A Three-Protein Panel to Support the Diagnosis of Sepsis in Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population: Inclusion and Exclusion Criteria
2.2. Data and Sample Collection
2.3. Proteomic Study
2.4. Selection of Proteins and Their Validation by ELISA
2.5. Statistical Analysis
3. Results
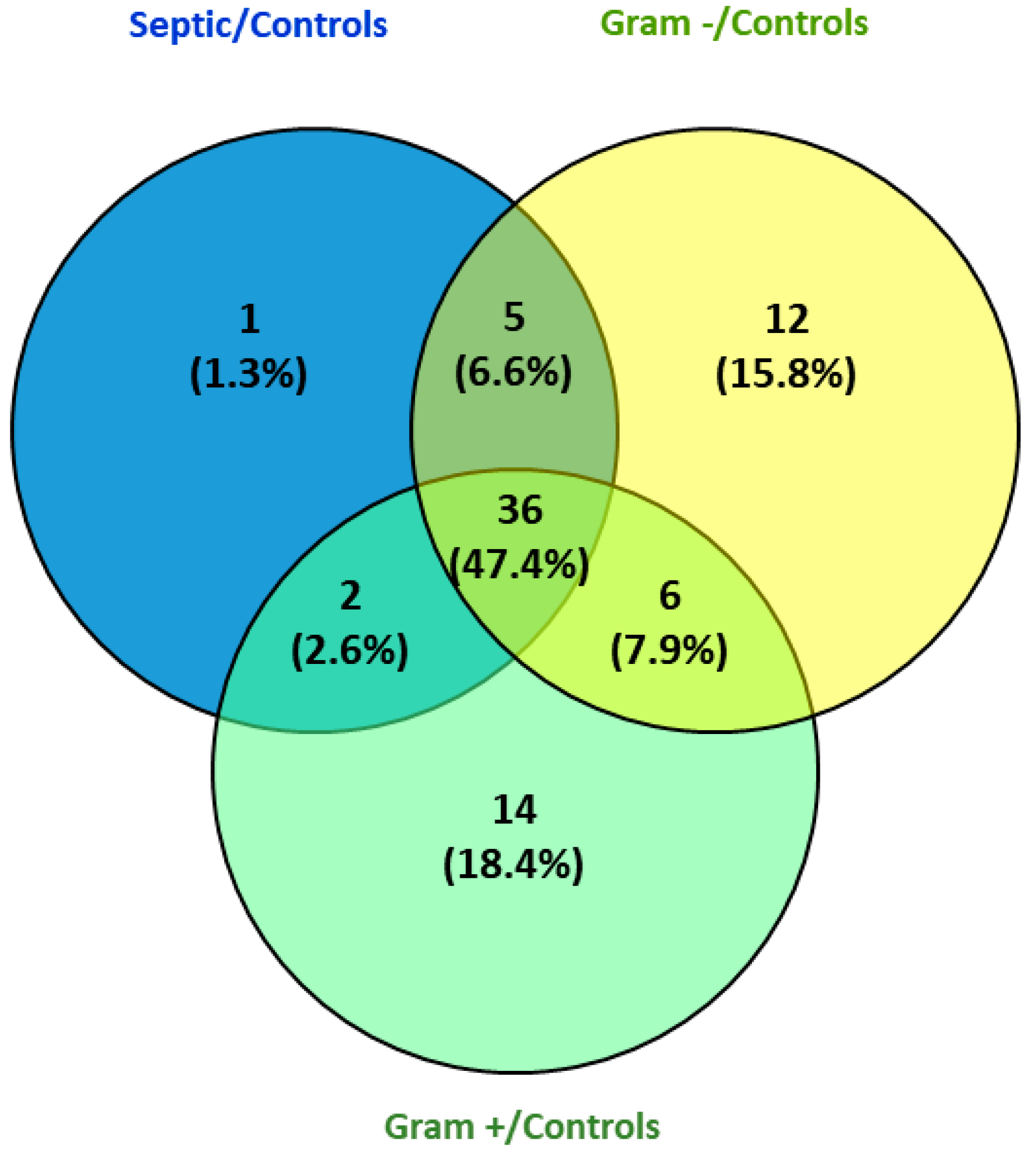
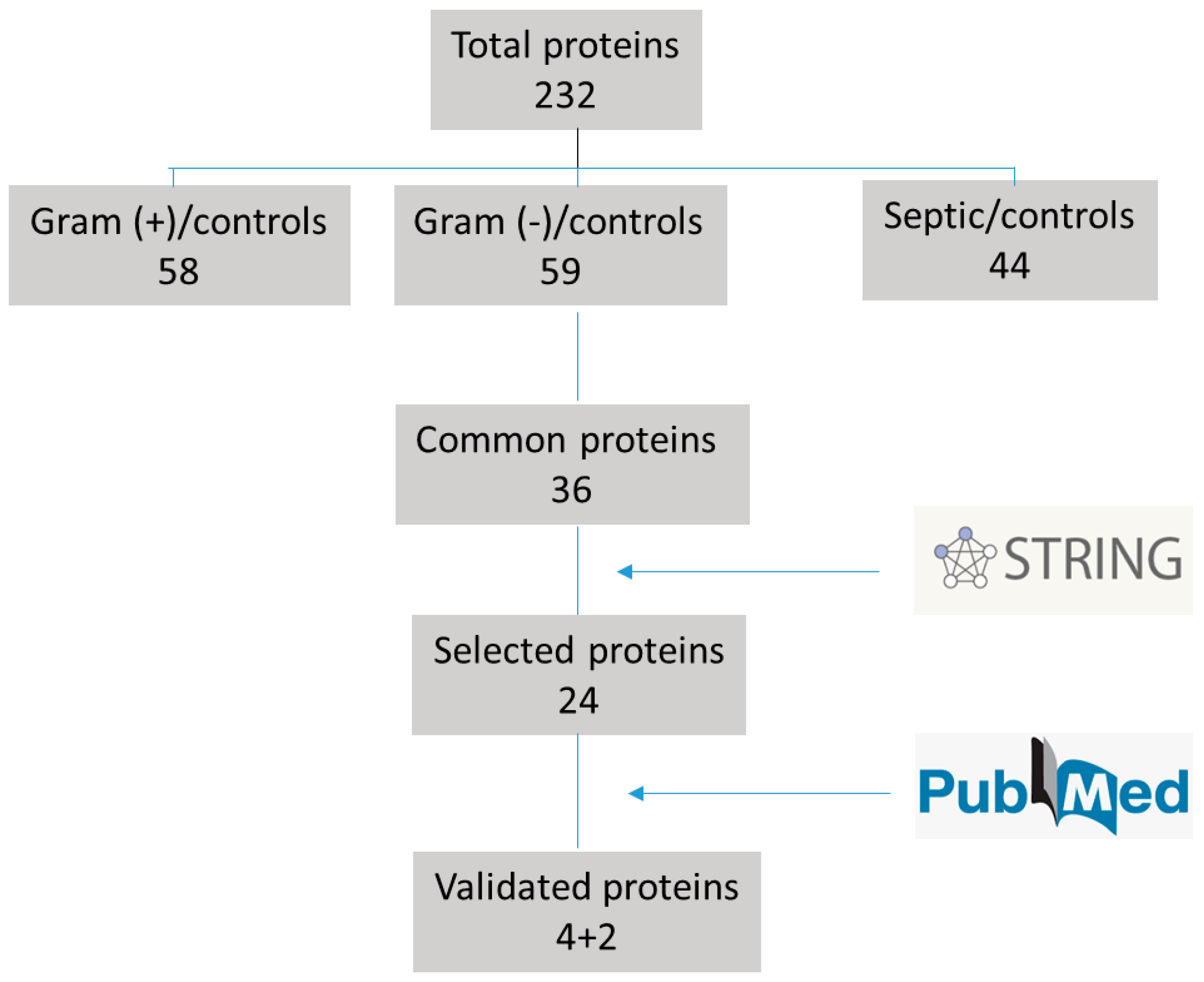
3.1. Proteomic Results
3.2. Selection of Proteins and Their Validation by ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Matics, T.J.; Sanchez-Pinto, L.N. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr. 2017, 171, e172352. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr. Crit. Care Med. 2020, 21, e52–e106. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Goldfarb, D.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Odetola, F.O.; Gebremariam, A.; Freed, G.L. Patient and Hospital Correlates of Clinical Outcomes and Resource Utilization in Severe Pediatric Sepsis. Pediatrics 2007, 119, 487–494. [Google Scholar] [CrossRef]
- Prout, A.J.; Talisa, V.B.; Carcillo, J.A.; Mayr, F.B.; Angus, D.C.; Seymour, C.W.; Chang, C.-C.H.; Yende, S. Children with Chronic Disease Bear the Highest Burden of Pediatric Sepsis. J. Pediatr. 2018, 199, 194–199. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; MacLaren, G.; Festa, M.; Alexander, J.; Erickson, S.; Beca, J.; Slater, A.; Schibler, A.; Pilcher, D.; Millar, J.; et al. Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. 2017, 43, 1085–1096. [Google Scholar] [CrossRef]
- Giannakopoulos, K.; Hoffmann, U.; Ansari, U.; Bertsch, T.; Borggrefe, M.; Akin, I.; Behnes, M. The Use of Biomarkers in Sepsis: A Systematic Review. Curr. Pharm. Biotechnol. 2017, 18, 499–507. [Google Scholar] [CrossRef]
- Ray, S.; Reddy, P.J.; Jain, R.; Gollapalli, K.; Moiyadi, A.; Srivastava, S. Proteomic technologies for the identification of disease biomarkers in serum: Advances and challenges ahead. Proteomics 2011, 11, 2139–2161. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Robinson, R.A.S. The role of proteomics in understanding biological mechanisms of sepsis. Proteom. Clin. Appl. 2014, 8, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.R.; Hummon, A.B. Mass spectrometry for the discovery of biomarkers of sepsis. Mol. Biosyst. 2017, 13, 648–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Weitzman, S. Approach to Hemophagocytic Syndromes. Hematol. Am. Soc. Hematol. Educ. Program. 2011, 2011, 178–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tothova, Z.; Berliner, N. Hemophagocytic syndrome and critical illness: New insights into diagnosis and management. J. Intensive Care Med. 2015, 30, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Salomao, R. Sepsis Through the Eyes of Proteomics: The Progress in the Last Decade. Shock 2017, 47 (Suppl. S1), 17–25. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.-L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Pontrelli, G.; De Crescenzo, F.; Buzzetti, R.; Jenkner, A.; Balduzzi, S.; Carducci, F.C.; Amodio, D.; De Luca, M.; Chiurchiù, S.; Davies, E.H.; et al. Accuracy of serum procalcitonin for the diagnosis of sepsis in neonates and children with systemic inflammatory syndrome: A meta-analysis. BMC Infect. Dis. 2017, 17, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, M.G.; García, R.P.; Gamero, D.B.; González-Tomé, M.I.; Romero, P.C.; Ferrer, M.M.; Contreras, J.R. Lack of Accuracy of Biomarkers and Physical Examination to Detect Bacterial Infection in Febrile Infants. Pediatr. Emerg. Care 2016, 32, 664–668. [Google Scholar] [CrossRef]
- Arnon, S.; Litmanovitz, I.; Regev, R.H.; Bauer, S.; Shainkin-Kestenbaum, R.; Dolfin, T. Serum amyloid A: An early and accurate marker of neonatal early-onset sepsis. J. Perinatol. 2007, 27, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashida, T.; Nakada, T.-A.; Satoh, M.; Tomita, K.; Kawaguchi, R.; Nomura, F.; Oda, S. Proteome analysis of hemofilter adsorbates to identify novel substances of sepsis: A pilot study. J. Artif. Organs 2017, 20, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Shirai, R.; Hirano, F.; Ohkura, N.; Ikeda, K.; Inoue, S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem. Biophys. Res. Commun. 2009, 382, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Druhan, L.J.; Lance, A.; Li, S.; Price, A.E.; Emerson, J.T.; Baxter, S.A.; Gerber, J.M.; Avalos, B.R. Leucine Rich α-2 Glycoprotein: A Novel Neutrophil Granule Protein and Modulator of Myelopoiesis. PLoS ONE 2017, 12, e0170261. [Google Scholar] [CrossRef]
- Llewelyn, M.J.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matera, G.; Puccio, R.; Giancotti, A.; Quirino, A.; Pulicari, M.C.; Zicca, E.; Caroleo, S.; Renzulli, A.; Liberto, M.C.; Focà, A. Impact of interleukin-10, soluble CD25 and interferon-γ on the prognosis and early diagnosis of bacteremic systemic inflammatory response syndrome: A prospective observational study. Crit. Care 2013, 17, R64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Obregon, S.; Azkargorta, M.; Seijas, I.; Pilar-Orive, J.; Borrego, F.; Elortza, F.; Boyano, M.D.; Astigarraga, I. Identification of a panel of serum protein markers in early stage of sepsis and its validation in a cohort of patients. J. Microbiol. Immunol. Infect. 2018, 51, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Wagatsuma, T.; Toyama, H.; Ejima, Y.; Hoshi, K.; Shibusawa, M.; Kato, M.; Kurosawa, S. Sepsis is Characterized by the Increases in Percentages of Circulating CD4+CD25+ Regulatory T Cells and Plasma Levels of Soluble CD25. Tohoku J. Exp. Med. 2008, 216, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Lvovschi, V.; Arnaud, L.; Parizot, C.; Freund, Y.; Juillien, G.; Ghillani-Dalbin, P.; Bouberima, M.; Larsen, M.; Riou, B.; Gorochov, G.; et al. Cytokine Profiles in Sepsis Have Limited Relevance for Stratifying Patients in the Emergency Department: A Prospective Observational Study. PLoS ONE 2011, 6, e28870. [Google Scholar] [CrossRef] [PubMed]
- Mearelli, F.; Fiotti, N.; Giansante, C.; Casarsa, C.; Orso, D.; De Helmersen, M.; Altamura, N.; Ruscio, M.; Castello, L.M.; Colonetti, E.; et al. Derivation and Validation of a Biomarker-Based Clinical Algorithm to Rule Out Sepsis From Noninfectious Systemic Inflammatory Response Syndrome at Emergency Department Admission: A Multicenter Prospective Study. Crit. Care Med. 2018, 46, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Kofoed, K.; Andersen, O.; Kronborg, G.; Tvede, M.; Petersen, J.; Eugen-Olsen, J.; Larsen, K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: A prospective study. Crit. Care 2007, 11, R38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.R.; Salisbury, S.; Xiao, Q.; Cvijanovich, N.Z.; Hall, M.; Allen, G.L.; Thomas, N.J.; Freishtat, R.J.; Anas, N.; Meyer, K.; et al. The pediatric sepsis biomarker risk model. Crit. Care 2012, 16, R174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patients (n= 40) | Severe Sepsis (n = 7) | Septic Shock (n = 33) | |
|---|---|---|---|
| Gender (% female) | 18/22 (55%) | 5/2 (71.4%) | 17/16 (51.5%) |
| Age (in years) (median P25–P75) | 3.83 (1.6–8.3) | 1.04 (0.38–8.7) | 4.02 (2.28–8.5) |
| Weight (kg) (median P25–P75) | 16 (11–29) | 8.7 (7–35) | 17 (12–29) |
| PRISM (median P25–P75) | 7 (4–11) | 4 (2.7–4.5) | 8 (4.5–13.5) |
| Comorbidities: | |||
| Prematurity | 3 (7.5%) | 1 (3%) | |
| Heart Disease | 3 (7.5%) | 2 (28.6%) | 3 (9%) |
| Chronic respiratory failure | 4 (10%) | 3 (9%) | |
| Neurological disease | 4 (10%) | 1 (14.3%) | 4 (12%) |
| Origin | |||
| Emergency | 28 (70%) | 24 (72.7%) | |
| Ward | 2 (5%) | 4 (57.1%) | 2 (6.1%) |
| Other hospital | 9 (22.5%) | 6 (18.2%) | |
| Theatre room | 1 (2.5%) | 3 (42.9%) | 1 (3%) |
| Admission | |||
| Medical | 38 (95%) | 7 (100%) | 31(94%) |
| Surgical | 2 (2.5%) | 2 (6%) | |
| Organ dysfunction | |||
| Cardiovascular | 33 (82.5%) | 33 (100%) | |
| Respiratory | 12 (30%) | 10 (30%) | |
| Renal | 8 (20%) | 2 (28.6%) | 8 (24%) |
| Coagulopathy | 7 (17.5%) | 7 (21%) | |
| Neurological | 5 (12.5%) | 5 (15%) | |
| Liver | 3 (7.5%) | 3 (9%) | |
| Thrombocytopenia | 2 (5%) | 2 (6%) | |
| Infection | |||
| Microbiologically proven | 21 (52%) | 4 (57.1%) | 17 (51.5%) |
| Site of infection | |||
| Endovascular | 21 (52.5%) | 2 (28.6%) | 19 (57.6%) |
| Pneumonia | 8 (20%) | 5 (71.4%) | 3 (9%) |
| Intra-abdominal | 3 (7.5%) | 3 (9%) | |
| Others | 8 (20%) | 8 (18.3%) | |
| Treatment at PICU | |||
| Vasoactive n (%) | 33 (82.5%) | 33 (100%) | |
| NE n, dose µg/k/min | 16 (0.3; 0.05–1) | 16 (0.3; 0.05–1) | |
| EPI n, dose µg/k/min | 14 (0.3; 0.1–1) | 14 (0.3; 0.1–1) | |
| DOP n, dose µg/k/min | 26 (10; 5–20) | 26 (10; 5–20) | |
| MV n (%) | 13 (32.5%) | 1 (14.3%) | 12 (36.4%) |
| NIV n (%) | 1 (2.5%) | 1 (14.3%) | |
| AKI n (%) | 7 (17.5%) | 7 (21%) | |
| Hydrocortisone n (%) | 11 (27.5%) | 1 (14.3%) | 10 (30.3%) |
| Insulin n (%) | 1 (2.5%) | 1 (3%) | |
| Length of support (d) | |||
| Vasoactives (median P25–P75) | 2 (1–3) | 2 (1–3) | |
| MV (median P25–P75) | 3 (2–5) | 12 | 3 (2–5) |
| Length of stay | |||
| PICU | 3 (2–6) | 2 (1–5) | 3 (2–6) |
| Hospital | 7 (6–9.5) | 7 (6–14) | 7 (6–9) |
| Outcome | |||
| Survivors | 39 | 7 | 32 |
| Non-survivors | 1 | 1 |
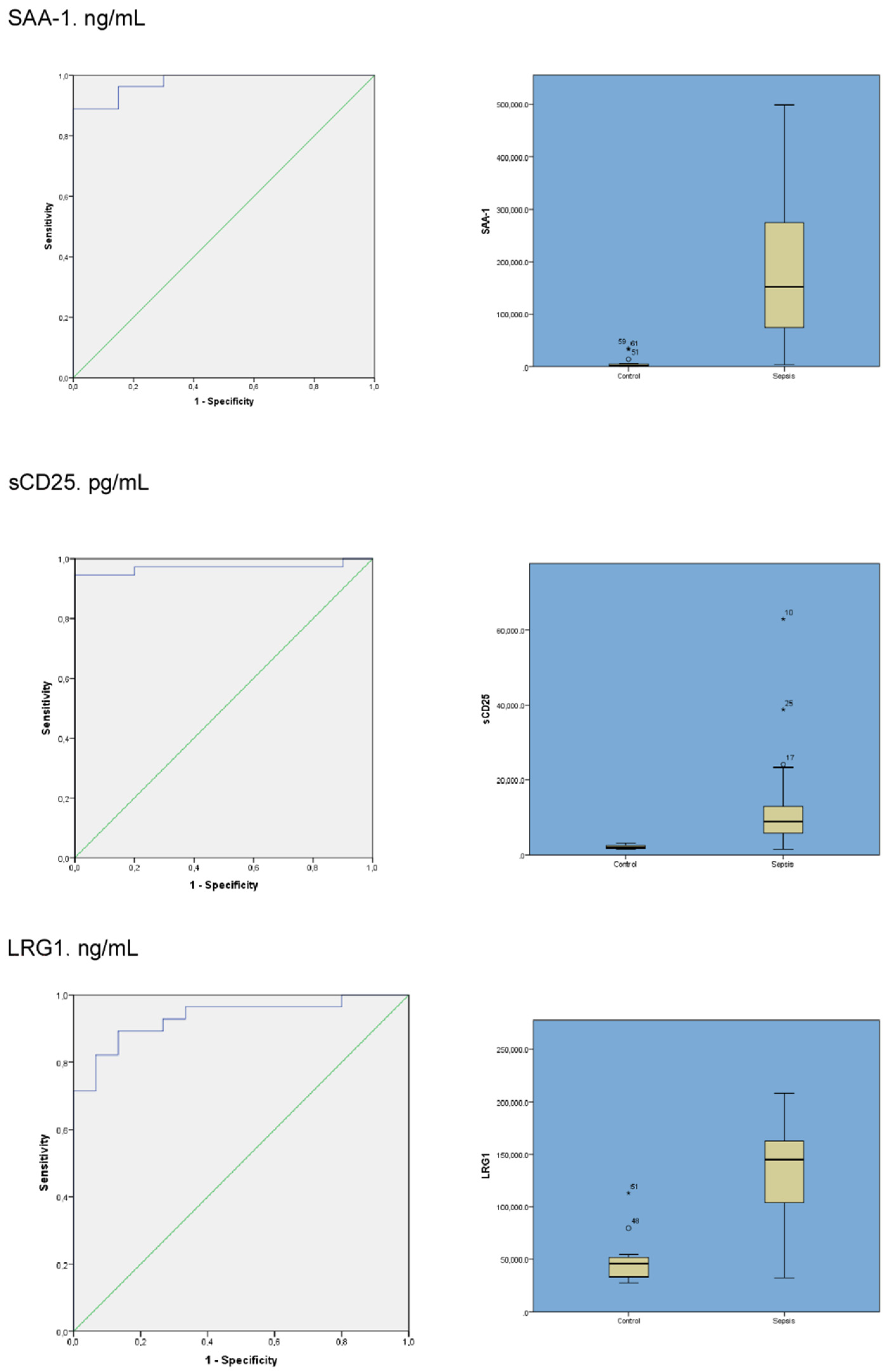
| Protein (Units) | Sepsis (Mean/SD) | Control (Mean/SD) | p-Value | Sensitivity | Specificity | Area Under the Curve (AUROC) (95% CI) (DeLong) |
|---|---|---|---|---|---|---|
| SAA-1 ng/mL | 188,472.7 ± 148,147.6 | 5965.7 ± 10,308.8 | <0.001 | 0.889 | 1 | 0.978 (0.946–1) |
| sCD25 pg/mL | 11,886.1 ± 11,334.7 | 2119.1 ± 500.5 | <0.001 | 0.946 | 1 | 0.97 (0.92–1) |
| LRG1 ng/mL | 131,052.8 ± 46,256.1 | 48,119.2 ± 22,320.2 | <0.001 | 0.867 | 0.89 | 0.933 (0.86–1) |
| LTF ng/mL | 51,940.2 ± 71,933.6 | 9640.8 ± 9840.9 | <0.001 | 0.724 | 0.9 | 0.83 (0.71–0.94) |
| sCD163 pg/mL | 1291.4 ± 6444 | 915.3 ± 377 | 0.079 | 0.595 | 0.8 | 0.68 (0.51–0.85) |
| CFAB ng/mL | 188,472.7 ± 148,147.6 | 316,717.6 ± 431,551.4 | 0.078 | 0.621 | 0.73 | 0.65 (0.49–0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilar-Orive, F.J.; Astigarraga, I.; Azkargorta, M.; Elortza, F.; Garcia-Obregon, S. A Three-Protein Panel to Support the Diagnosis of Sepsis in Children. J. Clin. Med. 2022, 11, 1563. https://doi.org/10.3390/jcm11061563
Pilar-Orive FJ, Astigarraga I, Azkargorta M, Elortza F, Garcia-Obregon S. A Three-Protein Panel to Support the Diagnosis of Sepsis in Children. Journal of Clinical Medicine. 2022; 11(6):1563. https://doi.org/10.3390/jcm11061563
Chicago/Turabian StylePilar-Orive, Francisco J., Itziar Astigarraga, Mikel Azkargorta, Felix Elortza, and Susana Garcia-Obregon. 2022. "A Three-Protein Panel to Support the Diagnosis of Sepsis in Children" Journal of Clinical Medicine 11, no. 6: 1563. https://doi.org/10.3390/jcm11061563
APA StylePilar-Orive, F. J., Astigarraga, I., Azkargorta, M., Elortza, F., & Garcia-Obregon, S. (2022). A Three-Protein Panel to Support the Diagnosis of Sepsis in Children. Journal of Clinical Medicine, 11(6), 1563. https://doi.org/10.3390/jcm11061563






