Unreliability of Clinical Prediction Rules to Exclude without Echocardiography Infective Endocarditis in Staphylococcus aureus Bacteremia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Predefined Ruling Out IE Thresholds
2.3. Primary and Secondary Objectives
2.4. Statistical Analysis
3. Results
3.1. PREDICT Score (Day 5 Model) Evaluation
3.2. POSITIVE Score Evaluation
3.3. VIRSTA Score Evaluation
3.4. Echocardiographic Assessment and 30-Day Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuehl, R.; Morata, L.; Boeing, C.; Subirana, I.; Seifert, H.; Rieg, S.; Kern, W.V.; Kim, H.B.; Kim, E.S.; Liao, C.H.; et al. Defining persistent Staphylococcus aureus bacteraemia: Secondary analysis of a prospective cohort study. Lancet Infect. Dis. 2020, 20, 1409–1417. [Google Scholar] [CrossRef]
- Le Moing, V.; Alla, F.; Doco-Lecompte, T.; Delahaye, F.; Piroth, L.; Chirouze, C.; Tattevin, P.; Lavigne, J.-P.; Erpelding, M.-L.; Hoen, B.; et al. Staphylococcus aureus Bloodstream Infection and Endocarditis—A Prospective Cohort Study. PLoS ONE 2015, 10, e0127385. [Google Scholar]
- Hidalgo-Tenorio, C.; Gálvez, J.; Martínez-Marcos, F.J.; Plata-Ciezar, A.; De La Torre-Lima, J.; López-Cortés, L.E.; Noureddine, M.; Reguera, J.M.; Vinuesa, D.; García, M.V.; et al. Clinical and prognostic differences between methicillin-resistant and methicillin-susceptible Staphylococcus aureus infective endocarditis. BMC Infect. Dis. 2020, 20, 160. [Google Scholar] [CrossRef]
- Hsu, R.-B. Risk Factors for Nosocomial Infective Endocarditis in Patients with Methicillin-Resistant Staphylococcus Aureus Bacteremia. Infect. Control Hosp. Epidemiol. 2005, 26, 654–657. Available online: https://www.cambridge.org/core/product/identifier/S0899823X00198605/type/journal_article (accessed on 13 October 2021). [CrossRef]
- Mensa, J.; Soriano, A.; Llinares, P.; Barberán, J.; Montejo, M.; Salavert, M.; Alvarez-Rocha, L.; Maseda, E.; Moreno, A.; Pasquau, J.; et al. Guía de tratamiento antimicrobiano de la infección por Staphylococcus aureus. Rev. Esp. Quim. Publ. Soc. Esp. Quim. 2013, 26 (Suppl. 1), 1–84. [Google Scholar]
- Gudiol, F.; Aguado, J.M.; Almirante, B.; Bouza, E.; Cercenado, E.; Domínguez, M.Á.; Gasch, O.; Lora-Tamayo, J.; Miró, J.M.; Palomar, M.; et al. Diagnosis and treatment of bacteremia and endocarditis due to Staphylococcus aureus. A clinical guideline from the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm. Infecc. Microbiol. Clin. 2015, 33, 625.e1–625.e23. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. Available online: https://academic.oup.com/cid/article/52/3/e18/306145 (accessed on 13 October 2021). [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. Available online: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehv319 (accessed on 20 September 2021).
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler Jr, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000296 (accessed on 20 September 2021). [CrossRef]
- Schoenfeld, M.; Machhar, R.; Maw, A. Diagnosing Endocarditis in Patients With Staphylococcus aureus Bacteremia. JAMA 2015, 313, 420. Available online: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2014.16951 (accessed on 13 October 2021). [CrossRef]
- Ramos-Martínez, A.; Calderón-Parra, J.; Miró, J.M.; Muñoz, P.; Rodríguez-Abella, H.; Valerio, M.; de Alarcón, A.; Luque, R.; Ambrosioni, J.; Fariñas, M.C.; et al. Effect of the type of surgical indication on mortality in patients with infective endocarditis who are rejected for surgical intervention. Int. J. Cardiol. 2019, 282, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Heriot, G.S.; Tong, S.Y.C.; Cheng, A.C.; Liew, D. What risk of endocarditis is low enough to justify the omission of transoesophageal echocardiography in Staphylococcus aureus bacteraemia? A narrative review. Clin. Microbiol. Infect. 2018, 24, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Hilberath, J.N.; Oakes, D.A.; Shernan, S.K.; Bulwer, B.E.; D’Ambra, M.N.; Eltzschig, H.K. Safety of transesophageal echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Spencer, K.T.; Furlong, K.T.; DeCara, J.M.; Sugeng, L.; Ward, R.P.; Lang, R.M. Clinical features of complications from transesophageal echocardiography: A single-center case series of 10,000 consecutive examinations. J. Am. Soc. Echocardiogr. 2005, 18, 925–929. [Google Scholar] [CrossRef]
- Liu, E.; Guha, A.; Dunleavy, M.; Obarski, T. Safety of Transesophageal Echocardiography in Patients with Esophageal Varices. J. Am. Soc. Echocardiogr. 2019, 32, 676–677. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Kalluru, D.; Lunagariya, A.; Sanchez, L.; Yusuf, S.W.; Hassan, S.; Palaskas, N.; Mouhayar, E.; Banchs, J. Safety of Transesophageal Echocardiography in Patients with Thrombocytopenia. J. Am. Soc. Echocardiogr. 2019, 32, 1010–1015. [Google Scholar] [CrossRef]
- Heriot, G.S.; Tong, S.Y.C.; Cheng, A.C.; Liew, D. Benefit of Echocardiography in Patients With Staphylococcus aureus Bacteremia at Low Risk of Endocarditis. Open Forum. Infect. Dis. 2018, 5, ofy303. Available online: https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofy303/5238173 (accessed on 13 October 2021). [CrossRef]
- Palraj, B.R.; Baddour, L.M.; Hess, E.P.; Steckelberg, J.M.; Wilson, W.R.; Lahr, B.D.; Sohail, M.R. Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT): Scoring System to Guide Use of Echocardiography in the Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2015, 61, 18–28. Available online: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/civ235 (accessed on 13 October 2021). [CrossRef]
- Tubiana, S.; Duval, X.; Alla, F.; Selton-Suty, C.; Tattevin, P.; Delahaye, F.; Piroth, L.; Chirouze, C.; Lavigne, J.P.; Erpelding, M.L.; et al. The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J. Infect. 2016, 72, 544–553. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0163445316000517 (accessed on 13 October 2021). [CrossRef]
- Kahn, F.; Resman, F.; Bergmark, S.; Filiptsev, P.; Nilson, B.; Gilje, P.; Rasmussen, M. Time to blood culture positivity in Staphylococcus aureus bacteraemia to determine risk of infective endocarditis. Clin. Microbiol. Infect. 2021, 27, e7–e1345. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1198743X2030700X (accessed on 13 October 2021). [CrossRef]
- Bai, A.D.; Agarwal, A.; Steinberg, M.; Showler, A.; Burry, L.; Tomlinson, G.A.; Bell, C.M.; Morris, A.M. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2017, 23, 900–906. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1198743X17302367 (accessed on 13 October 2021). [CrossRef] [PubMed]
- Peinado-Acevedo, J.S.; Hurtado-Guerra, J.J.; Hincapié-Osorno, C.; Mesa-Abad, J.; Uribe-Delgado, J.R.; Giraldo-Ramírez, S.; Lengerke-Diaz, P.A.; Jaimes, F. Validation of VIRSTA and PREDICT scores to determine the priority of echocardiography in patients with Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2021, 73, e1151–e1157. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, T.W.; Prins, J.M.; Soetekouw, R.; van Twillert, G.; Veenstra, J.; Herpers, B.L.; Rozemeijer, W.; Jansen, R.R.; Bonten, M.J.; van der Meer, J. Prediction rules for ruling out endocarditis in patients with Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2021. online ahead of print ciab632. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, K.; Bakker, L.J.; Keijer, J.T.; de Vries, P.J. Implementing a hospital-wide protocol for Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol Infect. Dis. 2018, 37, 1553–1562. Available online: http://link.springer.com/10.1007/s10096-018-3284-9 (accessed on 13 October 2021). [CrossRef] [PubMed]
- Holden, E.; Bashir, A.; Das, I.; Morton, H.; Steadman, C.D.; Nightingale, P.; Steeds, R.P.; David, M.D. Staphylococcus aureus bacteraemia in a UK tertiary referral centre: A “transoesophageal echocardiogram for all” policy. J. Antimicrob. Chemother. 2014, 69, 1960–1965. Available online: https://academic.oup.com/jac/article-lookup/doi/10.1093/jac/dku082 (accessed on 13 October 2021). [CrossRef]
- Heriot, G.S.; Cheng, A.C.; Tong, S.Y.C.; Liew, D. Clinical predictors and prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: Attention must be paid to the reference standard. Clin. Microbiol. Infect. 2018, 24, 314–316. Available online: https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(17)30536-0/fulltext#relatedArticles (accessed on 20 February 2022). [CrossRef]
- Joseph, J.P.; Meddows, T.R.; Webster, D.P.; Newton, J.D.; Myerson, S.G.; Prendergast, B.; Scarborough, M.; Herring, N. Prioritizing echocardiography in Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 2013, 68, 444–449. [Google Scholar] [CrossRef]
- Mun, S.J.; Kim, S.H.; Huh, K.; Cho, S.Y.; Kang, C.I.; Chung, D.R.; Peck, K.R. Role of echocardiography in uncomplicated Staphylococcus aureus catheter-related bloodstream infections. Medicine 2021, 100, e25679. [Google Scholar] [CrossRef]
- Heriot, G.S.; Tong, S.Y.C.; Cheng, A.C.; Liew, D. A Scenario-Based Survey of Expert Echocardiography Recommendations for Patients With Staphylococcus aureus Bacteremia at Varying Risk for Endocarditis. JAMA Netw. Open 2020, 3, e202401. [Google Scholar] [CrossRef]
- Parra, J.C.; De Castro-Campos, D.; García, P.M.; Samperio, M.O.; Arriaza, M.M.; De Alarcón, A.; Gutierrez-Carretero, E.; Alvarez, M.C.; Meda, J.M.; Sanchez, M.Á.; et al. Non-HACEK gram negative bacilli endocarditis: Analysis of a national prospective cohort. Eur. J. Intern. Med. 2021, 92, 71–78. Available online: https://www.ejinme.com/article/S0953-6205(21)00144-8/fulltext#relatedArticles (accessed on 11 September 2021). [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.D.; Showler, A.; Burry, L.; Steinberg, M.; Tomlinson, G.A.; Bell, C.M.; Morris, A.M. Clinical prediction rules in Staphylococcus aureus bacteremia demonstrate the usefulness of reporting likelihood ratios in infectious diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1393–1398. Available online: http://link.springer.com/10.1007/s10096-016-2711-z (accessed on 13 October 2021). [CrossRef] [PubMed]
- Abu Saleh, O.; Fida, M.; Asbury, K.; Narichania, A.; Sotello, D.; Bosch, W.; Vikram, H.R.; Palraj, R.; Lahr, B.; Baddour, L.M.; et al. Prospective Validation of PREDICT and Its Impact on the Transesophageal Echocardiography Use in Management of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2021, 73, e1745–e1753. Available online: https://academic.oup.com/cid/article/73/7/e1745/5860918 (accessed on 13 October 2021). [CrossRef] [PubMed]
- Heriot, G.S.; Cronin, K.; Tong, S.Y.C.; Cheng, A.C.; Liew, D. Criteria for Identifying Patients With Staphylococcus aureus Bacteremia Who Are at Low Risk of Endocarditis: A Systematic Review. Open Forum. Infect. Dis. 2017, 4, ofx261. Available online: https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofx261/4657117 (accessed on 13 October 2021). [CrossRef]
- Simeon, S.; Le Moing, V.; Tubiana, S.; Duval, X.; Fournier, D.; Lavigne, J.P.; Erpelding, M.L.; Gustave, C.A.; Desage, S.; Chirouze, C.; et al. Time to blood culture positivity: An independent predictor of infective endocarditis and mortality in patients with Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2019, 25, 481–488. [Google Scholar] [CrossRef]
- Vos, F.J.; Bleeker-Rovers, C.P.; Sturm, P.D.; Krabbe, P.F.M.; Kullberg, B.J. Endocarditis: Effects of routine echocardiography during Gram-positive bacteraemia. Neth. J. Med. 2011, 69, 335–340. [Google Scholar]
- Lau, L.; Wiens, E.J.; Karlowsky, J.A.; Keynan, Y.; Jassal, D.S. Clinical utility of echocardiography for the diagnosis of native valve infective endocarditis in Staphylococcus aureus bacteremia. Echocardiography 2019, 36, 1852–1858. Available online: https://onlinelibrary.wiley.com/doi/10.1111/echo.14480 (accessed on 13 October 2021). [CrossRef]
- Urja, P.; Walters, R.W.; Vivekanandan, R.; Kumar, M.; Abdulghani, S.; Hari Belbase, R.; Zook, N.; Mahesh Alla, V. Trends in the use of echocardiography in patients with Staphylococcus aureus bacteremia: An analysis using the Nationwide Inpatient Sample data. Echocardiography 2019, 36, 1625–1632. Available online: https://onlinelibrary.wiley.com/doi/10.1111/echo.14473 (accessed on 13 October 2021). [CrossRef]
- Thorlacius-Ussing, L.; Sandholdt, H.; Nissen, J.; Rasmussen, J.; Skov, R.; Frimodt-Møller, N.; Dahl Knudsen, J.; Østergaard, C.; Benfield, T. Comparable Outcomes of Short-Course and Prolonged-Course Therapy in Selected Cases of Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Pooled Cohort Study. Clin. Infect. Dis. 2021, 73, 866–872. [Google Scholar] [CrossRef]
| Variable | Total (n = 404) | IE (n = 50) | Non-IE (n = 354) | p | Missing | |
|---|---|---|---|---|---|---|
| Demographic and comorbidity | ||||||
| Age | 69 (56–79) | 68 (58–77) | 69 (55–80) | 0.927 | 0 | |
| Sex (female) | 31.4% (127) | 26.0% (13) | 32.2% (1149) | 0.420 | 0 | |
| Charlson index | 2 (1–5) | 2 (1–4) | 2 (1–5) | 0.635 | 3 | |
| Age-adjusted Charlson index | 5 (3–7) | 5 (3–7) | 5 (3–7) | 0.968 | 3 | |
| Arterial hypertension | 55.9% (226) | 72.0% (36) | 53.7% (190) | 0.015 | 0 | |
| Diabetes mellitus | 29.2% (118) | 34.0% (17) | 28.5% (101) | 0.506 | 0 | |
| Chronic heart failure | 30.4% (123) | 48.0% (24) | 28.0% (99) | 0.005 | 0 | |
| Ischemic heart disease | 18.1% (73) | 22.0% (11) | 17.5% (62) | 0.556 | 0 | |
| Natural cardiac valve disease | 18.3% (74) | 29.7% (22) | 14.7% (52) | <0.001 | 0 | |
| Prosthetic heart valve disease | 4.7% (19) | 14.0% (7) | 3.4% (12) | 0.005 | 0 | |
| CIED | 6.2% (25) | 18.0% (9) | 4.5% (16) | 0.001 | 0 | |
| Chronic renal failure | 22.8% (92) | 28.0% (14) | 22.0% (78) | 0.369 | 0 | |
| Hemodialysis | 7.1% (29) | 8.0% (4) | 7.1% (25) | 0.794 | 0 | |
| Liver cirrhosis | 3.2% (13) | 2.0% (1) | 3.4% (12) | 0.715 | 1 | |
| Solid organ malignancy | 21.6% (87) | 12.0% (6) | 22.9% (81) | 0.098 | 1 | |
| Parenteral drug user | 1.0% (4) | 2.0% (1) | 0.8% (3) | 0.413 | 2 | |
| Clinical presentation | ||||||
| Acquisition | Nosocomial | 51.5% (208) | 40.0% (20) | 53.1% (188) | Ref. | 0 |
| Healthcare-associated | 16.8% (68) | 14.0% (7) | 17.2% (61) | 0.870 | ||
| Community | 31.7% (128) | 46.0% (23) | 29.7% (105) | 0.026 | ||
| Source of infection | Primary/unknown | 29.4% (116) | 49.0% (24) | 26.7% (92) | <0.001 | 10 |
| Catheter-related | 34.2% (135) | 28.0% (14) | 35.2% (121) | 0.571 | ||
| Other | 36.3% (143) | 24.0% (12) | 38.1% (131) | ref | ||
| Fever | 89.8% (362) | 92.0% (46) | 89.5% (316) | 0.635 | 1 | |
| Sepsis/septic shock | 28.3% (114) | 48.0% (24) | 25.5% (90) | 0.001 | 1 | |
| Fever defervescence within 72 h | 89.2% (330) | 79.2% (38) | 90.6% (292) | 0.050 | 34 | |
| Septic emboli | 13.4% (54) | 46.0% (23) | 8.8% (31) | <0.001 | 0 | |
| Acute kidney injury | 40.5% (162) | 60.0% (30) | 37.7% (132) | 0.003 | 0 | |
| Acute cardiac failure | 20.9% (84) | 54.0% (27) | 16.2% (57) | <0.001 | 2 | |
| Pitt’s bacteremia score | 0 (0–3) | 1 (0–3) | 0 (0–2) | 0.004 | 2 | |
| SOFA | 2 (0–4) | 3 (1–5) | 2 (0–4) | 0.038 | 3 | |
| Microbiology | ||||||
| Time to positivity (hours) | 12 (9–16) | 11 (8–14) | 12 (10–16) | 0.023 | 0 | |
| Persistent bacteriemia | 31.8% (99) | 62.2% (28) | 26.7% (71) | <0.001 | 93 | |
| Methicillin-resistant SAB | 19.6% (79) | 14.0% (7) | 20.3% (72) | 0.345 | 0 | |
| Diagnostic workup | ||||||
| TTE | 62.3% (250) | 80.0% (40) | 59.8% (210) | 0.007 | 3 | |
| TEE | 32.2% (128) | 72.0% (36) | 26.4% (92) | <0.001 | 6 | |
| PET-CT | 10.2% (41) | 26.0% (13) | 8.0% (28) | <0.001 | 3 | |
| Outcomes | ||||||
| 30-day mortality | 15.4% (62) | 20.0% (10) | 14.8% (52) | 0.401 | 2 | |
| In-hospital mortality | 20.3% (82) | 28.0% (14) | 19.3% (68) | 0.187 | 0 | |
| SAB relapse | 4.5% (17) | 4.5% (2) | 4.5% (15) | 1.000 | 62 | |
| PREDICT (5-Day Model) | POSITIVE | VIRSTA | ||
|---|---|---|---|---|
| High risk patients | IE | 16.7% (45) | 23.6% (38) | 20.8% (46) |
| Non IE | 83.3% (224) | 76.4% (123) | 79.2% (181) | |
| Total | 66.3% (269) | 39.7% (161) | 54.4% (221) | |
| Low risk patients | IE | 3.6% (5) | 4.9% (12) | 2.2% (4) |
| Non IE | 96.4% (132) | 95.1% (233) | 97.8% (181) | |
| Total | 33.7% (137) | 60.3% (245) | 45.6% (185) | |
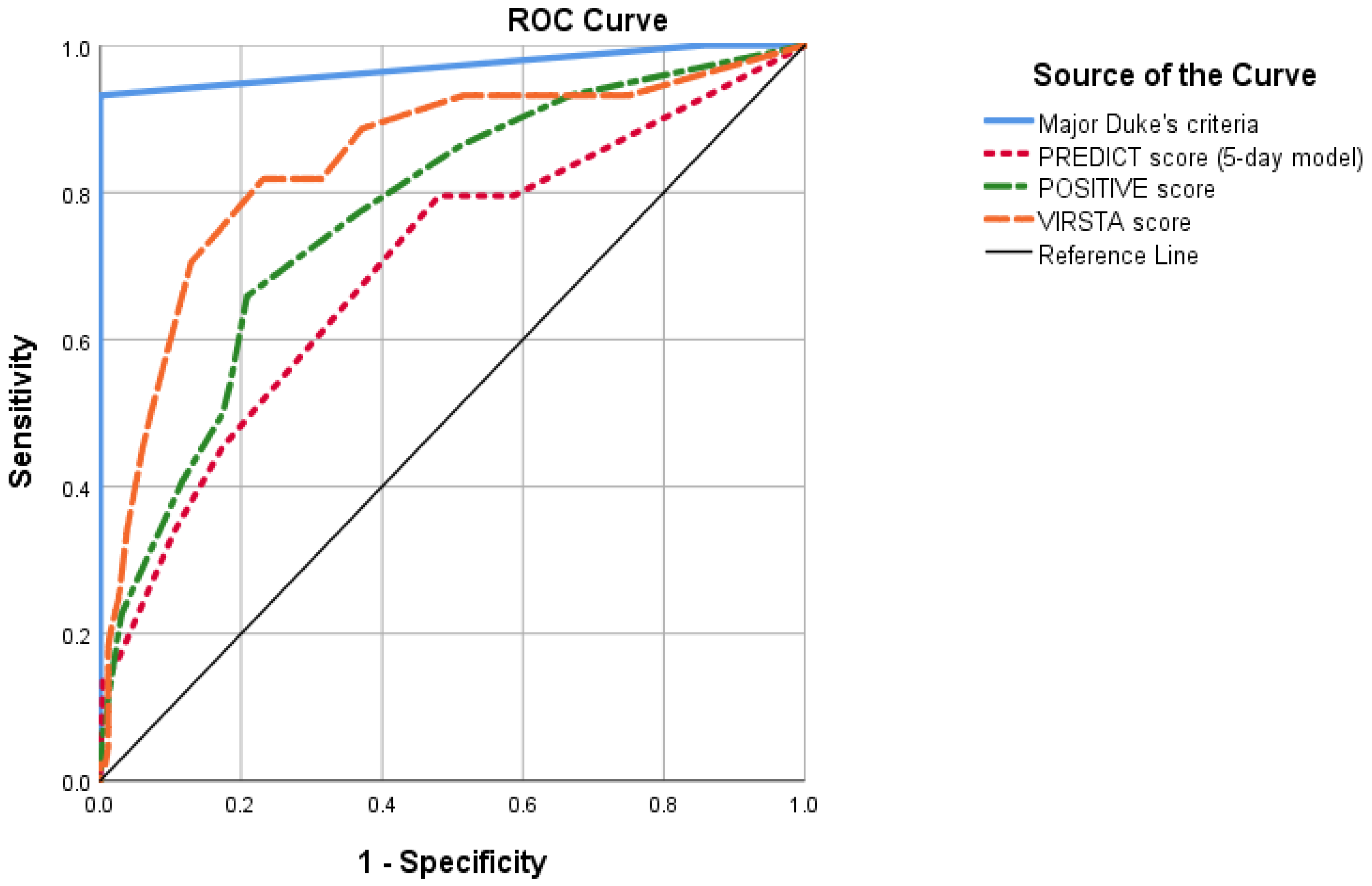
| Cut-Off | Sens. | Spec. | PPV | NPV | PLR | NLR | AUC | |
|---|---|---|---|---|---|---|---|---|
| PREDICT (5-day model) | >1 point | 90% | 37.1% | 16.7% | 96.4% | 1.43 | 0.27 | 0.70 |
| POSITIVE | >4 points | 76% | 65.5% | 23.6% | 95.1% | 2.17 | 0.37 | 0.78 |
| VIRSTA | >2 points | 92.0% | 50.8% | 20.8% | 97.8% | 1.84 | 0.16 | 0.85 |
| PREDICT Low Risk | POSITIVE Low Risk | VIRSTA Low Risk | ||||
|---|---|---|---|---|---|---|
| IE Prevalence | CI 95% | IE Prevalence | CI 95% | IE Prevalence | CI 95% | |
| All patients (n = 404) | 3.6% (5/137) | 0.1–6.9% | 4.9% (12/245) | 2.2–7.7% | 2.2% (4/185) | 0.1–4.3% |
| Patients with TTE and/or TEE (n = 289) | 4.8% (5/104) | 0.1–9.0% | 7.5% (12/160) | 3.4–11.6% | 3.5% (4/116) | 0–6.8% |
| Patients with TEE (n = 128) | 5.3% (2/39) | 0–12.4% | 12.0% (6/50) | 2.7–21.3% | 5.7% (2/35) | 0–13.8% |
| Patients with negative TTE (n = 235) | 3.4% (3/89) | 0–7.2% | 2.8% (4/141) | 0.1–5.6% | 0.9% (1/103) | 0–2.8% |
| 30-Day Mortality | OR | 95% CI | p | |
|---|---|---|---|---|
| Age (each year) | 1.04 | 1.01–1.07 | 0.008 | |
| Charlson index (each point) | 1.07 | 0.95–1.23 | 0.329 | |
| Unknown source of infection | 3.70 | 1.67–8.20 | 0.001 | |
| SOFA (each point) | 1.22 | 1.03–1.46 | 0.026 | |
| Complicated bacteriemia | 2.69 | 1.18–6.17 | 0.019 | |
| Low-risk VIRSTA score | 0.44 | 0.19–0.99 | 0.048 | |
| Echocardiographic evaluation | TTE and/or TEE | 0.24 | 0.10–0.54 | 0.001 |
| TTE | 0.28 | 0.13–0.60 | 0.001 | |
| TEE | 0.59 | 0.25–1.39 | 0.232 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Parra, J.; Diego-Yagüe, I.; Santamarina-Alcantud, B.; Mingo-Santos, S.; Mora-Vargas, A.; Vázquez-Comendador, J.M.; Fernández-Cruz, A.; Muñez-Rubio, E.; Gutiérrez-Villanueva, A.; Sánchez-Romero, I.; et al. Unreliability of Clinical Prediction Rules to Exclude without Echocardiography Infective Endocarditis in Staphylococcus aureus Bacteremia. J. Clin. Med. 2022, 11, 1502. https://doi.org/10.3390/jcm11061502
Calderón-Parra J, Diego-Yagüe I, Santamarina-Alcantud B, Mingo-Santos S, Mora-Vargas A, Vázquez-Comendador JM, Fernández-Cruz A, Muñez-Rubio E, Gutiérrez-Villanueva A, Sánchez-Romero I, et al. Unreliability of Clinical Prediction Rules to Exclude without Echocardiography Infective Endocarditis in Staphylococcus aureus Bacteremia. Journal of Clinical Medicine. 2022; 11(6):1502. https://doi.org/10.3390/jcm11061502
Chicago/Turabian StyleCalderón-Parra, Jorge, Itziar Diego-Yagüe, Beatriz Santamarina-Alcantud, Susana Mingo-Santos, Alberto Mora-Vargas, José Manuel Vázquez-Comendador, Ana Fernández-Cruz, Elena Muñez-Rubio, Andrea Gutiérrez-Villanueva, Isabel Sánchez-Romero, and et al. 2022. "Unreliability of Clinical Prediction Rules to Exclude without Echocardiography Infective Endocarditis in Staphylococcus aureus Bacteremia" Journal of Clinical Medicine 11, no. 6: 1502. https://doi.org/10.3390/jcm11061502
APA StyleCalderón-Parra, J., Diego-Yagüe, I., Santamarina-Alcantud, B., Mingo-Santos, S., Mora-Vargas, A., Vázquez-Comendador, J. M., Fernández-Cruz, A., Muñez-Rubio, E., Gutiérrez-Villanueva, A., Sánchez-Romero, I., & Ramos-Martínez, A. (2022). Unreliability of Clinical Prediction Rules to Exclude without Echocardiography Infective Endocarditis in Staphylococcus aureus Bacteremia. Journal of Clinical Medicine, 11(6), 1502. https://doi.org/10.3390/jcm11061502







