Cardiovascular Health Does Not Change Following High-Intensity Interval Training in Women with Polycystic Ovary Syndrome
Abstract
:1. Introduction
2. Materials and Methods
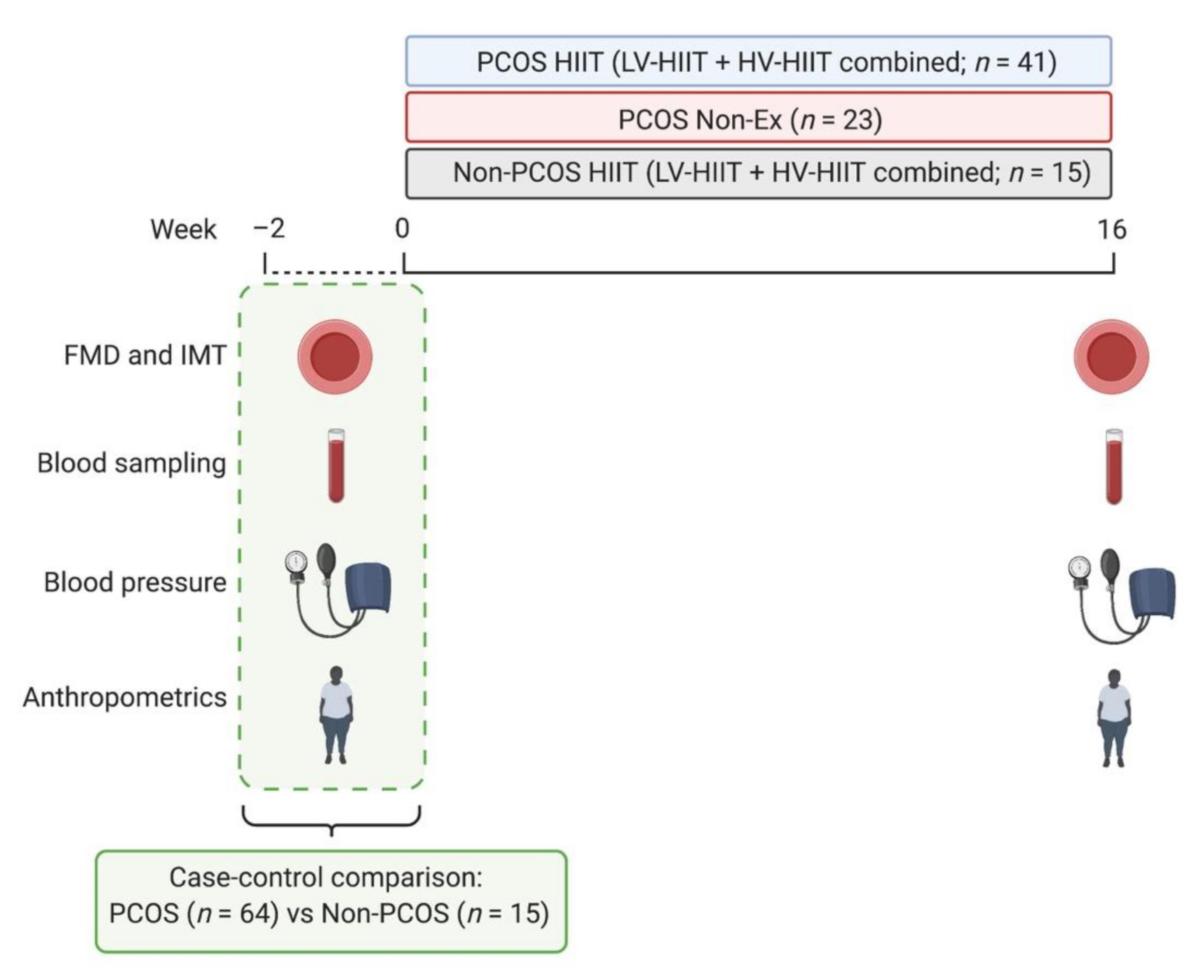
2.1. Study Design
2.2. Participants
2.3. Interventions
2.4. Outcomes
2.4.1. Flow-Mediated Dilatation and Intima-Media Thickness
2.4.2. Blood Pressure and Circulating Markers
2.4.3. Statistical Analyses
3. Results
3.1. Baseline Comparisons between Women with and without PCOS
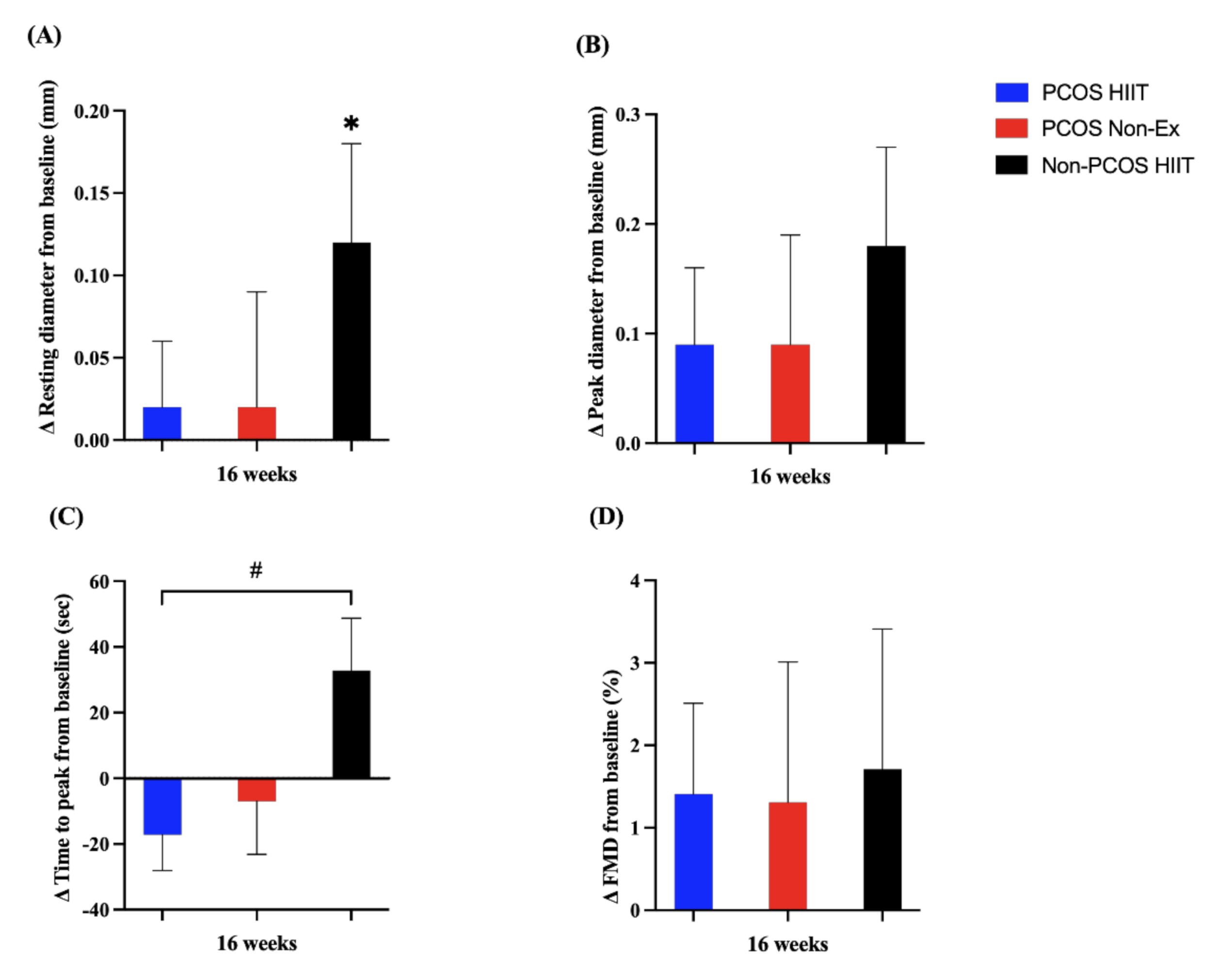
3.2. Cardiovascular Responses to High-Intensity Interval Training
4. Discussion
4.1. CVD Risk Factors between Women with and without PCOS
4.2. The Effects of HIIT on CVD Risk Factors
4.3. Strength and Limitations
4.4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wekker, V.; van Dammen, L.; Koning, A.; Heida, K.Y.; Painter, R.C.; Limpens, J.; Laven, J.S.E.; Roeters van Lennep, J.E.; Roseboom, T.J.; Hoek, A. Long-term cardiometabolic disease risk in women with PCOS: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 942–960. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.A.; Carmina, E.; Diamanti-Kandarakis, E.; Dokras, A.; Escobar-Morreale, H.F.; Futterweit, W.; Lobo, R.; Norman, R.J.; Talbott, E.; Dumesic, D.A. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 2010, 95, 2038–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111, quiz 189–190. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Sprung, V.S.; Atkinson, G.; Cuthbertson, D.J.; Pugh, C.J.; Aziz, N.; Green, D.J.; Cable, N.T.; Jones, H. Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: A meta-analysis of the observational studies. Clin. Endocrinol. 2013, 78, 438–446. [Google Scholar] [CrossRef]
- Sprung, V.S.; Jones, H.; Pugh, C.J.; Aziz, N.F.; Daousi, C.; Kemp, G.J.; Green, D.J.; Cable, N.T.; Cuthbertson, D.J. Endothelial dysfunction in hyperandrogenic polycystic ovary syndrome is not explained by either obesity or ectopic fat deposition. Clin. Sci. (Lond.) 2014, 126, 67–74. [Google Scholar] [CrossRef]
- Meyer, M.L.; Malek, A.M.; Wild, R.A.; Korytkowski, M.T.; Talbott, E.O. Carotid artery intima-media thickness in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 112–126. [Google Scholar] [CrossRef]
- Dambala, K.; Paschou, S.A.; Michopoulos, A.; Siasos, G.; Goulis, D.G.; Vavilis, D.; Tarlatzis, B.C. Biomarkers of Endothelial Dysfunction in Women with Polycystic Ovary Syndrome. Angiology 2019, 70, 797–801. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Shalev, E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front. Biosci. 2004, 9, 2474–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylus, A.M.; Nandeesha, H.; Chitra, T. Matrix metalloproteinase-9 increases and Interleukin-10 reduces with increase in body mass index in polycystic ovary syndrome: A cross-sectional study. Int. J. Reprod. Biomed. 2020, 18, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, K.C.; Komorowski, J.; O’Callaghan, C.J.; Tan, B.K.; Chen, J.; Prelevic, G.M.; Randeva, H.S. Increased circulating levels of matrix metalloproteinase-2 and -9 in women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 1173–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbaran, J.; Farimani, M.; Tavilani, H.; Ghorbani, M.; Karimi, J.; Poormonsefi, F.; Khodadadi, I. Matrix metalloproteinases 2 and 9 and MMP9/NGAL complex activity in women with PCOS. Reproduction 2016, 151, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Cai, L.Y.; Lv, H.M.; Xia, L.; Zhang, Y.J.; Zhang, H.X.; Guan, Y.M. Raised serum levels of matrix metalloproteinase-9 in women with polycystic ovary syndrome and its association with insulin-like growth factor binding protein-1. Gynecol. Endocrinol. 2008, 24, 285–288. [Google Scholar] [CrossRef]
- Orio, F.; Muscogiuri, G.; Giallauria, F.; Savastano, S.; Bottiglieri, P.; Tafuri, D.; Predotti, P.; Colarieti, G.; Colao, A.; Palomba, S. Oral contraceptives versus physical exercise on cardiovascular and metabolic risk factors in women with polycystic ovary syndrome: A randomized controlled trial. Clin. Endocrinol. 2016, 85, 764–771. [Google Scholar] [CrossRef]
- Sprung, V.S.; Cuthbertson, D.J.; Pugh, C.J.; Aziz, N.; Kemp, G.J.; Daousi, C.; Green, D.J.; Cable, N.T.; Jones, H. Exercise training in polycystic ovarian syndrome enhances flow-mediated dilation in the absence of changes in fatness. Med. Sci. Sports Exerc. 2013, 45, 2234–2242. [Google Scholar] [CrossRef]
- Almenning, I.; Rieber-Mohn, A.; Lundgren, K.M.; Shetelig Løvvik, T.; Garnæs, K.K.; Moholdt, T. Effects of High Intensity Interval Training and Strength Training on Metabolic, Cardiovascular and Hormonal Outcomes in Women with Polycystic Ovary Syndrome: A Pilot Study. PLoS ONE 2015, 10, e0138793. [Google Scholar] [CrossRef] [Green Version]
- Rauramaa, R.; Hassinen, M. Exercise Training and Endothelial Function. Curr. Cardiovasc. Risk Rep. 2011, 5, 323–330. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Vrabas, I.S.; Sailer, N.; Kapelouzou, A.; Fotiadis, G.; Noussios, G.; Karayannacos, P.E.; Angelopoulou, N. Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 2010, 36, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kiel, I.A.; Lionett, S.; Parr, E.B.; Jones, H.; Røset, M.A.H.; Salvesen, Ø.; Vanky, E.; Moholdt, T. Improving reproductive function in women with polycystic ovary syndrome with high-intensity interval training (IMPROV-IT): Study protocol for a two-centre, three-armed randomised controlled trial. BMJ Open 2020, 10, e034733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiel, I.A.; Lionett, S.; Parr, E.B.; Jones, H.; Røset, M.A.H.; Salvesen, Ø.; Hawley, J.A.; Vanky, E.; Moholdt, T. High-Intensity Interval Training in Polycystic Ovary Syndrome: A Two-Center, Three-Armed Randomized Controlled Trial. Med. Sci. Sports Exerc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lionett, S.; Kiel, I.A.; Camera, D.M.; Vanky, E.; Parr, E.B.; Lydersen, S.; Hawley, J.A.; Moholdt, T. Circulating and Adipose Tissue miRNAs in Women with Polycystic Ovary Syndrome and Responses to High-Intensity Interval Training. Front. Physiol. 2020, 11, 904. [Google Scholar] [CrossRef]
- Lionett, S.; Kiel, I.A.; Røsbjørgen, R.; Lydersen, S.; Larsen, S.; Moholdt, T. Absent Exercise-Induced Improvements in Fat Oxidation in Women with Polycystic Ovary Syndrome after High-Intensity Interval Training. Front. Physiol. 2021, 12, 649794. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, G.; Batterham, A.M. The percentage flow-mediated dilation index: A large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vasc. Med. 2013, 18, 354–365. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Gautam, K.; Pyakurel, D. Comparison of calculated LDL-cholesterol using the Friedewald formula and de Cordova formula with a directly measured LDL-cholesterol in Nepalese population. Pract. Lab. Med. 2020, 20, e00165. [Google Scholar] [CrossRef]
- Gerlach, R.F.; Demacq, C.; Jung, K.; Tanus-Santos, J.E. Rapid separation of serum does not avoid artificially higher matrix metalloproteinase (MMP)-9 levels in serum versus plasma. Clin. Biochem. 2007, 40, 119–123. [Google Scholar] [CrossRef]
- Lee, K.; Kang, I.; Mack, W.J.; Mortimer, J.; Sattler, F.; Salem, G.; Dieli-Conwright, C.M. Effect of High Intensity Interval Training on Matrix Metalloproteinases in Women with Breast Cancer Receiving Anthracycline-Based Chemotherapy. Sci. Rep. 2020, 10, 5839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twisk, J.; Bosman, L.; Hoekstra, T.; Rijnhart, J.; Welten, M.; Heymans, M. Different ways to estimate treatment effects in randomised controlled trials. Contemp. Clin. Trials Commun. 2018, 10, 80–85. [Google Scholar] [CrossRef]
- Cussons, A.J.; Watts, G.F.; Stuckey, B.G. Dissociation of endothelial function and arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2009, 71, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Lobo, R.A. Is There Really Increased Cardiovascular Morbidity in Women with Polycystic Ovary Syndrome? J. Womens Health 2018, 27, 1385–1388. [Google Scholar] [CrossRef]
- Daskalopoulos, G.N.; Karkanaki, A.; Karagiannis, A.; Mikhailidis, D.P.; Athyros, V.G. Is the risk for cardiovascular disease increased in all phenotypes of the polycystic ovary syndrome? Angiology 2011, 62, 285–290. [Google Scholar] [CrossRef]
- Gomes, V.A.; Vieira, C.S.; Jacob-Ferreira, A.L.; Belo, V.A.; Soares, G.M.; Fernandes, J.B.; Ferriani, R.A.; Tanus-Santos, J.E. Imbalanced circulating matrix metalloproteinases in polycystic ovary syndrome. Mol. Cell. Biochem. 2011, 353, 251–257. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Woodward, A.; Klonizakis, M.; Broom, D. Exercise and Polycystic Ovary Syndrome. Adv. Exp. Med. Biol. 2020, 1228, 123–136. [Google Scholar] [CrossRef]
- Benham, J.L.; Yamamoto, J.M.; Friedenreich, C.M.; Rabi, D.M.; Sigal, R.J. Role of exercise training in polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Obes. 2018, 8, 275–284. [Google Scholar] [CrossRef]
- Patten, R.K.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K. Exercise Interventions in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef]
- Park, J.; Park, H. Effects of 6 months of aerobic and resistance exercise training on carotid artery intima media thickness in overweight and obese older women. Geriatr. Gerontol. Int. 2017, 17, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Jee, J.H.; Park, W.H. Effects of Aerobic and Resistance Exercise Training on Carotid Intima-Media Thickness in Abdominal Obese Women. Metab. Syndr. Relat. Disord. 2021, 19, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Orio, F.; Palomba, S.; Longo, R.A.; Cascella, T.; Colao, A.; Lombardi, G.; Rini, G.B.; Lobo, R.A. Endothelial dysfunction in PCOS: Role of obesity and adipose hormones. Am. J. Med. 2006, 119, 356.e1–356.e6. [Google Scholar] [CrossRef] [PubMed]
| PCOS | Non-PCOS | p-Values | |||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Age (years) | 64 | 30 (5) | 15 | 31 (6) | 0.50 |
| Body weight (kg) | 64 | 85.1 (19.6) | 15 | 81.2 (17.1) | 0.48 |
| Body mass index (kg/m2) | 64 | 30.5 (6.5) | 15 | 28.4 (5.6) | 0.24 |
| Systolic blood pressure (mmHg) | 64 | 117.2 (9.8) | 15 | 114.8 (8.4) | 0.35 |
| Diastolic blood pressure (mmHg) | 64 | 75.2 (8.3) | 15 | 76.1 (6.8) | 0.70 |
| Resting heart rate (beats/min) | 64 | 64.7 (9.9) | 15 | 57.1 (12.2) | 0.011 |
| MMP-9 (ng/mL) * | 62 | 44.5 (27.0) | 11 | 50.4 (27.6) | 0.61 |
| Total cholesterol (mmol/L) | 64 | 4.4 (0.8) | 13 | 4.3 (0.6) | 0.62 |
| HDL cholesterol (mmol/L) | 64 | 1.3 (0.3) | 13 | 1.4 (0.2) | 0.65 |
| LDL cholesterol (mmol/L) | 63 | 2.6 (0.7) | 13 | 2.5 (0.5) | 0.60 |
| Triglycerides (mmol/L) | 63 | 1.1 (0.6) | 13 | 0.9 (0.4) | 0.23 |
| Glucose (mmol/L) | 63 | 5.0 (0.5) | 13 | 4.8 (0.4) | 0.40 |
| Insulin (pmol/L) | 64 | 117 (89) | 12 | 84 (61) | 0.28 |
| HOMA-IR | 63 | 4.5 (3.8) | 12 | 3.0 (2.3) | 0.24 |
| Glucose area under the curve (mmol/L * min) | 61 | 833 (199) | 12 | 818 (151) | 0.63 |
| Glucose incremental area under the curve (mmol/L * min) | 61 | 191 (147) | 12 | 197 (106) | 0.97 |
| Insulin area under the curve (pmol/L * min) | 59 | 69,652 (45,772) | 6 | 62,069 (31,704) | 0.71 |
| Insulin incremental area under the curve (pmol/L * min) | 59 | 46,803 (32,377) | 6 | 43,001 (18,508) | 0.79 |
| Absolute FMD (mm) | 33 | 0.19 (0.13) | 10 | 0.28 (0.11) | 0.064 |
| FMD (%) | 33 | 5.5 (4.1) | 10 | 8.2 (3.9) | 0.067 |
| Resting Diameter (mm) | 34 | 3.4 (0.3) | 10 | 3.4 (0.2) | 0.91 |
| Peak Diameter (mm) | 33 | 3.6 (0.3) | 10 | 3.7 (0.2) | 0.36 |
| Time to peak (s) | 33 | 44 (42) | 10 | 19 (20) | 0.073 |
| IMT (mm) | 20 | 0.56 (0.06) | 4 | 0.57 (0.03) | 0.96 |
| PCOS HIIT | PCOS Non-Ex | Non-PCOS HIIT | PCOS HIIT vs. PCOS Non-Ex (Group x Time Interaction) | PCOS HIIT vs. Non-PCOS HIIT (Group x Time Interaction) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| Body weight (kg) | −0.44 (−1.36, 0.49) | 0.35 | −2.19 (−3.35, −1.03) | <0.001 | −1.52 (−2.88, −0.17) | 0.028 | 1.75 (0.27, 3.24) | 0.021 | −1.09 (−2.73, 0.55) | 0.19 |
| Body mass index (kg/m2) | −0.20 (−0.53, 0.13) | 0.22 | −0.79 (−1.20, −0.37) | <0.001 | −0.54 (−1.02, −0.06) | 0.029 | 0.58 (0.06, 1.1) | 0.031 | −0.34 (−0.92, 0.25) | 0.25 |
| Resting heart rate (beats/min) | −2.8 (−5.4, −0.2) | 0.037 | −1.1 (−4.2, 2.1) | 0.50 | −1.1 (−4.9, 2.8) | 0.58 | −1.7 (−5.7, 2.3) | 0.40 | 1.7 (−2.9, 6.4) | 0.46 |
| Systolic blood pressure (mmHg) | −1.5 (−3.9, 0.8) | 0.20 | −4.8 (−7.6, −1.9) | 0.001 | −2.1 (−5.6, 1.3) | 0.22 | −3.2 (−6.8, 0.4) | 0.078 | −0.5(−4.7, 3.7) | 0.81 |
| Diastolic blood pressure (mmHg) | −1.7 (−4.0, 0.6) | 0.14 | −0.1 (−2.9, 2.6) | 0.93 | −0.7 (−4.1, 2.7) | 0.68 | −1.6 (−5.0, 1.8) | 0.36 | −1.3 (−2.8, 5.5) | 0.53 |
| Total cholesterol (mmol/L) | −0.07 (−0.25, 0.11) | 0.42 | −0.11 (−0.33, 0.11) | 0.30 | 0.04 (−0.25, 0.32) | 0.80 | −0.04 (−0.32, 0.24) | 0.77 | 0.12 (−0.22, 0.46) | 0.48 |
| HDL cholesterol (mmol/L) | −0.03 (−0.10, 0.04) | 0.41 | −0.12 (−0.20, −0.03) | 0.010 | −0.01 (−0.12, 0.11) | 0.91 | −0.09 (−0.20, 0.02) | 0.12 | 0.02 (−0.11, 0.16) | 0.74 |
| LDL cholesterol (mmol/L) | −0.02 (−0.17, 0.13) | 0.76 | 0.07 (−0.11, 0.26) | 0.43 | 0.03 (−0.20, 0.27) | 0.77 | −0.10 (−0.33, 0.14) | 0.41 | 0.06 (−0.22, 0.34) | 0.67 |
| Triglycerides (mmol/L) | −0.01 (−0.15, 0.16) | 0.91 | −0.14 (−0.33, 0.05) | 0.15 | 0.03 (−0.22, 0.28) | 0.82 | −0.15 (−0.38, 0.09) | 0.22 | 0.02 (−0.27, 0.32) | 0.88 |
| Glucose (mmol/L) | −0.11 (−0.25, 0.03) | 0.11 | −0.09 (−0.26, 0.08) | 0.30 | 0.10 (−0.13, 0.32) | 0.38 | −0.03 (−0.24, 0.19) | 0.81 | 0.20 (−0.06, 0.47) | 0.13 |
| Insulin (pmol/L) | −13.6 (−31.6, 4.4) | 0.14 | −25.1 (−47.2, −3.0) | 0.026 | −11.5 (−41.3, 18.3) | 0.44 | 11.5 (−16.4, 39.4) | 0.42 | 4.1 (−30.8, 39.1) | 0.81 |
| HOMA-IR | −0.66 (−1.48, 0.16) | 0.11 | −1.10 (−2.11, −0.10) | 0.032 | −0.35 (−1.72, 1.01) | 0.61 | 0.44 (−0.83, 1.71) | 0.49 | 0.39 (−1.21, 1.99) | 0.63 |
| Glucose area under the curve (mmol/L * min) | −34.7 (−84.0, 14.6) | 0.17 | −11.6 (−72.6, 49.4) | 0.71 | 41.9 (−39.8, 123.6) | 0.31 | −23.1 (−98.8, 52.6) | 0.55 | 70.4 (−25.6, 166.4) | 0.15 |
| Glucose incremental area under the curve (mmol/L * min) | −10.6 (−51.5, 30.4) | 0.61 | 3.9 (−46.6, 54.4) | 0.88 | 22.7 (−45.7, 91.1) | 0.51 | −14.5 (−76.7, 47.8) | 0.65 | 26.1 (−54.1, 106.4) | 0.52 |
| Insulin area under the curve (pmol/L * min) | −3609.9 (−13,074.4, 5854.6) | 0.45 | −8898.2 (−20,769.0, 2972.7) | 0.14 | −12804.2 (−33,481.4, 7873.0) | 0.22 | −5288.3 (−20,193.7, 9617.1) | 0.48 | −8031.2 (−30,807.1, 14744.8) | 0.48 |
| Insulin incremental area under the curve (pmol/L * min) | −1240.4 (−8788.6, 6307.7) | 0.74 | −6499.5 (−15,949.9, 2950.9) | 0.17 | −7748.3 (−24,392.6, 8895.9) | 0.35 | −5259.0 (−17,060.2, 6542.1) | 0.38 | −5620.9 (−23,949.1, 12707.4) | 0.54 |
| MMP-9 (ng/mL) | −0.79 (−10.52, 8.94) | 0.87 | 2.30 (−9.42, 14.02) | 0.70 | 2.73 (−14.44, 19.90) | 0.75 | −3.09 (−17.30, 11.12) | 0.67 | 4.48 (−15.49, 24.46) | 0.66 |
| IMT (mm) | −0.01 (−0.05, 0.03) | 0.44 | −0.00 (−0.08, 0.07) | 0.96 | 0.03 (−0.02, 0.08) | 0.14 | 0.01 (−0.07, 0.10) | 0.73 | 0.04 (−0.02, 0.10) | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiel, I.A.; Jones, H.; Lionett, S.; Røsbjørgen, R.; Lydersen, S.; Vanky, E.; Moholdt, T. Cardiovascular Health Does Not Change Following High-Intensity Interval Training in Women with Polycystic Ovary Syndrome. J. Clin. Med. 2022, 11, 1626. https://doi.org/10.3390/jcm11061626
Kiel IA, Jones H, Lionett S, Røsbjørgen R, Lydersen S, Vanky E, Moholdt T. Cardiovascular Health Does Not Change Following High-Intensity Interval Training in Women with Polycystic Ovary Syndrome. Journal of Clinical Medicine. 2022; 11(6):1626. https://doi.org/10.3390/jcm11061626
Chicago/Turabian StyleKiel, Ida Almenning, Helen Jones, Sofie Lionett, Ragnhild Røsbjørgen, Stian Lydersen, Eszter Vanky, and Trine Moholdt. 2022. "Cardiovascular Health Does Not Change Following High-Intensity Interval Training in Women with Polycystic Ovary Syndrome" Journal of Clinical Medicine 11, no. 6: 1626. https://doi.org/10.3390/jcm11061626
APA StyleKiel, I. A., Jones, H., Lionett, S., Røsbjørgen, R., Lydersen, S., Vanky, E., & Moholdt, T. (2022). Cardiovascular Health Does Not Change Following High-Intensity Interval Training in Women with Polycystic Ovary Syndrome. Journal of Clinical Medicine, 11(6), 1626. https://doi.org/10.3390/jcm11061626






