, E.; Wrona, A.; Gudowska, M.; Panasiuk, A.; Cylwik, B.; Lipartowska-Klimuk, K.; Flisiak, R.; Chrostek, L. The transferrin isoforms in chronic hepatitis. Clin. Biochem. 2017, 50, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Gruszewska, E.; Sienkiewicz, M.; Abramowicz, P.; Konstantynowicz, J.; Gudowska-Sawczuk, M.; Chrostek, L.; Cylwik, B. Serum profile of transferrin isoforms in JIA: A preliminary study. Rheumatol. Int. 2018, 38, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Sarner, M.; Cotton, P.B. Clasiffication of pancreatitis. Gut. 1984, 25, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, E.; Adjarov, D.; Etarska, M.; Stankushev, T.; Brumbarov, K.; Kerimova, M. Elevated liver gamma-glutamyl transferase in chronic alcoholics. Enzyme 1980, 25, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Milstein, H.J.; Bloomer, J.R.; Klatskin, G. Serum bile acids in alcoholic liver disease. Comparison with histological features of the disease. Am. J. Dig. Dis. 1976, 21, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Trinchet, J.C.; Gerhardt, M.F.; Balkau, B.; Munz, C.; Poupon, R.E. Serum bile acids and cholestasis in alcoholic hepatitis. Relationship with usual liver tests and histological features. J. Hepatol. 1994, 21, P235–P240. [Google Scholar] [CrossRef]

| Test | Controls (n = 30) | Acute Pancreatitis (n = 84) | Chronic Pancreatitis (n = 42) |
|---|---|---|---|
| MCV [fl] | 87.1 ± 4.5 | 90.7 ± 5.8 * | 88.0 ± 4.4 |
| PLT [109/L] | 243.2 ± 55.1 | 258.1 ± 138.7 | 192.4 ± 66.7 |
| PT [sec] | 12.42 ± 0.42 | 13.04 ± 1.35 | 11.8 ± 1.5 |
| INR | 0.93 ± 0.04 | 1.09 ± 0.11 * | 0.98 ± 0.12 |
| CDT [%] | 0.62 ± 0.19 | 1.53 ± 1.86 * | 1.38 ± 2.07 |
| 5-sialoTf [%] | 18.61 ± 6.03 | 13.76 ± 2.20 * | 12.95 ± 2.58 * |
| 4-sialoTf [%] | 76.84 ± 5.62 | 80.82 ± 2.87 * | 80.23 ± 3.36 |
| 3-sialoTf [%] | 3.61 ± 1.16 | 3.80 ± 1.17 | 5.43 ± 3.18 |
| Transferrin [g/L] | 3.14 ± 0.57 | 1.74 ± 0.50 *# | 2.23 ± 0.48 * |
| BA [µMol/L] | 3.55 ± 2.14 | 10.75 ± 20.22 | 18.75 ± 28.34 |
| Glucose [mM/L] | 5.22 ± 0.42 | 5.56 ± 2.32 | 6.22 ± 2.33 |
| AST [IU] | 23.2 ± 5.3 | 70.6 ± 73.7 * | 41.8 ± 20.8 |
| ALT [IU/L] | 17.6 ± 8.3 | 74.3 ± 79.1 * | 57.5 ± 54.2 |
| GGT [IU/L] | 23.3 ± 7.3 | 221.7 ± 238.5 * | 210.0 ± 168.2 * |
| Amylase [IU/L] | 58.6 ± 14.3 | 627.7 ± 714.41 *# | 94.5 ± 85.8 |
| Lipase [IU/L] | 36.4 ± 13.3 | 1263 ± 1931 *# | 91.0 ± 118.1 |
| ALP [IU/L] | 61.4 ± 11.2 | 113.3 ± 110.3 * | 194.5 ± 130.8 * |
| CRP [mg/L] | 1.03 ± 0.78 | 96.0 ± 112.5 *# | 4.5 ± 3.5 * |
| Bilirubin [µM/L] | 12.48 ± 5.30 | 17.61 ± 11.6 | 11.45 ± 1.54 |
| Cholesterol [mM/L] | 5.13 ± 0.81 | 3.94 ± 1.18 * | 4.77 ± 2.04 |
| TG [mM/L] | 1.12 ± 0.32 | 1.49 ± 0.92 | 1.43 ± 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucha, A.; Zaczek, M.; Kralisz, M.; Gruszewska, E.; Cylwik, B.; Panasiuk, A.; Chrostek, L. The Serum Profile of Transferrin Isoforms in Pancreatitis. J. Clin. Med. 2022, 11, 1638. https://doi.org/10.3390/jcm11061638
Mucha A, Zaczek M, Kralisz M, Gruszewska E, Cylwik B, Panasiuk A, Chrostek L. The Serum Profile of Transferrin Isoforms in Pancreatitis. Journal of Clinical Medicine. 2022; 11(6):1638. https://doi.org/10.3390/jcm11061638
Chicago/Turabian StyleMucha, Agnieszka, Malgorzata Zaczek, Michal Kralisz, Ewa Gruszewska, Bogdan Cylwik, Anatol Panasiuk, and Lech Chrostek. 2022. "The Serum Profile of Transferrin Isoforms in Pancreatitis" Journal of Clinical Medicine 11, no. 6: 1638. https://doi.org/10.3390/jcm11061638
APA StyleMucha, A., Zaczek, M., Kralisz, M., Gruszewska, E., Cylwik, B., Panasiuk, A., & Chrostek, L. (2022). The Serum Profile of Transferrin Isoforms in Pancreatitis. Journal of Clinical Medicine, 11(6), 1638. https://doi.org/10.3390/jcm11061638






