Changes in Ventilation Practices for Bronchiolitis in the Hospital Ward and Need for ICU Transfer over the Last Decade
Abstract
:1. Introduction
2. Materials and Methods
- -
- Non-support group—admitted without respiratory support and stable for at least 12 h. Failure means to be started on SOT, HFNC, CPAP/BLPAP or ICU transfer.
- -
- SOT group—stability reached on SOT. Failure means escalation of respiratory support (HFNC/CPAP/BLPAP) or ICU transfer.
- -
- HFNC group—stability achieved on HFNC. Failure means that CPAP/BLPAP is subsequently required or ICU transfer.
- -
- CDP cohort—initial stabilisation with a continuous distension pressure (CDP) device in the airway (CPAP and/or BLPAP). Failure means the need for ICU transfer.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muñoz-Quiles, C.; López-Lacort, M.; Úbeda-Sansano, I.; Alemán-Sánchez, S.; Pérez-Vilar, S.; Puig-Barberà, J.; Díez-Domingo, J. Population-based analysis of bronchiolitis epidemiology in Valencia. Spain. Pediatr. Infect. Dis. J. 2016, 35, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. American Academy of Pediatrics. Clinical practice guideline: The diagnosis, management and prevention of bronchiolitis. Pediatrics 2014, 5, 1474–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkilä, P.; Sokuri, P.; Mecklin, M.; Nuolivirta, K.; Tapiainen, T.; Peltoniemi, O.; Renko, M.; Korppi, M. Using high-flow nasal cannulas for infants with bronchiolitis admitted to paediatric wards is safe and feasible. Acta Paediatr. 2018, 107, 1971–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riese, J.; Porter, T.; Fierce, J.; Riese, A.; Richardson, T.; Alverson, B.K. Clinical Outcomes of Bronchiolitis After Implementation of a General Ward High Flow Nasal Cannula Guideline. Hosp. Pediatr. 2017, 7, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes Domínguez, S.; del Villar Guerra, P.; Oñate Vergara, E.; Miñambres Rodríguez, M. ¿Y si la oxigenoterapia de alto flujo no es el tratamiento idóneo para la bronquiolitis en las plantas de hospitalización? An. Pediatr. 2020, 92, 60–61. [Google Scholar] [CrossRef] [PubMed]
- González Martínez, F.; González Sánchez, M.; Pérez Moreno, J.; Rodríguez Fernández, R. La oxigenoterapia de alto flujo sí tiene un papel en el tratamiento de la bronquiolitis en las plantas de pediatría. An. Pediatr. 2020, 92, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Pintile, M. Competing Risk. A Practical Perspective, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470870686. [Google Scholar]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Bueno Campaña, M.; Olivares Ortiz, J.; Notario Muñoz, C.; Rupérez Lucas, M.; Fernández Rincón, A.; Patiño Hernández, O.; Calvo Rey, C. High flow therapy versus hypertonic saline in bronchiolitis: Randomised controlled trial. Arch. Dis. Child. 2014, 99, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Fujiogi, M.; Goto, T.; Yasunaga, H.; Fujishiro, J.; Mansbach, J.M.; Camargo, C.A.; Hasegawa, K. Trends in Bronchiolitis Hospitalizations in the United States: 2000–2016. Pediatrics 2019, 144, e20192614. [Google Scholar] [CrossRef]
- Pelletier, J.H.; Au, A.K.; Fuhrman, D.; Clark, R.S.B.; Horvat, C. Trends in Bronchiolitis ICU Admissions and Ventilation Practices: 2010–2019. Pediatrics 2021, 147, e2020039115. [Google Scholar] [CrossRef] [PubMed]
- Coon, E.R.; Stoddard, G.; Brady, P.W. Intensive Care Unit Utilization after Adoption of a Ward-Based High-Flow Nasal Cannula Protocol. J. Hosp. Med. 2020, 15, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Garland, H.; Gunz, A.C.; Miller, M.R.; Lim, R.K. High-flow nasal cannula implementation has not reduced intubation rates for bronchiolitis in Canada. Paediatr. Child Health 2021, 26, e194–e198. [Google Scholar] [CrossRef] [PubMed]
- Milési, C.; Essouri, S.; Pouyau, R.; Liet, J.M.; Afanetti, M.; Portefaix, A.; Baleine, J.; Durand, S.; Combes, C.; Douillard, A.; et al. High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: A multicenter randomized controlled trial (TRAMONTANE study). Intensive Care Med. 2017, 43, 209–216. [Google Scholar] [CrossRef]
- Milési, C.; Pierre, A.-F.; Deho, A.; Pouyau, R.; Liet, J.-M.; Guillot, C.; Guilbert, A.-S.; Rambaud, J.; Millet, A.; for the GFRUP Respiratory Study Group; et al. A multicenter randomized controlled trial of a 3-L/kg/min versus 2-L/kg/min high-flow nasal cannula flow rate in young infants with severe viral bronchiolitis (TRAMONTANE 2). Intensive Care Med. 2018, 44, 1870–1878. [Google Scholar] [CrossRef]
- Vahlkvist, S.; Jürgensen, L.; La Cour, A.; Markoew, S.; Petersen, T.H.; Kofoed, P.-E. High flow nasal cannula and continuous positive airway pressure therapy in treatment of viral bronchiolitis: A randomized clinical trial. Eur. J. Pediatr. 2019, 179, 513–518. [Google Scholar] [CrossRef]
- Franklin, D.; Babl, F.E.; Schlapbach, L.J.; Oakley, E.; Craig, S.; Neutze, J.; Furyk, J.; Fraser, J.F.; Jones, M.; Whitty, J.A.; et al. A Randomized Trial of High-Flow Oxygen Therapy in Infants with Bronchiolitis. N. Engl. J. Med. 2018, 378, 1121–1131. [Google Scholar] [CrossRef]
- Meskill, S.D.; Moore, R.H. High-Flow Oxygen Therapy in Infants with Bronchiolitis. N. Engl. J. Med. 2018, 378, 2444. [Google Scholar]
- Gc, V.S.; Franklin, D.; Whitty, J.A.; Dalziel, S.R.; Babl, F.E.; Schlapbach, L.J.; Fraser, J.F.; Craig, S.; Neutze, J.; Oakley, E.; et al. First-line oxygen therapy with high-flow in bronchiolitis is not cost saving for the health service. Arch. Dis. Child. 2020, 105, 975–980. [Google Scholar] [CrossRef]
- Durand, P.; Guiddir, T.; Kyheng, C.; Blanc, F.; Vignaud, O.; Epaud, R.; Dugelay, F.; Breant, I.; Badier, I.; Degas-Bussière, V.; et al. A randomised trial of high-flow nasal cannula in infants with moderate bronchiolitis. Eur. Respir. J. 2020, 56, 1901926. [Google Scholar] [CrossRef]
- Kepreotes, E.; Whitehead, B.; Attia, J.; Oldmeadow, C.; Collison, A.; Searles, A.; Goddard, B.; Hilton, J.; Lee, M.; Mattes, J. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): An open, phase 4, randomised controlled trial. Lancet 2017, 389, 930–939. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Xiong, L.; Liu, S.; Gong, C.; Dai, J. High-flow nasal cannula therapy for children with bronchiolitis: A systematic review and meta-analysis. Arch. Dis. Child. 2019, 104, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Tortosa, F.; Izcovich, A.; Carrasco, G.; Varone, G.; Haluska, P.; Sanguine, V. High-flow oxygen nasal cannula for treating acute bronchiolitis in infants: A systematic review and meta-analysis. Medwave 2021, 21, e8190. [Google Scholar] [CrossRef] [PubMed]
- Cataño-Jaramillo, M.L.; Jaramillo-Bustamante, J.C.; Florez, I.D. Continuous Positive Airway Pressure vs. High Flow Nasal Can-nula in children with acute severe or moderate bronchiolitis. A systematic review and Meta-analysis. Med. Intensiva 2020, 46, 72–80. [Google Scholar] [CrossRef]
- Tang, G.; Lin, J.; Zhang, Y.; Shi, Q. The Effects and Safety of Continuous Positive Airway Pressure in Children with Bronchiolitis: A Systematic Review and Meta-Analysis. J. Trop. Pediatr. 2021, 67, 128. [Google Scholar] [CrossRef]
- Moreel, L.; Proesmans, M. High flow nasal cannula as respiratory support in treating infant bronchiolitis: A systematic review. Eur. J. Pediatr. 2020, 179, 711–718. [Google Scholar] [CrossRef]
- Siraj, S.; Compton, B.; Russell, B.; Ralston, S. Reducing High-flow Nasal Cannula Overutilization in Viral Bronchiolitis. Pediatr. Qual. Saf. 2021, 6, e420. [Google Scholar] [CrossRef]
- Nguyen, T.-L.; Collins, G.S.; Spence, J.; Daurès, J.-P.; Devereaux, P.J.; Landais, P.; Le Manach, Y. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 2017, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
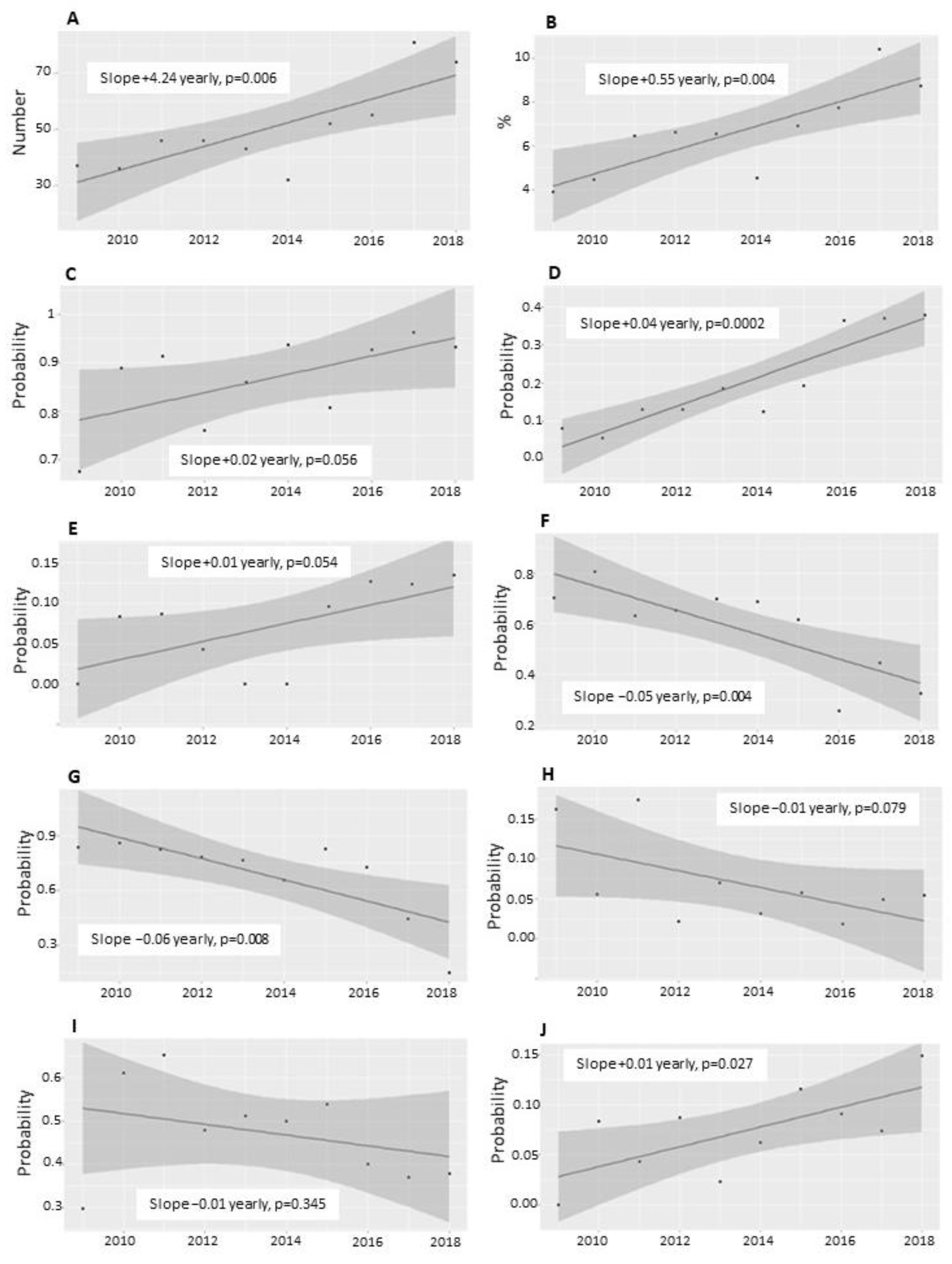
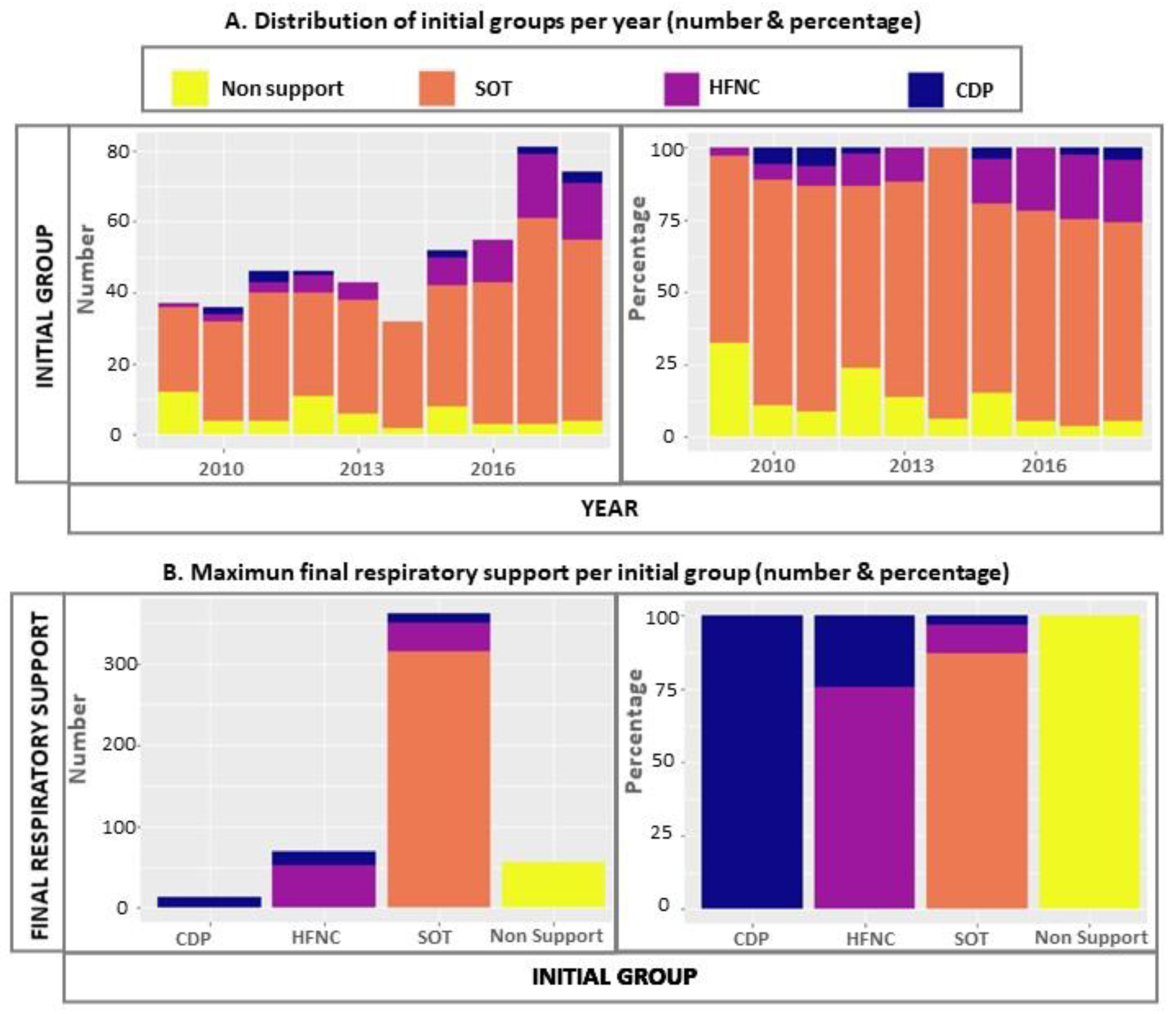
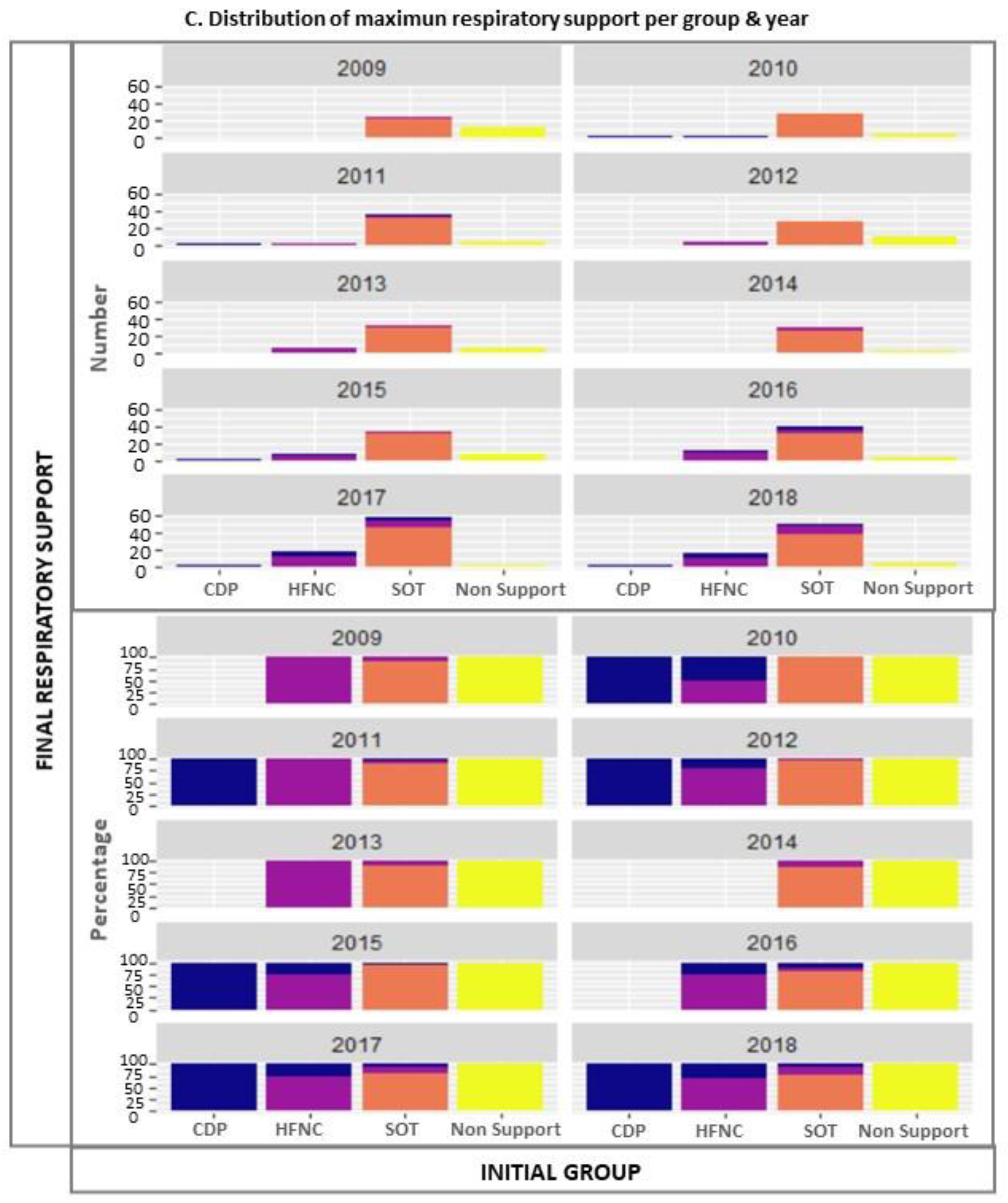

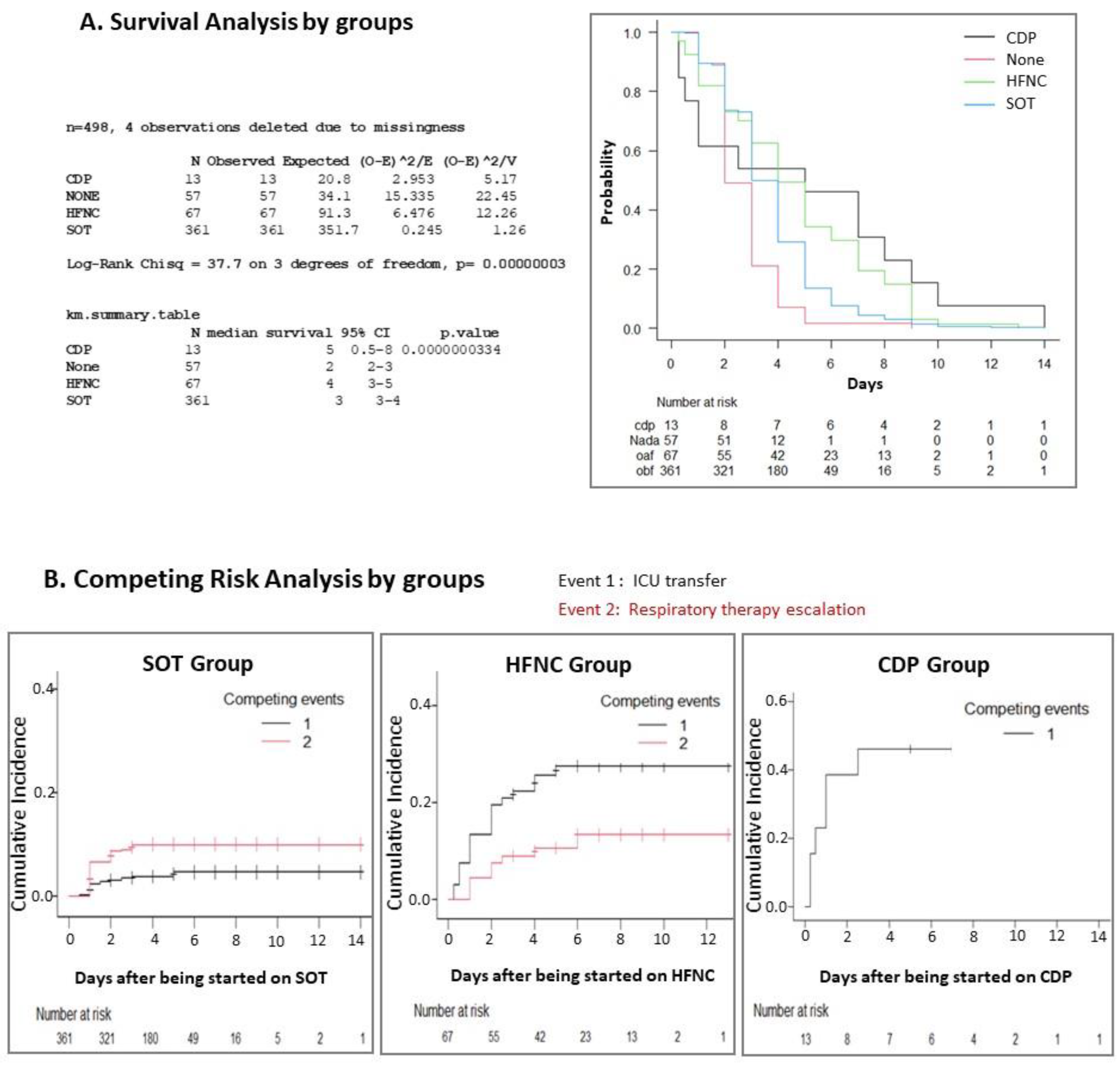
| Variables | Total | Non-Support Group | SOT Group | HFNC Group | CDP Group | p-Value |
|---|---|---|---|---|---|---|
| n (%) | 502 | 57 (11.3) | 362 (72.1) | 70 (13.9) | 13 (2.6) | |
| Demographic variables | ||||||
| Male (%) | 274 (54.6) | 37 (64.9) | 196 (54.1) | 36 (51.4) | 5 (38.5) | 0.25 |
| Age (months) (median [IQR]) | 3 [2; 5] | 3 [2; 4] | 3 [2; 4] | 2.5 [2; 4] | 1 [1; 2] | 0.0012 |
| <1 months (%) | 60 (12) | 8 (14.0) | 33 (9.1) | 11 (15.7) | 9 (69.2) | |
| 1–3 months (%) | 236 (47) | 32 (56.1) | 167 (46.1) | 34 (48.6) | 3 (23.1) | |
| 3–6 months (%) | 134 (26.7) | 11 (19.3) | 108 (29.8) | 14 (20.0) | 1 (7.7) | |
| >6 months (%) | 72 (14.3) | 6 (10.5) | 54 (14.9) | 11 (15.7) | 0 | |
| Prematurity < 37 weeks (%) | 42 (8.4) | 3 (5.3) | 31 (8.6) | 7 (10.0) | 1 (7.7) | 0.80 |
| Gestational age (weeks) if prematurity (median [IQR]) | 34.5 (33; 36) | 36 (33; 36) | 34 (33; 35) | 33 (28; 36) | 35 | 0.71 |
| Medical treatment | ||||||
| Bronchodilators (%) | 418 (83.3) | 47 (82.5) | 312 (86.2) | 52 (74.3) | 7 (53.9) | 0.004 |
| Beta2-mimetics (%) | 272 (54.2) | 28 (49.1) | 213 (58.8) | 27 (38.6) | 4 (30.8) | 0.004 |
| Adrenaline (%) | 320 (63.7) | 40 (70.2) | 233 (64.4) | 41 (58.6) | 6 (46.2) | 0.30 |
| Corticoids (%) | 33 (6.6) | 0 | 31 (8.6) | 1 (1.4) | 1 (7.7) | 0.10 |
| Antibiotics (%) | 113 (22.5) | 10 (17.4) | 70 (19.3) | 25 (35.7) | 8 (61.5) | <0.0001 |
| Fluid therapy (%) | 231 (45.6) | 17 (29.8) | 142 (39.2) | 60 (85.7) | 12 (92.3) | <0.0001 |
| Clinical evolution | ||||||
| Complications (%) | 126 (25.1) | 12 (21.1) | 78 (21.6) | 27 (38.6) | 9 (69.2) | <0.0001 |
| Atelectasis (%) | 33 (6.6) | 0 (0) | 15 (4.1) | 15 (21.4) | 3 (23.1) | <0.0001 |
| Viral pneumonia (%) | 6 (1.2) | 1 (1.8) | 3 (0.8) | 2 (2.9) | 0 | 0.30 |
| Pneumothorax (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Death (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Coinfections (%) | 124 (24.7) | 11 (19.3) | 77 (21.3) | 27 (38.6) | 9 (69.2) | <0.0001 |
| Bordetella pertussis (%) | 6 (1.2) | 1 (1.8) | 4 (1.1) | 0 | 1 (7.7) | 0.15 |
| Bacterial pneumonia (%) | 44 (8.8) | 3 (5.3) | 25 (6.9) | 11 (15.7) | 5 (38.5) | 0.001 |
| Acute otitis media (%) | 35 (6.7) | 4 (7.0) | 31 (8.6) | 0 | 0 | 0.03 |
| Urinary tract infection (%) | 4 (0.8) | 1 (1.8) | 3 (0.8) | 0 | 0 | 0.52 |
| Sepsis (%) | 2 (0.4) | 1 (1.8) | 0 | 0 | 1 (7.7) | 0.12 |
| Total duration of respiratory support (days) (median [IC95%]) | 3 (3–3) | 0 (0) | 3 (2.5–3) | 4 (3.5–5.5) | 4 (2-NA *) | <0.0001 |
| Hospital length of stay (days) (median [IC95%]) | 4 (4–4) | 2 (2–3) | 4 (4–4) | 8 (6–9) | 10 (7-NA) | <0.0001 |
| Failure of initial respiratory support (%) | 84 (16.6) | 0 (0) | 49 (13.5) | 29 (41.4) | 6 (46.2) | <0.0001 |
| ICU transfer (%) | 39 (7.8) | 0 (0) | 14 (3.9) | 19 (27.1) | 6 (46.2) | <0.0001 |
| Invasive mechanical ventilation (% Total) (% ICU transferred) | 8 (1.5) (20.5) | NA | 3 (0.8) (21.4) | 3 (4.2) (15.8) | 2 (15.3) (33.3) | 0.004 |
| ICU length of stay (days) (median [IC95%]) | 4 (3–6) | NA | 4.5 (3–7) | 5 (3–7) | 3 (2-NA) | 0.883 |
| Independent Variables | Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| PREDICTOR FACTORS FOR ICU TRANSFER | ||||
| GLOBAL SAMPLE | ||||
| Intercept | 2.7 × 10−127 | 1.59 × 10−254 | 4.57 | 0.051 |
| Epidemic season | 0.14 | 0.07 | 1.93 | 0.056 |
| Complications/Co-infections | 6.69 | 3.04 | 14.7 | <0.001 |
| Prematurity | 2.57 | 0.85 | 7.77 | 0.09 |
| Cohorts: | ||||
| • SOT (Ref) | - | - | - | - |
| • No support | 2.95 × 10−7 | 0 | Inf | 0.98 |
| • HFNC | 5.86 | 2.61 | 13.2 | <0.001 |
| • CDP | 10.6 | 2.84 | 39.6 | <0.001 |
| SOT GROUP | ||||
| Intercept | 0.004 | 0.001 | 0.021 | <0.001 |
| Complications/Co-infections | 3.92 | 1.06 | 14.5 | 0.04 |
| Escalation to: | ||||
| • HFNC | 29.1 | 5.63 | 150 | <0.001 |
| • CDP | 3.03 | 0.62 | 14.7 | 0.17 |
| HFNC GROUP | ||||
| Intercept | 1.22 | 0.32 | 4.66 | 0.77 |
| Complications/Co-infections | 5.11 | 1.38 | 18.9 | 0.01 |
| Days on HFNC | 0.48 | 0.3 | 0.78 | 0.002 |
| PREDICTOR FACTORS FOR RESPIRATORY SUPPORT ESCALATION | ||||
| GLOBAL SAMPLE | ||||
| Intercept | 2.42 × 10−203 | 9.55 × 10−298 | 6.15 × 10−109 | <0.001 |
| Epidemic season | 1.26 | 1.13 | 1.4 | <0.001 |
| Complications/Co-infections | 4.72 | 2.56 | 8.69 | <0.001 |
| Prematurity | 3.04 | 1.35 | 6.85 | 0.007 |
| Cohorts: | ||||
| • SOT (Ref) | - | - | - | - |
| • No support | 7.95 × 10−8 | 0 | Inf | 0.98 |
| • HFNC | 2.71 | 1.46 | 5.05 | 0.001 |
| • CDP | 3.16 | 0.93 | 1.07 | 0.07 |
| SOT GROUP | ||||
| Intercept | 4.13 × 10−313 | 0 | 3.61 × 10−173 | 1.21 × 10−5 |
| Epidemic season | 1.43 | 1.22 | 1.68 | <0.001 |
| Prematurity | 3.72 | 1.25 | 11.1 | 0.02 |
| Complications/Co-infections | 9.23 | 3.74 | 22.8 | <0.001 |
| Bronchodilators | 2.54 | 0.9 | 7.14 | 0.07 |
| Days on O2 | 0.31 | 0.2 | 0.48 | <0.001 |
| HFNC GROUP | ||||
| Intercept | 5.17 | 1.08 | 24.7 | 0.04 |
| Complications/Co-infections | 5.27 | 1.42 | 19.6 | 0.01 |
| Bronchodilators | 0.37 | 0.09 | 1.47 | 0.16 |
| Days on HFNC | 0.49 | 0.32 | 0.75 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solana-Gracia, R.; Modesto i Alapont, V.; Bueso-Inchausti, L.; Luna-Arana, M.; Möller-Díez, A.; Medina, A.; Pérez-Moneo, B. Changes in Ventilation Practices for Bronchiolitis in the Hospital Ward and Need for ICU Transfer over the Last Decade. J. Clin. Med. 2022, 11, 1622. https://doi.org/10.3390/jcm11061622
Solana-Gracia R, Modesto i Alapont V, Bueso-Inchausti L, Luna-Arana M, Möller-Díez A, Medina A, Pérez-Moneo B. Changes in Ventilation Practices for Bronchiolitis in the Hospital Ward and Need for ICU Transfer over the Last Decade. Journal of Clinical Medicine. 2022; 11(6):1622. https://doi.org/10.3390/jcm11061622
Chicago/Turabian StyleSolana-Gracia, Ruth, Vicent Modesto i Alapont, Leticia Bueso-Inchausti, María Luna-Arana, Ariadna Möller-Díez, Alberto Medina, and Begoña Pérez-Moneo. 2022. "Changes in Ventilation Practices for Bronchiolitis in the Hospital Ward and Need for ICU Transfer over the Last Decade" Journal of Clinical Medicine 11, no. 6: 1622. https://doi.org/10.3390/jcm11061622
APA StyleSolana-Gracia, R., Modesto i Alapont, V., Bueso-Inchausti, L., Luna-Arana, M., Möller-Díez, A., Medina, A., & Pérez-Moneo, B. (2022). Changes in Ventilation Practices for Bronchiolitis in the Hospital Ward and Need for ICU Transfer over the Last Decade. Journal of Clinical Medicine, 11(6), 1622. https://doi.org/10.3390/jcm11061622






