Neurological Presentation of Giant Pituitary Tumour Apoplexy: Case Report and Literature Review of a Rare but Life-Threatening Condition
Abstract
:1. Introduction
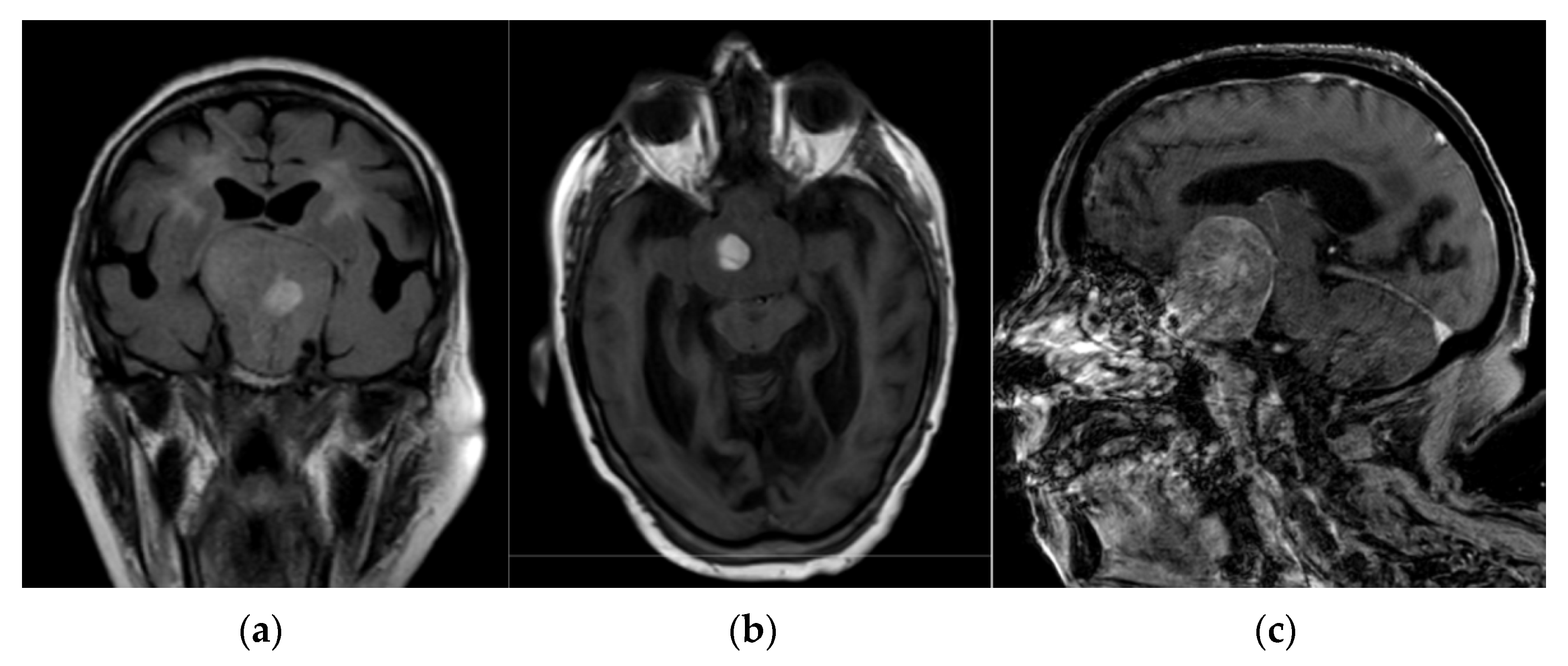
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iglesias, P.; Berrocal, V.R.; Díez, J.J. Giant pituitary adenoma: Histological types, clinical features and therapeutic approaches. Endocrine 2018, 61, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Hammer, S.; de Keizer, R.J.; Roelfsema, F.; Schutte, P.J.; Smit, J.W.; Romijn, J.A.; Pereira, A.M. The natural course of non-functioning pituitary macroadenomas. Eur. J. Endocrinol. 2007, 156, 217–224. [Google Scholar] [CrossRef]
- Matsuyama, J.; Kawase, T.; Yoshida, K.; Hasegawa, M.; Hirose, Y.; Nagahisa, S.; Watanabe, S.; Sano, H. Management of large and giant pituitary adenomas with suprasellar extensions. Asian J. Neurosurg. 2010, 5, 48–53. [Google Scholar] [PubMed]
- Joshi, S.M.; Chopra, I.S.; Powell, M. Hydrocephalus caused by giant pituitary tumors: Case series and guidelines for management. Br. J. Neurosurg. 2009, 23, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Wildemberg, L.E.; Glezer, A.; Bronstein, M.D.; Gadelha, M.R. Apoplexy in nonfunctioning pituitary adenomas. Pituitary 2018, 21, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Vanderpump, M.; Baldeweg, S.; Drake, W.; Reddy, N.; Lanyon, M.; Markey, A.; Plant, G.; Powell, M.; Sinha, S.; et al. UK guidelines for the management of pituitary apoplexy. Clin. Endocrinol. 2011, 74, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.; Gonzalez, B.; Ramirez, C.; Ferreira, A.; Espinosa, E.; Mendoza, V.; Guinto, G.; Lopez-Felix, B.; Zepeda, E.; Mercado, M. Clinical characteristics and treatment outcome of 485 patients with nonfunctioning pituitary macroadenomas. Int. J. Endocrinol. 2015, 2015, 756069. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.C.; Hamrahian, A.H.; Weil, R.J.; Kennedy, L. Pituitary tumor apoplexy. J. Clin. Neurosci. 2015, 22, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.H.; Lindholm, J.; Bjerre, P.; Christiansen, J.S.; Hagen, C.; Juul, S.; Jørgensen, J.; Kruse, A.; Laurberg, P. Frequent occurrence of pituitary apoplexy in patients with non-functioning pituitary adenoma. Clin. Endocrinol. 2006, 64, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Falhammar, H.; Tornvall, S.; Höybye, C. Pituitary apoplexy: A retrospective study of 33 cases from a single center. Front. Endocrinol. 2021, 12, 656950. [Google Scholar] [CrossRef] [PubMed]
- Okuda, O.; Umezawa, H.; Miyaoka, M. Pituitary apoplexy caused by endocrine stimulation tests: A case report. Surg. Neural. 1994, 42, 19–22. [Google Scholar] [CrossRef]
- Goel, A.; Deogaonkar, M.; Desai, K. Fatal postoperative “pituitary apoplexy”: Its cause and management. Br. J. Neurosurg. 1995, 9, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Perotti, V.; Dexter, M. Post-partum pituitary apoplexy with bilateral third nerve palsy and bilateral carotid occlusion. Case Rep. J. Clin. Neurosci. 2010, 17, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Fanous, A.A.; Quigley, E.P.; Chin, S.S.; Couldwell, W.T. Giant necrotic pituitary apoplexy. J. Clin. Neurosci. 2013, 20, 1462–1464. [Google Scholar] [CrossRef]
- Romano, A.; Ganau, M.; Zaed, I.; Scibilia, A.; Oretti, G.; Chibbaro, S. Primary endoscopic management of apoplexy in a giant pituitary adenoma. World Neurosurg. 2020, 142, 312–313. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.U.; Pandey, P.; Mahapatra, A.K. Post-operative “pituitary apoplexy” in giant pituitary adenomas: A series of cases. Neurol. India 2005, 53, 326–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Nadkarni, T.; Muzumdar, D.; Desai, K.; Phalke, U.; Sharma, P. Giant pituitary tumors: A study based on surgical treatment of 118 cases. Surg. Neurol. 2004, 61, 436–445. [Google Scholar] [CrossRef]
- Maiter, D.; Delgrange, E. Therapy of endocrine disease: The challenges in managing giant prolactinomas. Eur. J. Endocrinol. 2014, 170, R213–R227. [Google Scholar] [CrossRef] [Green Version]
- Nawar, R.N.; Mannan, A.D.; Selman, W.R.; Arafah, B.M. Pituitary tumor apoplexy: A review. J. Intens. Care Med. 2008, 23, 75–90. [Google Scholar] [CrossRef]
- Dubuisson, A.S.; Beckers, A.; Stevenaert, A. Classical pituitary tumour apoplexy: Clinical features, management and outcomes in a series of 24 patients. Clin. Neurol. Neurosurg. 2007, 109, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Karavitaki, N.; Wass, J.A. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Briet, C.; Salenave, S.; Bonneville, J.-F.; Laws, E.R.; Chanson, P. Pituitary apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef]
- Glezer, A.; Bronstein, M.D. Pituitary apoplexy: Pathophysiology, diagnosis and management. Arch. Endocrinol. Metab. 2015, 59, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randeva, H.S.; Schoebel, J.; Byrne, J.; Esiri, M.; Adams, C.B.; Wass, J.A. Classical pituitary apoplexy: Clinical features, management and outcome. Clin. Endocrinol. 1999, 51, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, J.; McGregor, E.J.; Mitchell, R.D.; Gittoes, N.J. Acute management of pituitary apoplexy-surgery or conservative management? Clin. Endocrinol. 2004, 61, 747–752. [Google Scholar] [CrossRef]
- Doglietto, F.; Costi, E.; Villaret, A.B.; Mardighian, D.; Fontanella, M.M.; Giustina, A. New oral anticoagulants and pituitary apoplexy. Pituitary 2016, 19, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Capatina, C.; Tenreiro, A.P.; Guardiola, P.D.; Byrne, J.V.; Cudlip, S.; Karavitaki, N.; Wass, J.A. Pituitary apoplexy in non-functioning pituitary adenomas: Long term follow up is important because of significant numbers of tumour recurrences. Clin. Endocrinol. 2011, 75, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.; Losa, M.; Mortini, P. Pituitary adenomas in elderly patients: Clinical and surgical outcome analysis in a large series. Endocrine 2019, 65, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Cantone, M.; Lanza, G.; Puglisi, V.; Vinciguerra, L.; Mandelli, J.; Fisicaro, F.; Pennisi, M.; Bella, R.; Ciurleo, R.; Bramanti, A. Hypertensive crisis in acute cerebrovascular diseases presenting at the emergency department: A narrative review. Brain Sci. 2021, 11, 70. [Google Scholar] [CrossRef]
- Lanza, G.; Vinciguerra, L.; Puglisi, V.; Giuffrida, S.; Foti, P.; Zelante, G.; Pennisi, G.; Bella, R. Acute isolated trochlear nerve palsy in a patient with cavernous carotid aneurysm and visit-to-visit variability in systolic blood pressure. Int. J. Stroke 2015, 10, E61. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Dunn, I.F.; Laws, E.R. Pituitary apoplexy. Endocrine 2015, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Arafah, B.M. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J. Clin. Endocrinol. Metab. 1986, 62, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Klibanski, A. Nonsecreting pituitary tumors. Endocrinol. Metab. Clin. N. Am. 1987, 16, 793–804. [Google Scholar] [CrossRef]
- Grossman, A.B. Clinical review: The diagnosis and management of central hypoadrenalism. J. Clin. Endocrinol. Metab. 2010, 95, 4855–4863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Cincu, R.; Goel, A. Current concepts and controversies in the management of non-functioning giant pituitary macroadenomas. Clin. Neurol. Neurosurg. 2007, 109, 6. [Google Scholar] [CrossRef] [PubMed]
- Buchfelder, M. Management of aggressive pituitary adenomas: Current treatment strategies. Pituitary 2009, 12, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kokshoorn, N.E.; Biermasz, N.R.; Roelfsema, F.; Smit, J.W.; Pereira, A.M.; Romijn, J.A. GH replacement therapy in elderly GH-deficient patients: A systematic review. Eur. J. Endocrinol. 2011, 164, 657–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappabianca, P.; Cavallo, L.M.; Esposito, F. Extended endoscopic endonasal approach to the midline skull base: The evolving role of transsphenoidal surger. Adv. Tech. Stand. Neurosurg. 2008, 33, 151–199. [Google Scholar] [CrossRef]

| Study, Year | Age, Years | Sex | Known Tumour | Precipitating Factors for PA | Clinical Presentation | Neuroimaging Findings | Management and Outcome |
|---|---|---|---|---|---|---|---|
| Okuda, et al., 1994 [9] | 60 | Female | Yes | Endocrine stimulation tests | Headache, stuporous status, hemiparesis | Haemorrhage intra- and extra-tumour | Surgery + radiation, with partial tumour residual; follow-up not available |
| Goel, et al., 1995 [10] | 40 | Male | Yes | Surgery of pituitary tumour | Coma | Diffuse swelling and haemorrhage | Re-surgery, followed by exitus (3 days after) |
| 17 | Male | Yes | Surgery of pituitary tumour | Visual deficit, coma | Diffuse swelling and haemorrhage | Re-surgery, followed by exitus (3 months after) | |
| Ahmad, et al., 2005 [14] | 50 | Male | Yes | Surgery of pituitary tumour | Altered mental status | Diffuse swelling and haemorrhage | Re-surgery, followed by exitus (3–20 days after) |
| 30 | Male | Yes | Altered mental status | ||||
| 35 | Female | Yes | Third nerve paresis, visual deficit | ||||
| 19 | Male | Yes | Altered mental status | ||||
| Perotti, et al., 2010 [11] | 29 | Female | No | Post-partum | Headache, vomiting, coma | Haemorrhage intra-tumour | Steroids + surgery; functionally independent at 6-month follow-up |
| Fanous, et al., 2013 [12] | 39 | Male | No | Spontaneous | Headache, diplopia, cranial nerve palsy | Necrotic apoplexy | Steroids + surgery, near complete resolution at 2-month follow-up |
| Romano, et al., 2020 [13] | 65 | Male | No | Spontaneous | Visual deficit, altered mental status, hemiparesis | Tumour apoplexy | Endoscopic approach; follow-up not available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglisi, V.; Morini, E.; Biasini, F.; Vinciguerra, L.; Lanza, G.; Bramanti, P. Neurological Presentation of Giant Pituitary Tumour Apoplexy: Case Report and Literature Review of a Rare but Life-Threatening Condition. J. Clin. Med. 2022, 11, 1581. https://doi.org/10.3390/jcm11061581
Puglisi V, Morini E, Biasini F, Vinciguerra L, Lanza G, Bramanti P. Neurological Presentation of Giant Pituitary Tumour Apoplexy: Case Report and Literature Review of a Rare but Life-Threatening Condition. Journal of Clinical Medicine. 2022; 11(6):1581. https://doi.org/10.3390/jcm11061581
Chicago/Turabian StylePuglisi, Valentina, Elisabetta Morini, Fiammetta Biasini, Luisa Vinciguerra, Giuseppe Lanza, and Placido Bramanti. 2022. "Neurological Presentation of Giant Pituitary Tumour Apoplexy: Case Report and Literature Review of a Rare but Life-Threatening Condition" Journal of Clinical Medicine 11, no. 6: 1581. https://doi.org/10.3390/jcm11061581
APA StylePuglisi, V., Morini, E., Biasini, F., Vinciguerra, L., Lanza, G., & Bramanti, P. (2022). Neurological Presentation of Giant Pituitary Tumour Apoplexy: Case Report and Literature Review of a Rare but Life-Threatening Condition. Journal of Clinical Medicine, 11(6), 1581. https://doi.org/10.3390/jcm11061581







