Accelerated HF-rTMS Modifies SERT Availability in the Subgenual Anterior Cingulate Cortex: A Canine [11C]DASB Study on the Serotonergic System
Abstract
1. Introduction
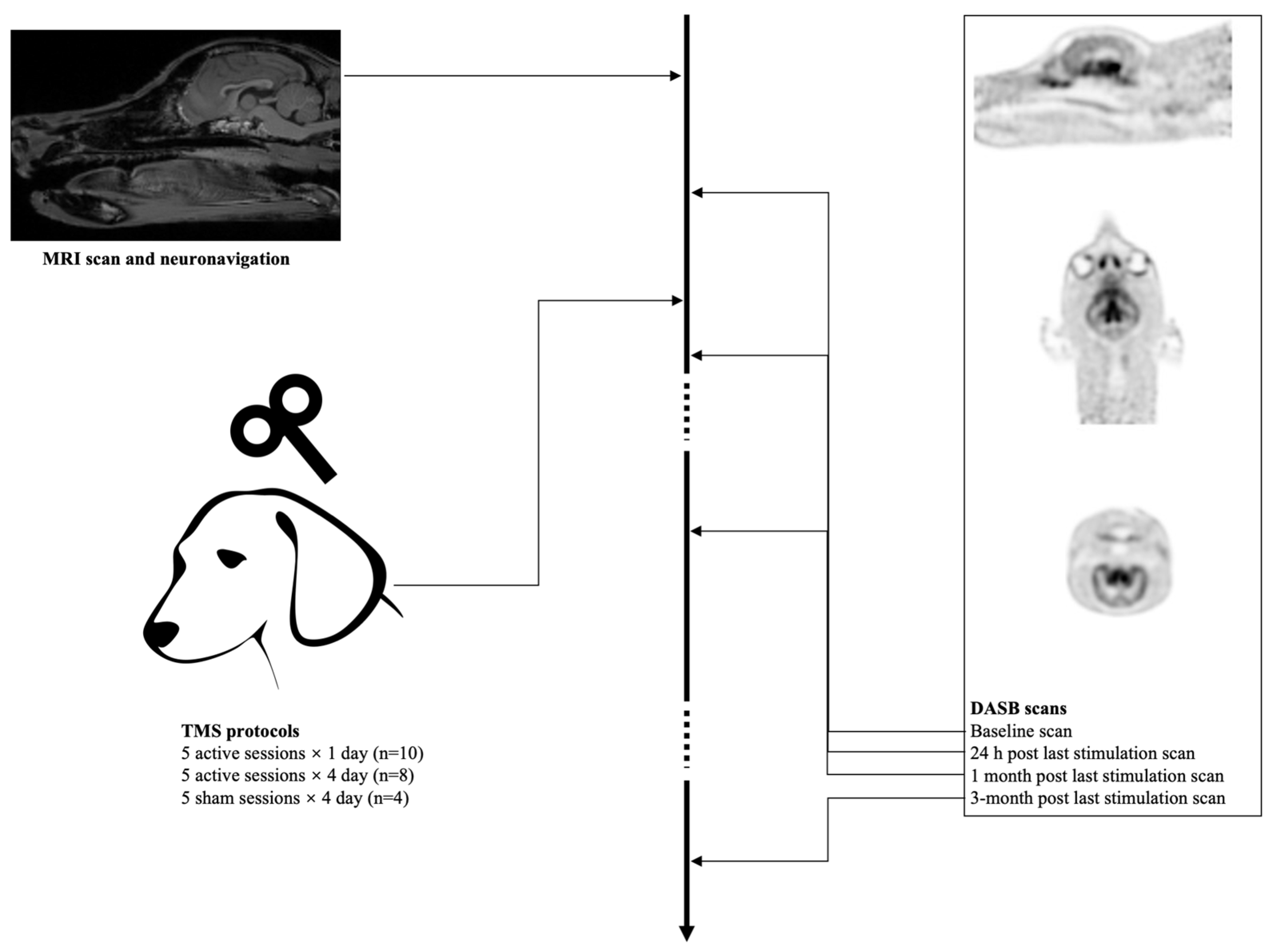
2. Materials and Methods
2.1. Animals
2.2. Neuronavigation
2.3. aHF-rTMS
2.4. Radiosynthesis
2.5. Imaging Procedure
2.6. PET Analysis
2.7. Statistical Analysis
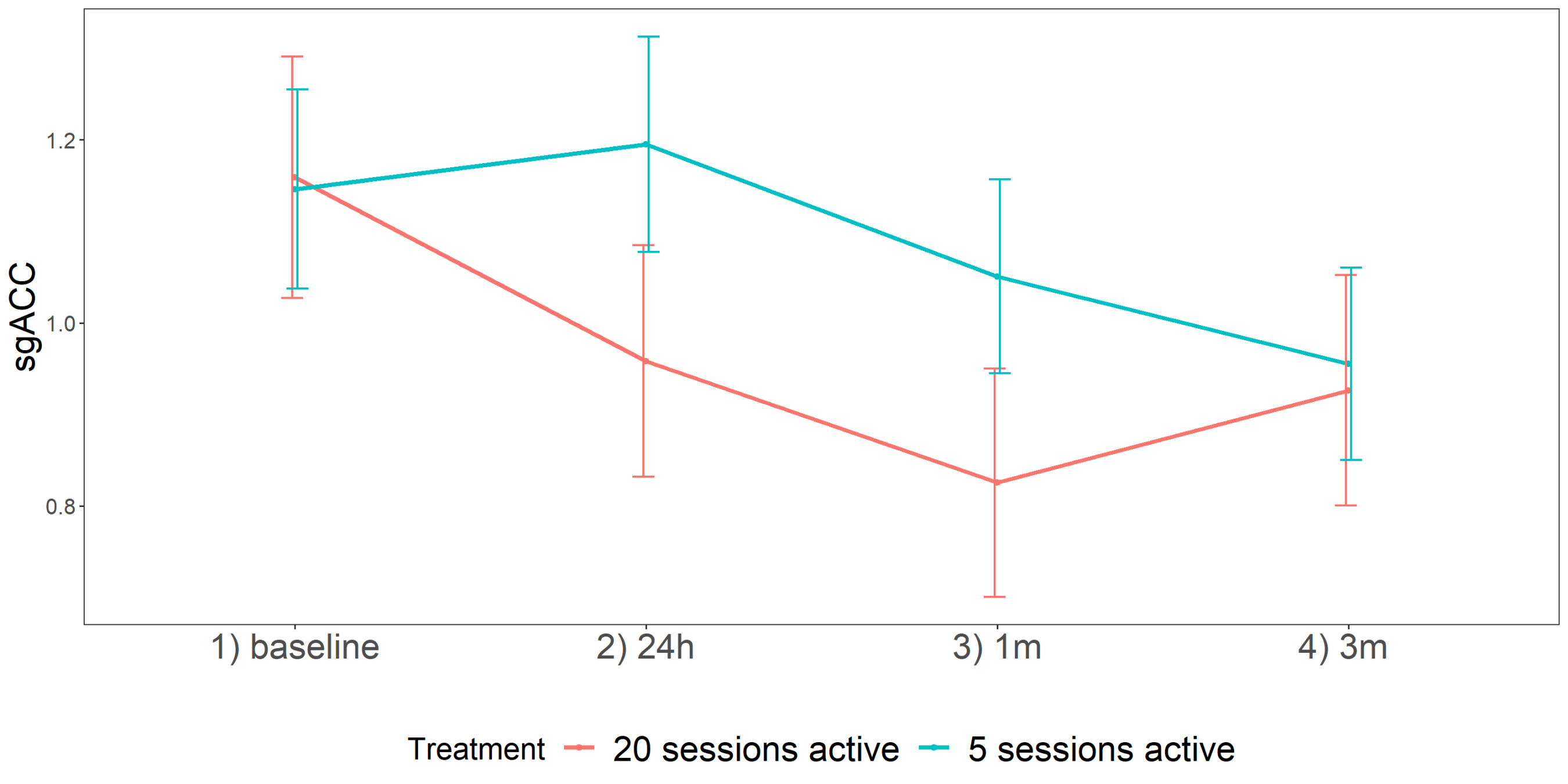
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tardieu, S.; Bottero, A.; Blin, P.; Bohbot, M.; Goni, S.; Gerard, A.; Gasquet, I. Roles and practices of general practitioners and psychiatrists in management of depression in the community. BMC Fam. Pract. 2006, 7, 5. [Google Scholar] [CrossRef][Green Version]
- Horschitz, S.; Hummerich, R.; Schloss, P. Structure, function and regulation of the 5-hydroxytryptamine (serotonin) transporter. Biochem. Soc. Trans. 2001, 29, 728–732. [Google Scholar] [CrossRef]
- Benmansour, S.; Cecchi, M.; Morilak, D.A.; Gerhardt, G.A.; Javors, M.A.; Gould, G.G.; Frazer, A. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J. Neurosci. 1999, 19, 10494–10501. [Google Scholar] [CrossRef]
- Pineyro, G.; Blier, P.; Dennis, T.; de Montigny, C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J. Neurosci. 1994, 14, 3036–3047. [Google Scholar] [CrossRef]
- Descarries, L.; Riad, M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2416–2425. [Google Scholar] [CrossRef]
- Taylor, O.; Van Laeken, N.; Polis, I.; Dockx, R.; Vlerick, L.; Dobbeleir, A.; Goethals, I.; Saunders, J.; Sadones, N.; Baeken, C.; et al. Estimation of the optimal dosing regimen of escitalopram in dogs: A dose occupancy study with [11C] DASB. PLoS ONE 2017, 12, e0179927. [Google Scholar] [CrossRef]
- Cortes, R.; Soriano, E.; Pazos, A.; Probst, A.; Palacios, J. Autoradiography of antidepressant binding sites in the human brain: Localization using [3H] imipramine and [3H] paroxetine. Neuroscience 1988, 27, 473–496. [Google Scholar] [CrossRef]
- Nord, M.; Finnema, S.J.; Halldin, C.; Farde, L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int. J. Neuropsychopharmacol. 2013, 16, 1577–1586. [Google Scholar] [CrossRef]
- Yang, K.-C.; Stepanov, V.; Amini, N.; Martinsson, S.; Takano, A.; Bundgaard, C.; Bang-Andersen, B.; Sanchez, C.; Halldin, C.; Farde, L.; et al. Effect of clinically relevant doses of vortioxetine and citalopram on serotonergic PET markers in the nonhuman primate brain. Neuropsychopharmacology 2019, 44, 1706–1713. [Google Scholar] [CrossRef]
- Voineskos, D.; Daskalakis, Z.J.; Blumberger, D.M. Management of treatment-resistant depression: Challenges and strategies. Neuropsychiatr. Dis. Treat. 2020, 16, 221–234. [Google Scholar] [CrossRef]
- Bartova, L.; Dold, M.; Kautzky, A.; Fabbri, C.; Spies, M.; Serretti, A.; Souery, D.; Mendlewicz, J.; Zohar, J.; Montgomery, S.; et al. Results of the European Group for the Study of Resistant Depression (GSRD)—basis for further research and clinical practice. World J. Biol. Psychiatry 2019, 20, 427–448. [Google Scholar] [CrossRef]
- Bystritsky, A. Treatment-resistant anxiety disorders. Mol. Psychiatry 2006, 11, 805–814. [Google Scholar] [CrossRef]
- Roy-Byrne, P. Treatment-refractory anxiety; definition, risk factors, and treatment challenges. Dialogues Clin. Neurosci. 2015, 17, 191–206. [Google Scholar]
- van der Linden, G.J.; Stein, D.J.; van Balkom, A.J. The efficacy of the selective serotonin reuptake inhibitors for social anxiety disorder (social phobia): A meta-analysis of randomized controlled trials. Int. Clin. Psychopharmacol. 2000, 15 (Suppl. S2), S15–S23. [Google Scholar] [CrossRef]
- Shen, H.w.; Numachi, Y.; Yoshida, S.; Fujiyama, K.; Toda, S.; Awata, S.; Matsuoka, H.; Sato, M. Electroconvulsive shock increases serotonin transporter in the rat frontal cortex. Neurosci. Lett. 2003, 341, 170–172. [Google Scholar] [CrossRef]
- Hayakawa, H.; Okamoto, Y.; Shimizu, M.; Nishida, A.; Motohashi, N.; Yamawaki, S. Single or repeated treatment with electroconvulsive shock increases number of serotonin uptake binding sites in the frontal cortex. Neuropsychobiology 1995, 31, 1–5. [Google Scholar] [CrossRef]
- Shen, H.; Numachi, Y.; Yoshida, S.; Toda, S.; Awata, S.; Matsuoka, H.; Mitsumoto, S. Electroconvulsive shock regulates serotonin transporter mRNA expression in rat raphe nucleus. Psychiatry Clin. Neurosci. 2001, 55, 75–77. [Google Scholar] [CrossRef]
- Cheetham, S.; Viggers, J.; Slater, N.; Heal, D.; Buckett, W. [3H] paroxetine binding in rat frontal cortex strongly correlates with [3H] 5-HT uptake: Effect of administration of various antidepressant treatments. Neuropharmacology 1993, 32, 737–743. [Google Scholar] [CrossRef]
- Gleiter, C.H.; Nutt, D.J. Repeated electroconvulsive shock does not change [3H]-paroxetine binding to the 5-HT uptake site in rat cortical membranes. Psychopharmacology 1988, 95, 68–70. [Google Scholar] [CrossRef]
- Ikeda, T.; Kurosawa, M.; Uchikawa, C.; Kitayama, S.; Nukina, N. Modulation of monoamine transporter expression and function by repetitive transcranial magnetic stimulation. Biochem. Biophys. Res. Commun. 2005, 327, 218–224. [Google Scholar] [CrossRef]
- Cambiaghi, M.; Buffelli, M.; Masin, L.; Valtorta, F.; Comai, S. Transcranial direct current stimulation of the mouse prefrontal cortex modulates serotonergic neural activity of the dorsal raphe nucleus. Brain Stimul. 2020, 13, 548–550. [Google Scholar] [CrossRef]
- Overall, K.L. Natural animal models of human psychiatric conditions: Assessment of mechanism and validity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2000, 24, 727–776. [Google Scholar] [CrossRef]
- De Cristofaro, M.T.R.; Sessarego, A.; Pupi, A.; Biondi, F.; Faravelli, C. Brain perfusion abnormalities in drug-naive, lactate-sensitive panic patients: A SPECT study. Biol. Psychiatry 1993, 33, 505–512. [Google Scholar] [CrossRef]
- Audenaert, K.; Van Laere, K.; Dumont, F.; Slegers, G.; Mertens, J.; van Heeringen, C.; Dierckx, R.A. Decreased frontal serotonin 5-HT 2a receptor binding index in deliberate self-harm patients. Eur. J. Nucl. Med. 2001, 28, 175–182. [Google Scholar] [CrossRef]
- Eren, İ.; Tükel, R.; Polat, A.; Karaman, R.; Ünal, S. Evaluation of regional cerebral blood flow changes in panic disorder with Tc99m-HMPAO SPECT. Psychiatry Res. Neuroimaging 2003, 123, 135–143. [Google Scholar] [CrossRef]
- Zitterl, W.; Aigner, M.; Stompe, T.; Zitterl-Eglseer, K.; Gutierrez-Lobos, K.; Wenzel, T.; Zettinig, G.; Hornik, K.; Pirker, W.; Thau, K. Changes in thalamus–hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive–compulsive disorder. Neuropsychopharmacology 2008, 33, 3126–3134. [Google Scholar] [CrossRef]
- Vermeire, S.; Audenaert, K.; Dobbeleir, A.; De Meester, R.; Vandermeulen, E.; Waelbers, T.; Peremans, K. Regional cerebral blood flow changes in dogs with anxiety disorders, measured with SPECT. Brain Imaging Behav. 2009, 3, 342–349. [Google Scholar] [CrossRef]
- Vermeire, S.T.; Audenaert, K.R.; Dobbeleir, A.A.; De Meester, R.H.; De Vos, F.J.; Peremans, K.Y. Evaluation of the brain 5-HT2A receptor binding index in dogs with anxiety disorders, measured with 123I-5I-R91150 and SPECT. J. Nucl. Med. 2009, 50, 284–289. [Google Scholar] [CrossRef]
- Irimajiri, M.; Miller, M.A.; Green, M.A.; Jaeger, C.B.; Luescher, A.U.; Hutchins, G.D. Cerebral metabolism in dogs assessed by 18F-FDG PET: A pilot study to understand physiological changes in behavioral disorders in dogs. J. Vet. Med. Sci. 2010, 72, 1–6. [Google Scholar] [CrossRef][Green Version]
- Wilson, A.A.; Ginovart, N.; Hussey, D.; Meyer, J.; Houle, S. In vitro and in vivo characterisation of [11C]-DASB: A probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl. Med. Biol. 2002, 29, 509–515. [Google Scholar] [CrossRef]
- Jensen, S.B.; Smith, D.F.; Bender, D.; Jakobsen, S.; Peters, D.; Nielsen, E.Ø.; Olsen, G.M.; Scheel-Krüger, J.; Wilson, A.; Cumming, P. [11C]-NS 4194 versus [11C]-DASB for PET imaging of serotonin transporters in living porcine brain. Synapse 2003, 49, 170–177. [Google Scholar] [CrossRef]
- Ginovart, N.; Wilson, A.A.; Meyer, J.H.; Hussey, D.; Houle, S. [11C]-DASB, a tool for in vivo measurement of SSRI-induced occupancy of the serotonin transporter: PET characterization and evaluation in cats. Synapse 2003, 47, 123–133. [Google Scholar] [CrossRef]
- Yokoyama, C.; Yamanaka, H.; Onoe, K.; Kawasaki, A.; Nagata, H.; Shirakami, K.; Doi, H.; Onoe, H. Mapping of serotonin transporters by positron emission tomography with [11C] DASB in conscious common marmosets: Comparison with rhesus monkeys. Synapse 2010, 64, 594–601. [Google Scholar] [CrossRef]
- Taylor, O.; Van Laeken, N.; De Vos, F.; Polis, I.; Bosmans, T.; Goethals, I.; Achten, R.; Dobbeleir, A.; Vandermeulen, E.; Baeken, C.; et al. In vivo quantification of the [11C] DASB binding in the normal canine brain using positron emission tomography. BMC Vet. Res. 2015, 11, 308. [Google Scholar] [CrossRef]
- Dockx, R.; Baeken, C.; Duprat, R.; De Vos, F.; Saunders, J.; Polis, I.; Audenaert, K.; Peremans, K. Changes in canine cerebral perfusion after accelerated high frequency repetitive transcranial magnetic stimulation (HF-rTMS): A proof of concept study. Vet. J. 2018, 234, 66–71. [Google Scholar] [CrossRef]
- Baeken, C. Accelerated rTMS: A potential treatment to alleviate refractory depression. Front. Psychol. 2018, 9, 2017. [Google Scholar] [CrossRef]
- Baeken, C.; Vanderhasselt, M.-A.; Remue, J.; Herremans, S.; Vanderbruggen, N.; Zeeuws, D.; Santermans, L.; De Raedt, R. Intensive HF-rTMS treatment in refractory medication-resistant unipolar depressed patients. J. Affect. Disord. 2013, 151, 625–631. [Google Scholar] [CrossRef]
- Duprat, R.; Desmyter, S.; van Heeringen, K.; Van den Abbeele, D.; Tandt, H.; Bakic, J.; Pourtois, G.; Dedoncker, J.; Vervaet, M.; Van Autreve, S.; et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission? J. Affect. Disord. 2016, 200, 6–14. [Google Scholar] [CrossRef]
- Cambiaghi, M.; Crupi, R.; Bautista, E.L.; Elsamadisi, A.; Malik, W.; Pozdniakova, H.; Han, Z.; Buffelli, M.; Battaglia, F. The Effects of 1-Hz rTMS on Emotional Behavior and Dendritic Complexity of Mature and Newly Generated Dentate Gyrus Neurons in Male Mice. Int. J. Environ. Res. Public Health 2020, 17, 4074. [Google Scholar] [CrossRef]
- Cambiaghi, M.; Cherchi, L.; Masin, L.; Infortuna, C.; Briski, N.; Caviasco, C.; Hazaveh, S.; Han, Z.; Buffelli, M.; Battaglia, F. High-frequency repetitive transcranial magnetic stimulation enhances layer II/III morphological dendritic plasticity in mouse primary motor cortex. Behav. Brain Res. 2021, 410, 113352. [Google Scholar] [CrossRef]
- Baeken, C.; De Raedt, R.; Bossuyt, A.; Van Hove, C.; Mertens, J.; Dobbeleir, A.; Blanckaert, P.; Goethals, I. The impact of HF-rTMS treatment on serotonin2A receptors in unipolar melancholic depression. Brain Stimul. 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Dockx, R.; Peremans, K.; Vlerick, L.; Van Laeken, N.; Saunders, J.H.; Polis, I.; De Vos, F.; Baeken, C. Anaesthesia, not number of sessions, influences the magnitude and duration of an aHF-rTMS in dogs. PLoS ONE 2017, 12, e0185362. [Google Scholar] [CrossRef]
- Dockx, R.; Peremans, K.; Duprat, R.; Vlerick, L.; Van Laeken, N.; Saunders, J.H.; Polis, I.; De Vos, F.; Baeken, C. Accurate external localization of the left frontal cortex in dogs by using pointer based frameless neuronavigation. PeerJ 2017, 5, e3425. [Google Scholar] [CrossRef][Green Version]
- Baeken, C.; Marinazzo, D.; Everaert, H.; Wu, G.R.; Van Hove, C.; Audenaert, K.; Goethals, I.; De Vos, F.; Peremans, K.; De Raedt, R. The impact of accelerated HF-rTMS on the subgenual anterior cingulate cortex in refractory unipolar major depression: Insights from 18FDG PET brain imaging. Brain Stimul. 2015, 8, 808–815. [Google Scholar] [CrossRef]
- Dua-Sharma, S.; Sharma, K.; Jacobs, H. The Canine Brain in Stereotaxic Coordinates; MIT Press: Cambridge, MA, USA, 1970. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Firth, D.; Friendly, M.; Gorjanc, G.; Graves, S.; et al. Package ‘Car’; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, AKA Least-Squares Means; R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Gershon, A.A.; Dannon, P.N.; Grunhaus, L. Transcranial magnetic stimulation in the treatment of depression. Am. J. Psychiatry 2003, 160, 835–845. [Google Scholar] [CrossRef]
- Mayberg, H.S. Targeted electrode-based modulation of neural circuits for depression. J. Clin. Investig. 2009, 119, 717–725. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J.; Trimble, M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef]
- Liston, C.; Chen, A.C.; Zebley, B.D.; Drysdale, A.T.; Gordon, R.; Leuchter, B.; Voss, H.U.; Casey, B.J.; Etkin, A.; Dubin, M.J. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry 2014, 76, 517–526. [Google Scholar] [CrossRef]
- Lundquist, P.; Roman, M.; Syvänen, S.; Hartvig, P.; Blomquist, G.; Hammarlund-Udenaes, M.; Långström, B. Effect on [11C] DASB binding after tranylcypromine-induced increase in serotonin concentration: Positron emission tomography studies in monkeys and rats. Synapse 2007, 61, 440–449. [Google Scholar] [CrossRef]
- Baldinger, P.; Kranz, G.S.; Haeusler, D.; Savli, M.; Spies, M.; Philippe, C.; Hahn, A.; Höflich, A.; Wadsak, W.; Mitterhauser, M.; et al. Regional differences in SERT occupancy after acute and prolonged SSRI intake investigated by brain PET. Neuroimage 2014, 88, 252–262. [Google Scholar] [CrossRef]
- Lanzenberger, R.; Kranz, G.S.; Haeusler, D.; Akimova, E.; Savli, M.; Hahn, A.; Mitterhauser, M.; Spindelegger, C.; Philippe, C.; Fink, M.; et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage 2012, 63, 874–881. [Google Scholar] [CrossRef]
- Dubin, M. Imaging TMS: Antidepressant mechanisms and treatment optimization. Int. Rev. Psychiatry 2017, 29, 89–97. [Google Scholar] [CrossRef]
- Sibon, I.; Strafella, A.P.; Gravel, P.; Ko, J.H.; Booij, L.; Soucy, J.-P.; Leyton, M.; Diksic, M.; Benkelfat, C. Acute prefrontal cortex TMS in healthy volunteers: Effects on brain 11C-αMtrp trapping. Neuroimage 2007, 34, 1658–1664. [Google Scholar] [CrossRef]
- Kuo, H.-I.; Paulus, W.; Batsikadze, G.; Jamil, A.; Kuo, M.-F.; Nitsche, M.A. Chronic enhancement of serotonin facilitates excitatory transcranial direct current stimulation-induced neuroplasticity. Neuropsychopharmacology 2016, 41, 1223–1230. [Google Scholar] [CrossRef]
- Melo, L.; Mosayebi-Samani, M.; Ghanavati, E.; Nitsche, M.A.; Kuo, M.F. Dosage-Dependent Impact of Acute Serotonin Enhancement on Transcranial Direct Current Stimulation Effects. Int. J. Neuropsychopharmacol. 2021, 24, 787–797. [Google Scholar] [CrossRef]
- Dockx, R.; Baeken, C.; De Bundel, D.; Saunders, J.; Van Eeckhaut, A.; Peremans, K. Accelerated high-frequency repetitive transcranial magnetic stimulation positively influences the behavior, monoaminergic system, and cerebral perfusion in anxious aggressive dogs: A case study. J. Vet. Behav. 2019, 33, 108–113. [Google Scholar] [CrossRef]
- Richieri, R.; Guedj, E.; Michel, P.; Loundou, A.; Auquier, P.; Lançon, C.; Boyer, L. Maintenance transcranial magnetic stimulation reduces depression relapse: A propensity-adjusted analysis. J. Affect. Disord. 2013, 151, 129–135. [Google Scholar] [CrossRef]
- Baeken, C.; Brem, A.-K.; Arns, M.; Brunoni, A.R.; Filipčić, I.; Ganho-Ávila, A.; Langguth, B.; Padberg, F.; Poulet, E.; Rachid, F.; et al. Repetitive transcranial magnetic stimulation treatment for depressive disorders: Current knowledge and future directions. Curr. Opin. Psychiatry 2019, 32, 409–415. [Google Scholar] [CrossRef]
- Lisanby, S.H.; Gutman, D.; Luber, B.; Schroeder, C.; Sackeim, H.A. Sham TMS: Intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol. Psychiatry 2001, 49, 460–463. [Google Scholar] [CrossRef]

| Pons | Left Thalamus | Presubgenual Cortex | Subgenual Cortex | |
|---|---|---|---|---|
| Intercept | 0.000 | 0.000 | 0.000 | 0.000 |
| 24 h | 0.297 | 0.258 | 0.639 | 0.580 |
| 1 month | 0.372 | 0.631 | 0.315 | 0.124 |
| 3 months | 0.058 | 0.209 | 0.064 | 0.765 |
| 20 sessions active | 0.826 | 0.907 | 0.778 | 0.144 |
| 5 sessions active | 0.067 | 0.462 | 0.532 | 0.033 * |
| 24 h: 20 sessions active | 0.044 * | 0.930 | 0.352 | 0.175 |
| 1 month: 20 sessions active | 0.603 | 0.036 * | 0.015 * | 0.002 ** |
| 3 months: 20 sessions active | 0.294 | 0.810 | 0.306 | 0.367 |
| 24 h: 5 sessions active | 0.488 | 0.301 | 0.817 | 0.879 |
| 1 month: 5 sessions active | 0.820 | 0.809 | 0.412 | 0.156 |
| 3 months: 5 sessions active | 0.570 | 0.495 | 0.362 | 0.316 |
| 20 Sessions Sham | 5 Sessions Active | 20 Sessions Active | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | LCL | UCL | p-Value | Estimate | SE | LCL | UCL | p-Value | Estimate | SE | LCL | UCL | p-Value | |
| T1-T0 | 0.01 | 0.18 | −0.27 | 0.47 | 0.58 | 0.13 | 0.11 | −0.09 | 0.35 | 0.24 | −0.20 | 0.12 | −0.44 | 0.04 | 0.10 |
| T2-T0 | 0.26 | 0.17 | −0.08 | 0.60 | 0.13 | −0.02 | 0.11 | −0.24 | 0.20 | 0.83 | −0.42 | 0.12 | −0.66 | −0.18 | 0.001 ** |
| T3-T0 | −0.05 | 0.16 | −0.37 | 0.27 | 0.77 | −0.24 | 0.10 | −0.44 | −0.04 | 0.03 | −0.22 | 0.11 | −0.44 | 0.00 | 0.05 * |
| T2-T1 | 0.16 | 0.18 | −0.21 | 0.53 | 0.37 | 0.16 | 0.11 | −0.38 | 0.06 | 0.17 | −0.23 | 0.12 | −0.47 | 0.01 | 0.06 |
| T3-T1 | −0.15 | 0.17 | −0.49 | 0.19 | 0.39 | −0.37 | 0.11 | −0.59 | −0.15 | 0.002 * | −0.03 | 0.11 | −0.25 | 0.19 | 0.80 |
| T3-T2 | −0.31 | 0.16 | −0.63 | 0.01 | 0.06 | −0.22 | 0.10 | −0.42 | −0.02 | 0.04 | 0.20 | 0.12 | −0.04 | 0.44 | 0.09 |
| 5 Sessions Active–20 Sessions Sham | 20 Sessions Active–20 Sessions Sham | 5 Sessions Active–20 Sessions Active | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | LCL | UCL | p-Value | Estimate | SE | LCL | UCL | p-Value | Estimate | SE | LCL | UCL | p-Value | |
| T0 | 0.42 | 0.19 | 0.03 | 0.81 | 0.03 * | 0.29 | 0.19 | −0.1 | 0.68 | 0.15 | 0.13 | 0.15 | −0.17 | 0.43 | 0.41 |
| T1 | 0.45 | 0.2 | 0.04 | 0.86 | 0.03 * | −0.01 | 0.2 | −0.42 | 0.4 | 0.98 | 0.46 | 0.15 | 0.16 | 0.76 | 0.01 * |
| T2 | 0.13 | 0.19 | −0.26 | 0.52 | 0.48 | −0.4 | 0.19 | −0.79 | −0.01 | 0.05 * | 0.53 | 0.15 | 0.23 | 0.83 | 0.00 ** |
| T3 | 0.22 | 0.18 | −0.15 | 0.59 | 0.21 | 0.11 | 0.18 | −0.26 | 0.48 | 0.53 | 0.11 | 0.14 | −0.17 | 0.39 | 0.44 |
| b | SE | z | p | |
|---|---|---|---|---|
| Baseline | 0.0132 | 0.169 | 0.078 | 0.938 |
| 24 h | −0.2367 | 0.171 | −1.381 | 0.167 |
| 1 month | −0.2254 | 0.161 | −1.399 | 0.162 |
| 3 months | −0.0288 | 0.161 | −0.178 | 0.859 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Kappen, M.; Peremans, K.; De Bundel, D.; Van Eeckhaut, A.; Van Laeken, N.; De Vos, F.; Dobbeleir, A.; Saunders, J.H.; Baeken, C. Accelerated HF-rTMS Modifies SERT Availability in the Subgenual Anterior Cingulate Cortex: A Canine [11C]DASB Study on the Serotonergic System. J. Clin. Med. 2022, 11, 1531. https://doi.org/10.3390/jcm11061531
Xu Y, Kappen M, Peremans K, De Bundel D, Van Eeckhaut A, Van Laeken N, De Vos F, Dobbeleir A, Saunders JH, Baeken C. Accelerated HF-rTMS Modifies SERT Availability in the Subgenual Anterior Cingulate Cortex: A Canine [11C]DASB Study on the Serotonergic System. Journal of Clinical Medicine. 2022; 11(6):1531. https://doi.org/10.3390/jcm11061531
Chicago/Turabian StyleXu, Yangfeng, Mitchel Kappen, Kathelijne Peremans, Dimitri De Bundel, Ann Van Eeckhaut, Nick Van Laeken, Filip De Vos, Andre Dobbeleir, Jimmy H. Saunders, and Chris Baeken. 2022. "Accelerated HF-rTMS Modifies SERT Availability in the Subgenual Anterior Cingulate Cortex: A Canine [11C]DASB Study on the Serotonergic System" Journal of Clinical Medicine 11, no. 6: 1531. https://doi.org/10.3390/jcm11061531
APA StyleXu, Y., Kappen, M., Peremans, K., De Bundel, D., Van Eeckhaut, A., Van Laeken, N., De Vos, F., Dobbeleir, A., Saunders, J. H., & Baeken, C. (2022). Accelerated HF-rTMS Modifies SERT Availability in the Subgenual Anterior Cingulate Cortex: A Canine [11C]DASB Study on the Serotonergic System. Journal of Clinical Medicine, 11(6), 1531. https://doi.org/10.3390/jcm11061531







