The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Device Specifications
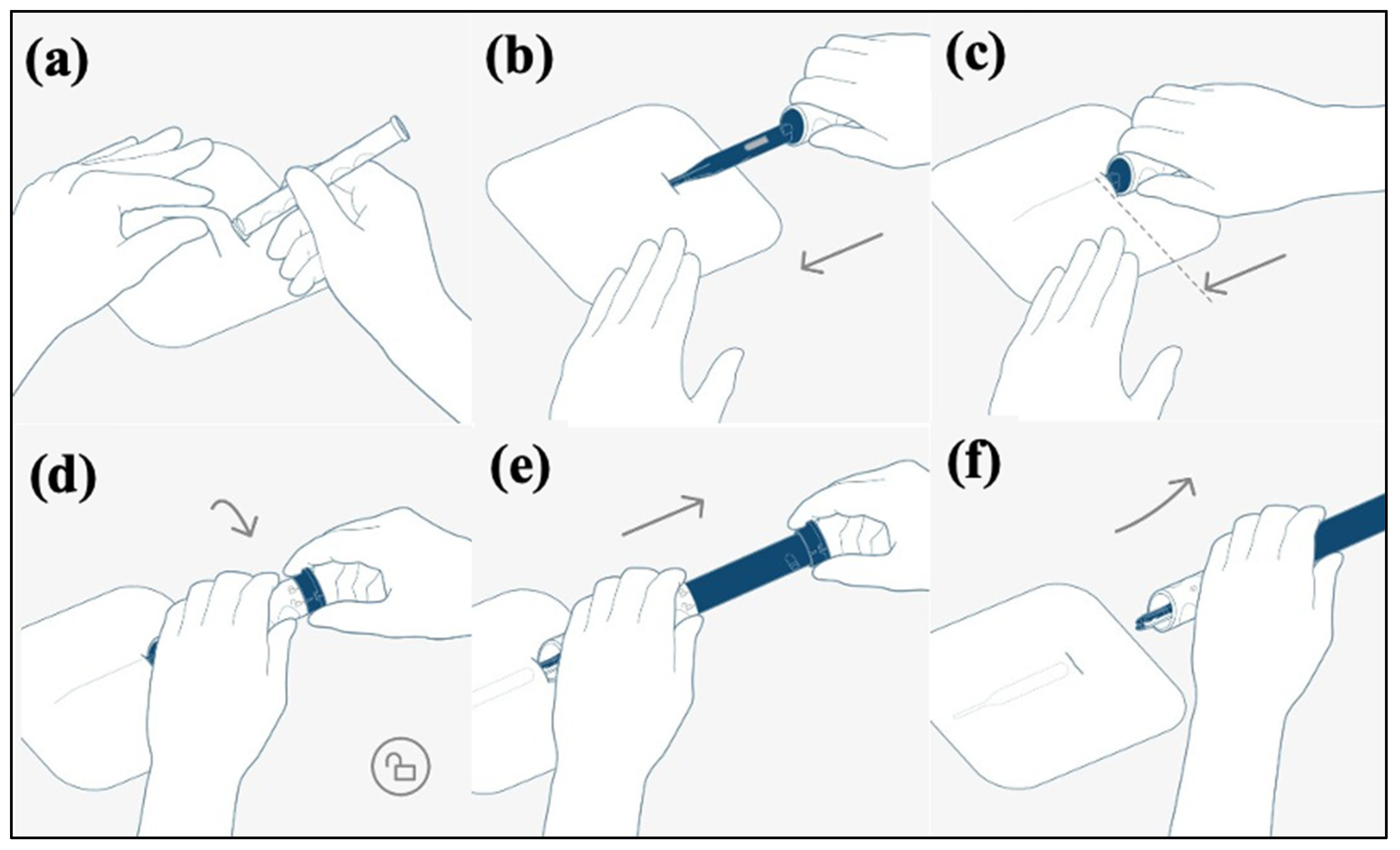
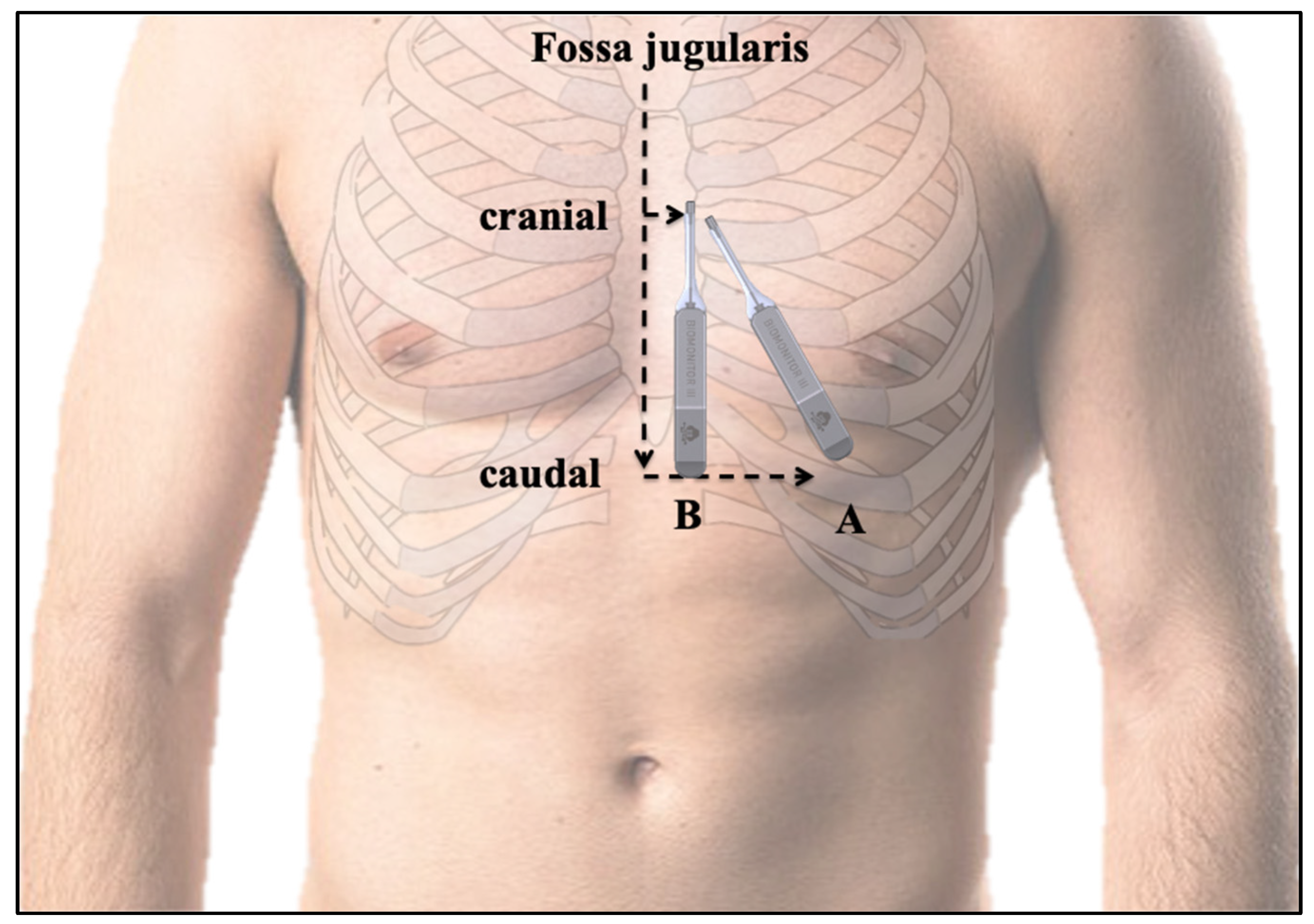
2.3. BIOMONITOR III Insertion
2.4. Data Evaluation and Consecutive Follow-Up
2.5. Patient Survey
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Insertion Procedure Results and Position of the ICM
3.3. Signal Quality
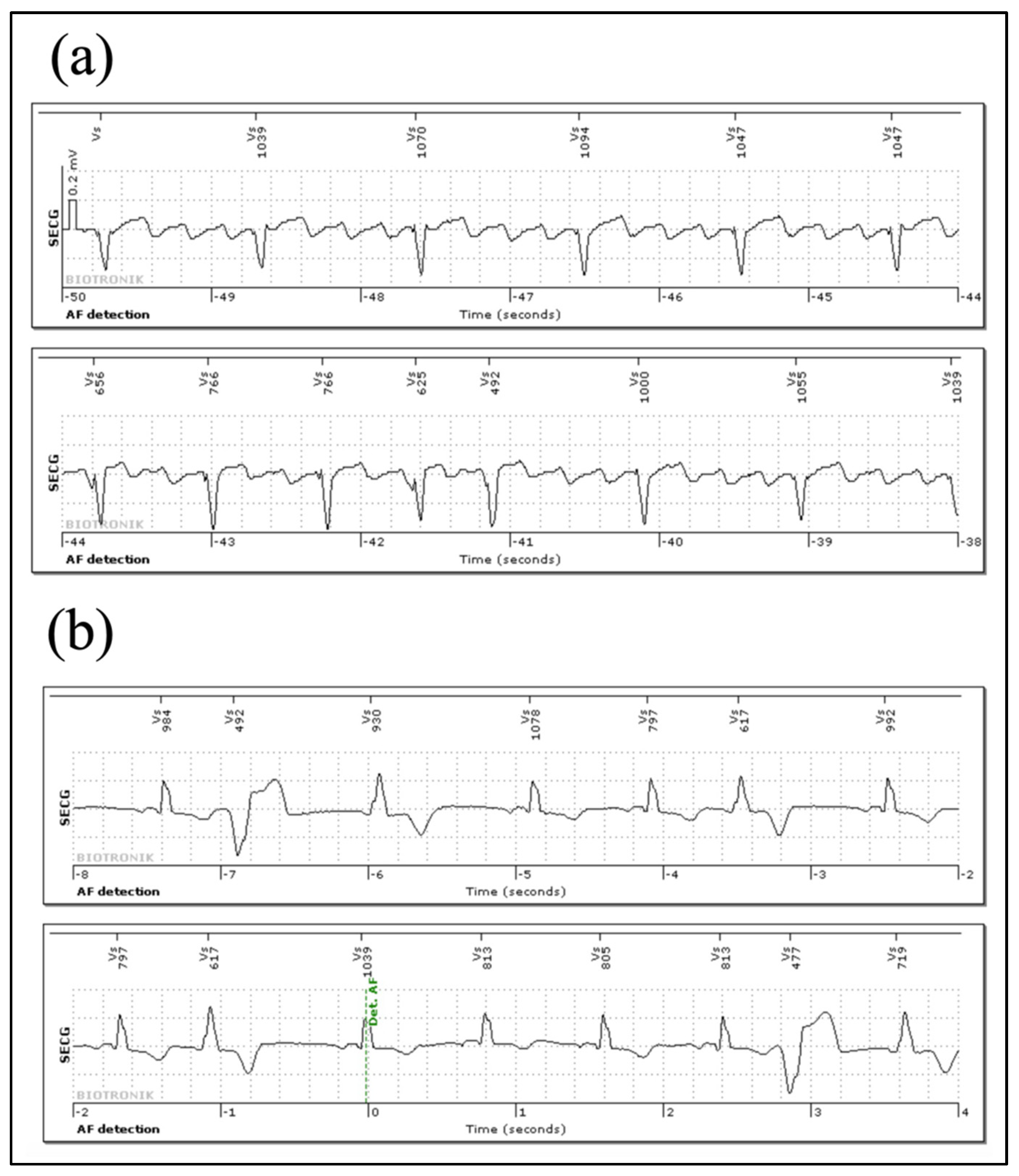
3.4. Arrhythmia Episodes Registered via Home Monitoring® and In-House Follow Up
3.5. Follow-Up and Therapeutic Consequences
3.6. Questionnaire and Patient Comfort
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| Injectable cardiac monitor | ICM |
| Electrocardiographic | ECG |
| Atrial fibrillation | AF |
| Fast insertion tool | FIT |
| Subcutaneous ECG | sECG |
| Premature ventricular contractions | PVC |
| Premature atrial contractions | PAC |
References
- Kapa, S.; Epstein, A.E.; Callans, D.J.; Garcia, F.C.; Lin, D.; Bala, R.; Riley, M.P.; Hutchinson, M.; Gerstenfeld, E.P.; Tzou, W.; et al. Assessing Arrhythmia Burden After Catheter Ablation of Atrial Fibrillation Using an Implantable Loop Recorder: The ABACUS Study. J. Cardiovasc. Electrophysiol. 2013, 24, 875–881. [Google Scholar] [CrossRef]
- Pecha, S.; Aydin, M.A.; Ahmadzade, T.; Hartel, F.; Hoffmann, B.; Steven, D.; Willems, S.; Reichenspurner, H.; Wagner, F.M. Implantable loop recorder monitoring after concomitant surgical ablation for atrial fibrillation (AF): Insights from more than 200 continuously monitored patients. Heart Vessel. 2016, 31, 1347–1353. [Google Scholar] [CrossRef]
- Ooi, S.-Y.; Ng, B.; Singarayar, S.; Hellestrand, K.; Illes, P.; Mohamed, U.; Razak, S.; Weerasooriya, R. BioMonitor 2 Pilot Study: Early Experience With Implantation of the Biotronik BioMonitor 2 Implantable Cardiac Monitor. Hear. Lung Circ. 2018, 27, 1462–1466. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; De Lange, F.J.; Deharo, J.-C.; Elliott, P.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martiın, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Kardiologia Polska 2018, 76, 1119–1198. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.; Rogers, J.; Sarkar, S.; Koehler, J.; Warman, E.N.; Tomson, T.T.; Passman, R.S. Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Hear. Rhythm 2016, 13, 1624–1630. [Google Scholar] [CrossRef] [Green Version]
- Podd, S.J.; Sugihara, C.; Furniss, S.S.; Sulke, N. Are implantable cardiac monitors the ‘gold standard’ for atrial fibrillation detection? A prospective randomized trial comparing atrial fibrillation monitoring using implantable cardiac monitors and DDDRP permanent pacemakers in post atrial fibrillation ablation patients. Europace 2015, 18, 1000–1005. [Google Scholar] [CrossRef]
- Pürerfellner, H.; Pokushalov, E.; Sarkar, S.; Koehler, J.; Zhou, R.; Urban, L.; Hindricks, G. P-wave evidence as a method for improving algorithm to detect atrial fibrillation in insertable cardiac monitors. Hear. Rhythm 2014, 11, 1575–1583. [Google Scholar] [CrossRef] [Green Version]
- Beinart, S.C.; Natale, A.; Verma, A.; Amin, A.; Kasner, S.; Diener, H.C.; Del Greco, M.; Wilkoff, B.L.; Pouliot, E.; Franco, N.; et al. Real-world comparison of in-hospital Reveal LINQ insertable cardiac monitor insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Am. Heart J. 2019, 207, 76–82. [Google Scholar] [CrossRef]
- Sakhi, R.; Theuns, D.A.; Szili-Torok, T.; Yap, S.-C. Insertable cardiac monitors: Current indications and devices. Expert Rev. Med. Devices 2019, 16, 45–55. [Google Scholar] [CrossRef]
- Steffel, J.; Wright, D.J.; Schäfer, H.; Rashid-Fadel, T.; Lewalter, T. Insertion of miniaturized cardiac monitors outside the catheter operating room: Experience and practical advice. Europace 2017, 19, 1624–1629. [Google Scholar] [CrossRef] [Green Version]
- Mariani, J.; Weerasooriya, R.; Brink, O.V.D.; Mohamed, U.; Gould, P.A.; Pathak, R.K.; Lin, T.; Conradie, A.; Illes, P.; Pavia, S.; et al. Miniaturized implantable cardiac monitor with a long sensing vector (BIOMONITOR III): Insertion procedure assessment, sensing performance, and home monitoring transmission success. J. Electrocardiol. 2020, 60, 118–125. [Google Scholar] [CrossRef]
- Reinsch, N.; Ruprecht, U.; Buchholz, J.; Diehl, R.R.; Kälsch, H.; Neven, K. The BioMonitor 2 insertable cardiac monitor: Clinical experience with a novel implantable cardiac monitor. J. Electrocardiol. 2018, 51, 751–755. [Google Scholar] [CrossRef]
- Bisignani, A.; De Bonis, S.; Mancuso, L.; Ceravolo, G.; Bisignani, G. Implantable loop recorder in clinical practice. J. Arrhythmia 2018, 35, 25–32. [Google Scholar] [CrossRef]
- Giancaterino, S.; Lupercio, F.; Nishimura, M.; Hsu, J.C. Current and Future Use of Insertable Cardiac Monitors. JACC Clin. Electrophysiol. 2018, 4, 1383–1396. [Google Scholar] [CrossRef]
- Wechselberger, S.; Piorkowski, C.; Pohl, M. Current rare indications and future directions for implantable loop recorders. Herzschrittmacherther. + Elektrophysiol. 2016, 27, 366–370. [Google Scholar] [CrossRef]
- Jons, C.; Sogaard, P.; Behrens, S.; Schrader, J.; Mrosk, S.; Bloch Thomsen, P.E. The clinical effect of arrhythmia monitoring after myocardial infarction (BIO-GUARDMI):study protocol for a randomized controlled trial. Trials 2019, 20, 563. [Google Scholar] [CrossRef]
- Lee, R.; Mittal, S. Utility and limitations of long-term monitoring of atrial fibrillation using an implantable loop recorder. Heart Rhythm 2018, 15, 287–295. [Google Scholar] [CrossRef]
- Piorkowski, C.; Busch, M.; Nölker, G.; Schmitt, J.; Roithinger, F.X.; Young, G.; Táborský, M.; Herrmann, G.; Schmitz, D. Clinical evaluation of a small implantable cardiac monitor with a long sensing vector. Pacing Clin. Electrophysiol. 2019, 42, 1038–1046. [Google Scholar] [CrossRef] [Green Version]
- Lortz, J.; Varnavas, V.; Weißenberger, W.; Erbel, R.; Reinsch, N. Maintaining Accurate Long-Term Sensing Ability Despite Significant Size Reduction of Implantable Cardiac Monitors. Pacing Clin. Electrophysiol. 2016, 39, 1344–1350. [Google Scholar] [CrossRef]
- Maines, M.; Zorzi, A.; Tomasi, G.; Angheben, C.; Catanzariti, D.; Piffer, L.; Del Greco, M. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQTM. Europace 2018, 20, 1050–1057. [Google Scholar] [CrossRef]
- Pürerfellner, H.; Sanders, P.; Pokushalov, E.; Di Bacco, M.; Bergemann, T.; Dekker, L.R. Miniaturized Reveal LINQ insertable cardiac monitoring system: First-in-human experience. Heart Rhythm 2015, 12, 1113–1119. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.R.; Mease, J.; Koppert, T.; Okabe, T.; Tyler, J.; Houmsse, M.; Augostini, R.S.; Weiss, R.; Hummel, J.D.; Kalbfleisch, S.J.; et al. Incidence of false-positive transmissions during remote rhythm monitoring with implantable loop recorders. Heart Rhythm 2020, 17, 75–80. [Google Scholar] [CrossRef]
- Davish, S.; Baker, C.; Fulks, M.; Godsey, J.; Parker, K. Drowning in Data: Workflow Changes Improve the Collection of Clinically Relevant and Actionable Data. Perspect. Health Inf. Manag. 2019, 16, 1d. [Google Scholar]
- Kawashima, A.; Tanimoto, F.; Nagao, T.; Toyoshima, T.; Okuyama, Y. Investigation of optimal position for implantable loop recorders by potential mapping with Reveal DX. J. Arrhythm. 2015, 31, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Lacour, P.; Dang, P.L.; Huemer, M.; Parwani, A.S.; Attanasio, P.; Pieske, B.; Boldt, L.; Haverkamp, W.; Blaschke, F. Performance of the New BioMonitor 2-AF Insertable Cardiac Monitoring System: Can Better be Worse? Pacing Clin. Electrophysiol. 2017, 40, 516–526. [Google Scholar] [CrossRef]
- Bisignani, G.; De Bonis, S.; Bisignani, A.; Mancuso, L.; Giacopelli, D. Sensing performance, safety, and patient acceptability of long-dipole cardiac monitor: An innovative axillary insertion. Pacing Clin. Electrophysiol. 2018, 41, 277–283. [Google Scholar] [CrossRef]

| Age | 67 ± 12 |
| Sex (male) | 12 (40%) |
| BMI (kg/m2) | 26 ± 4 |
| Coronary artery disease | 6 (20%) |
| Hypertension | 17 (57%) |
| Diabetes mellitus | 3 (10%) |
| Stroke | 6 (20%) |
| Atrial fibrillation | 5 (16%) |
| LVEF (%) | 58 ± 5 |
| CV drug use | |
| 6 (20%) |
| 11 (36%) |
| 14 (46%) |
| 12 (40%) |
| 0 (0%) |
| n= | Mean ± SD | Range | |
|---|---|---|---|
| R-wave sensing | |||
| Post-op | 30 | 0.73 ± 0.32 mV | 0.27–1.47 mV |
| Day 1 | 30 | 0.78 ± 0.38 mV | 0.25–1.52 mV |
| Follow-up | 30 | 0.81 ± 0.39 mV | 0.20–2.00 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinsch, N.; Füting, A.; Höwel, D.; Neven, K. The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor. J. Clin. Med. 2022, 11, 1634. https://doi.org/10.3390/jcm11061634
Reinsch N, Füting A, Höwel D, Neven K. The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor. Journal of Clinical Medicine. 2022; 11(6):1634. https://doi.org/10.3390/jcm11061634
Chicago/Turabian StyleReinsch, Nico, Anna Füting, Dennis Höwel, and Kars Neven. 2022. "The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor" Journal of Clinical Medicine 11, no. 6: 1634. https://doi.org/10.3390/jcm11061634
APA StyleReinsch, N., Füting, A., Höwel, D., & Neven, K. (2022). The BIOMONITOR III Injectable Cardiac Monitor: Clinical Experience with a Novel Injectable Cardiac Monitor. Journal of Clinical Medicine, 11(6), 1634. https://doi.org/10.3390/jcm11061634






