Retinal Vessel Density in Age-Related Macular Degeneration Patients with Geographic Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
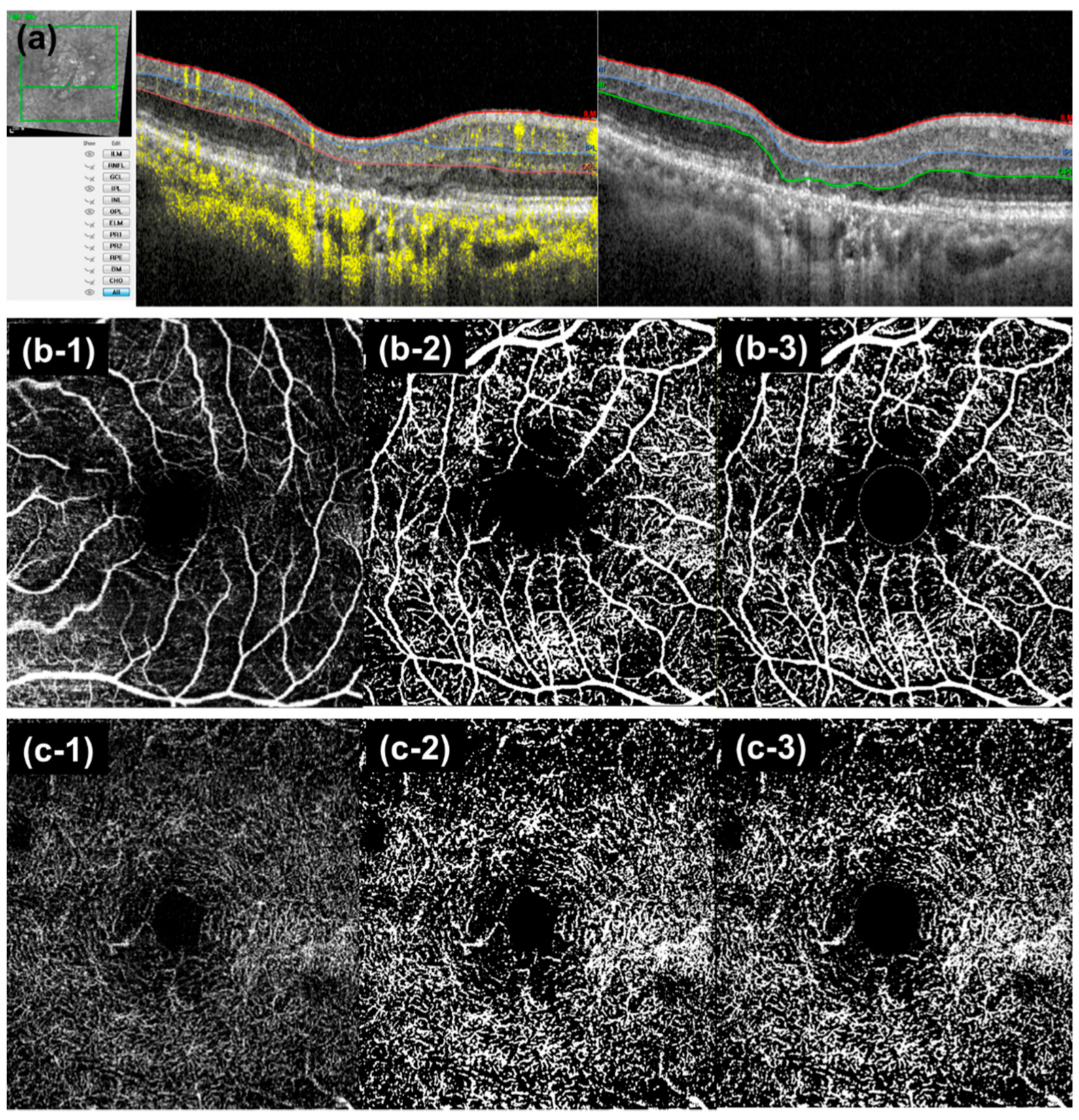
2.2. Image Acquisition and Analysis
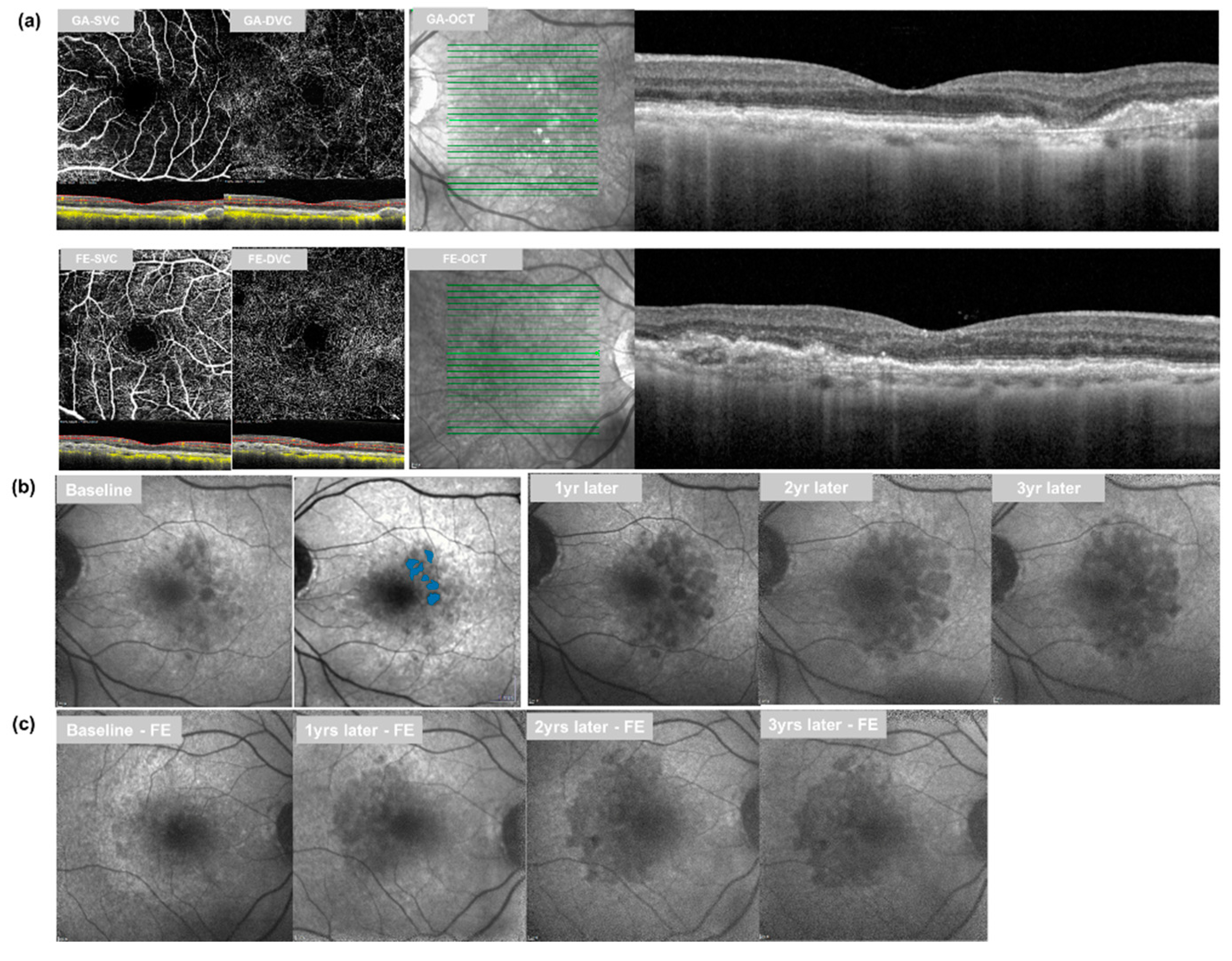
2.3. Follow-Up Analysis
2.4. Statistical Analyses
3. Results
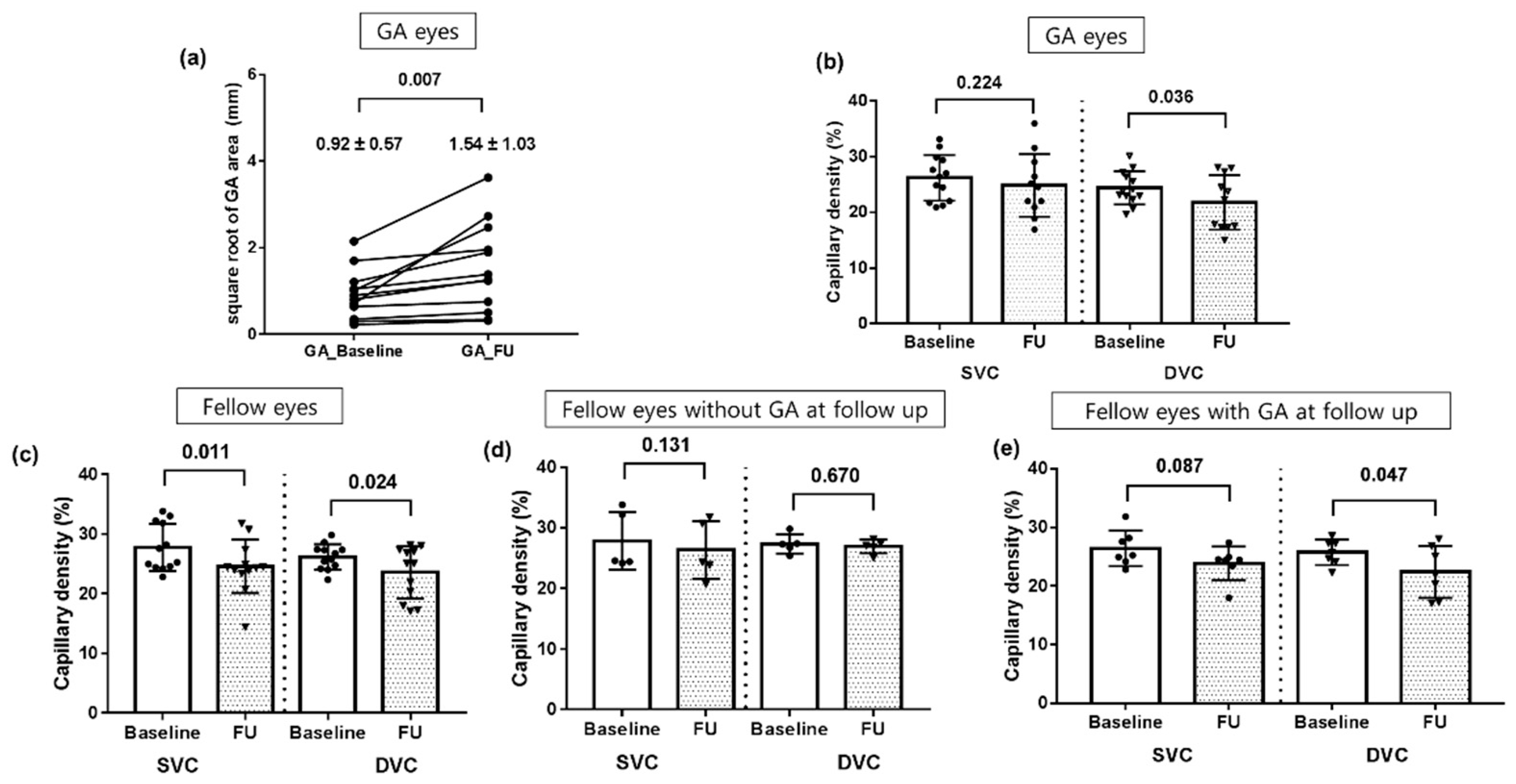
3.1. Retinal Vessel Density and GA Growth
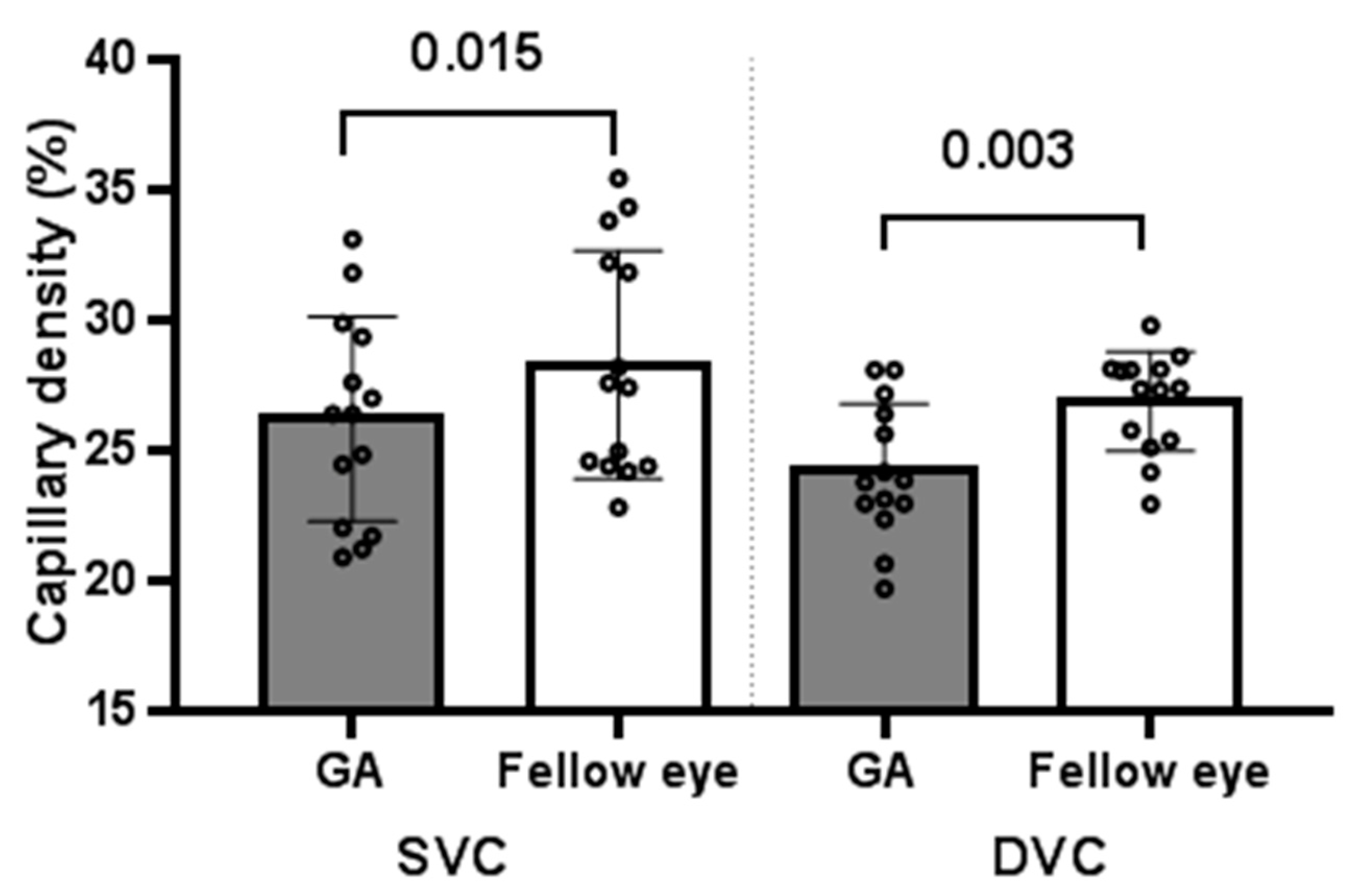
3.1.1. GA Eyes vs. Fellow Eyes at Baseline
3.1.2. Follow-Up Analysis in Eyes with GA at Baseline
3.1.3. Follow-Up Analysis in Fellow Eyes
3.2. Inner Retinal Thickness (mm)
3.2.1. GA Eyes vs. Fellow Eyes at Baseline
3.2.2. Follow-Up Analysis in Eyes with GA at Baseline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L., 3rd; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.; Bressler, N.; Bressler, S.; Chisholm, I.; Coscas, G.; Davis, M.; de Jong, P.; Klaver, C.; Klein, B.; Klein, R.; et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv. Ophthalmol. 1995, 39, 367–374. [Google Scholar] [CrossRef]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Guymer, R.H.; Rosenfeld, P.J.; Curcio, C.A.; Holz, F.G.; Staurenghi, G.; Freund, K.B.; Schmitz-Valckenberg, S.; Sparrow, J.; Spaide, R.F.; Tufail, A.; et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy in Age-Related Macular Degeneration: Classification of Atrophy Meeting Report 4. Ophthalmology 2020, 127, 394–409. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Nguyen, B.; Bartsch, D.U.; Saunders, L.J.; Muftuoglu, I.K.; You, Q.; Freeman, W.R. Reduced ganglion cell volume on optical coherence tomography in patients with geographic atrophy. Retina 2018, 38, 2159. [Google Scholar] [CrossRef]
- Zucchiatti, I.; Parodi, M.B.; Pierro, L.; Cicinelli, M.V.; Gagliardi, M.; Castellino, N.; Bandello, F. Macular ganglion cell complex and retinal nerve fiber layer comparison in different stages of age-related macular degeneration. Am. J. Ophthalmol. 2015, 160, 602–607. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sadda, S.; Humayun, M.S.; de Juan, E., Jr.; Melia, B.M.; Green, W.R. Morphometric analysis of the macula in eyes with geographic atrophy due to age-related macular degeneration. Retina 2002, 22, 464–470. [Google Scholar] [CrossRef]
- You, Q.S.; Wang, J.; Guo, Y.; Flaxel, C.J.; Hwang, T.S.; Huang, D.; Jia, Y.; Bailey, S.T. Detection of Reduced Retinal Vessel Density in Eyes with Geographic Atrophy Secondary to Age-Related Macular Degeneration Using Projection-Resolved Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2019, 209, 206–212. [Google Scholar] [CrossRef]
- Toto, L.; Borrelli, E.; Di Antonio, L.; Carpineto, P.; Mastropasqua, R. Retinal vascular plexuses’ changes in dry age-related macular degeneration, evaluated by means of optical coherence tomography angiography. Retina 2016, 36, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; Borrelli, E.; Mastropasqua, R.; Di Antonio, L.; Doronzo, E.; Carpineto, P.; Mastropasqua, L. Association between outer retinal alterations and microvascular changes in intermediate stage age-related macular degeneration: An optical coherence tomography angiography study. Br. J. Ophthalmol. 2017, 101, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Kim, K.M.; Lim, H.B.; Jo, Y.J.; Kim, J.Y. Repeatability of vessel density measurements using optical coherence tomography angiography in retinal diseases. Br. J. Ophthalmol. 2019, 103, 704–710. [Google Scholar] [CrossRef]
- Sampson, D.M.; Gong, P.; An, D.; Menghini, M.; Hansen, A.; Mackey, D.A.; Sampson, D.; Chen, F.K. Axial Length Variation Impacts on Superficial Retinal Vessel Density and Foveal Avascular Zone Area Measurements Using Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2017, 58, 3065–3072. [Google Scholar] [CrossRef]
- Holmen, I.C.; Konda, S.M.; Pak, J.W.; McDaniel, K.W.; Blodi, B.; Stepien, K.E.; Domalpally, A. Prevalence and Severity of Artifacts in Optical Coherence Tomographic Angiograms. JAMA Ophthalmol. 2020, 138, 119–126. [Google Scholar] [CrossRef]
- Campbell, J.P.; Zhang, M.; Hwang, T.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, srep42201. [Google Scholar] [CrossRef]
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative optical coherence tomography angiography: A review. Exp. Biol. Med. 2020, 245, 301–312. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R.; Rahimi, M.; Iafe, N.A.; Freund, K.B.; Sadda, S.R.; Sarraf, D. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Investig. Opthalmol. Vis. Sci. 2017, 58, 2063–2069. [Google Scholar] [CrossRef]
- Mehta, N.; Braun, P.X.; Gendelman, I.; Alibhai, A.Y.; Arya, M.; Duker, J.S.; Waheed, N.K. Repeatability of binarization thresholding methods for optical coherence tomography angiography image quantification. Sci. Rep. 2020, 10, 15368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Gao, S.; Liu, L.; Lauer, A.K.; Bailey, S.T.; Flaxel, C.J.; Wilson, D.J.; Huang, D.; Jia, Y. Automated Quantification of Capillary Nonperfusion Using Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA Ophthalmol. 2016, 134, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J.; Pechauer, A.D.; Hwang, T.S.; Gao, S.S.; Liu, L.; Liu, L.; Bailey, S.T.; Wilson, D.J.; Huang, D.; et al. Advanced image processing for optical coherence tomographic angiography of macular diseases. Biomed. Opt. Express 2015, 6, 4661–4675. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.L.; Sun, M.; Ahluwalia, A.; Young, B.K.; Park, M.M.; Del Priore, L.V. Geographic Atrophy Growth Is Strongly Related to Lesion Perimeter: Unifying Effects of Lesion Area, Number, and Circularity on Growth. Ophthalmol. Retin. 2021, 5, 868–878. [Google Scholar] [CrossRef]
- Budzynski, E.; Smith, J.H.; Bryar, P.; Birol, G.; Linsenmeier, R.A. Effects of photocoagulation on intraretinal PO2 in cat. Investig. Ophthalmol. Vis. Sci. 2008, 49, 380–389. [Google Scholar] [CrossRef][Green Version]
- Grunwald, J.E.; Riva, C.E.; Brucker, A.J.; Sinclair, S.H.; Petrig, B.L. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology 1986, 93, 590–595. [Google Scholar] [CrossRef]
- Sadigh, S.; Cideciyan, A.V.; Sumaroka, A.; Huang, W.C.; Luo, X.; Swider, M.; Steinberg, J.D.; Stambolian, D.; Jacobson, S.G. Abnormal thickening as well as thinning of the photoreceptor layer in intermediate age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1603–1612. [Google Scholar] [CrossRef]
- Nam, K.T.; Chung, H.W.; Jang, S.; Hwang, S.-Y.; Kim, S.-W.; Oh, J.; Yun, C. Ganglion cell—inner plexiform layer thickness in eyes with nonexudative age-related macular degeneration of different drusen subtypes. Retina 2021, 41, 1686–1696. [Google Scholar] [CrossRef]
- Borrelli, E.; Abdelfattah, N.; Uji, A.; Nittala, M.G.; Boyer, D.S.; Sadda, S.R. Postreceptor Neuronal Loss in Intermediate Age-related Macular Degeneration. Am. J. Ophthalmol. 2017, 181, 1–11. [Google Scholar] [CrossRef]
- Beck, M.; Munk, M.R.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. Retinal Ganglion Cell Layer Change in Patients Treated with Anti–Vascular Endothelial Growth Factor for Neovascular Age-related Macular Degeneration. Am. J. Ophthalmol. 2016, 167, 10–17. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Gharbiya, M.; Formisano, M.; Bertini, F.; Cerini, A.; Pacella, E. Anti-Vascular Endothelial Growth Factor Intravitreal Therapy and Macular Ganglion Cell Layer Thickness in Patients with Neovascular Age-Related Macular Degeneration. Curr. Eye Res. 2019, 44, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Sunness, J.S.; Margalit, E.; Srikumaran, D.; Applegate, C.A.; Tian, Y.; Perry, D.; Hawkins, B.S.; Bressler, N.M. The long-term natural history of geographic atrophy from age-related macular degeneration: Enlargement of atrophy and implications for interventional clinical trials. Ophthalmology 2007, 114, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Batıoğlu, F.; Gedik Oğuz, Y.; Demirel, S.; Ozmert, E. Geographic atrophy progression in eyes with age-related macular degeneration: Role of fundus autofluorescence patterns, fellow eye and baseline atrophy area. Ophthalmic Res. 2014, 52, 53–59. [Google Scholar] [CrossRef]
- Lindblad, A.S.; Lloyd, P.C.; Clemons, T.E.; Gensler, G.R.; Ferris, F.L., 3rd; Klein, M.L.; Armstrong, J.R.; Age-Related Eye Disease Study Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch. Ophthalmol. 2009, 127, 1168–1174. [Google Scholar] [PubMed]
- Seddon, J.M.; McLeod, D.S.; Bhutto, I.A.; Villalonga, M.B.; Silver, R.E.; Wenick, A.S.; Edwards, M.M.; Lutty, G.A. Histopathological Insights into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Povazay, B.; Bizheva, K.; Hermann, B.; Unterhuber, A.; Sattmann, H.; Fercher, A.; Drexler, W.; Schubert, C.; Ahnelt, P.; Mei, M.; et al. Enhanced visualization of choroidal vessels using ultrahigh resolution ophthalmic OCT at 1050 nm. Opt. Express 2003, 11, 1980–1986. [Google Scholar] [CrossRef]
- Unterhuber, A.; Povazay, B.; Hermann, B.; Sattmann, H.; Chavez-Pirson, A.; Drexler, W. In vivo retinal optical coherence tomography at 1040 nm—enhanced penetration into the choroid. Opt. Express 2005, 13, 3252–3258. [Google Scholar] [CrossRef]
- Alten, F.; Lauermann, J.L.; Clemens, C.R.; Heiduschka, P.; Eter, N. Signal reduction in choriocapillaris and segmentation errors in spectral domain OCT angiography caused by soft drusen. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2347–2355. [Google Scholar] [CrossRef]
| Eyes with GA (n = 14) | Fellow Eyes (n = 14) | p-Value * | |
|---|---|---|---|
| Age, years | 76.6 ± 5.1 | ||
| Sex, male (%) | 4 (28.6) | ||
| HTN (%) | 8 (57.1) | ||
| DM (%) | 4 (28.6) | ||
| Eyes with GA, OD (%) | 9 (64.3%) | ||
| Follow up period (months) | 25.11 ± 5.93 | ||
| Logmar BCVA (baseline) | 0.43 ± 0.50 | 0.30 ± 0.21 | 0.466 |
| LogMAR BCVA (last follow-up) | 0.70 ± 0.62 | 0.31 ± 0.27 | 0.050 |
| GA development in fellow eye during follow-up (n = 12) (%) | 7 (58) | ||
| Eyes with GA (n = 14) | Fellow Eyes (n = 14) | p-Value * | ||
|---|---|---|---|---|
| Center ring 1 mm | NFL | 12.14 ± 5.844 | 10.21 ± 2.26 | 0.246 |
| GCIPL | 26.81 ± 6.82 | 34.00 ± 13.93 | 0.032 | |
| INL | 18.8 ± 7.29 | 17.9 ± 8.17 | 0.384 | |
| ONL | 63.29 ± 17.89 | 81.79 ± 22.77 | 0.024 | |
| Inner ring 1–3 mm | NFL | 22.26 ± 3.61 | 21.71 ± 5.79 | 0.421 |
| GCIPL | 75.68 ± 11.06 | 79.41 ± 11.03 | 0.029 | |
| INL | 37.02 ± 3.27 | 36.75 ± 4.64 | 0.919 | |
| ONL | 45.43 ± 7.02 | 51.96 ± 10.58 | 0.065 | |
| Outer ring 3–6 mm | NFL | 36.62 ± 5.33 | 35.92 ± 9.75 | 0.310 |
| GCIPL | 65.01 ± 6.65 | 64.36 ± 9.17 | 0.594 | |
| INL | 31.27 ± 1.81 | 30.55 ± 2.23 | 0.326 | |
| ONL | 43.77 ± 10.24 | 44.8 ± 9.74 | 0.786 | |
| Total (mean) | NFL | 23.68 ± 3.92 | 22.63 ± 5.51 | 0.330 |
| GCIPL | 55.84 ± 6.00 | 59.26 ± 9.17 | 0.033 | |
| INL | 29.03 ± 2.161 | 28.40 ± 2.95 | 0.285 | |
| ONL | 50.83 ± 6.42 | 59.52 ± 12.19 | 0.026 |
| Baseline (n = 12) | Follow-Up (n = 12) | p-Value * | ||
|---|---|---|---|---|
| Center ring 1 mm | NFL | 13.33 ± 6.96 | 14.22 ± 5.95 | 0.891 |
| GCIPL | 26.22 ± 6.74 | 24.88 ± 12.44 | 0.327 | |
| INL | 16.6 ± 5.45 | 18.44 ± 7.31 | 0.725 | |
| ONL | 62.6 ± 19.75 | 55.0 ± 18.44 | 0.162 | |
| Inner ring 1–3 mm | NFL | 21.44 ± 4.05 | 35.53 ± 7.27 | 0.866 |
| GCIPL | 75.47 ± 10.14 | 67.55 ± 18.91 | 0.233 | |
| INL | 35.93 ± 4.52 | 36.11 ± 4.69 | 0.779 | |
| ONL | 46.86 ± 12.76 | 43.28 ± 7.64 | 0.184 | |
| Outer ring 3–6 mm | NFL | 35.25 ± 5.46 | 35.53 ± 7.27 | 0.779 |
| GCIPL | 64.67 ± 7.39 | 57.61 ± 10.41 | 0.050 | |
| INL | 30.83 ± 2.67 | 30.25 ± 3.53 | 0.326 | |
| ONL | 46.7 ± 11.82 | 44.13 ± 12.42 | 0.072 | |
| Total (mean) | NFL | 23.34 ± 4.80 | 23.76 ± 5.54 | 0.674 |
| GCIPL | 55.45 ± 5.76 | 53.35 ± 10.41 | 0.594 | |
| INL | 27.81 ± 3.01 | 28.19 ± 4.15 | 0.889 | |
| ONL | 52.04 ± 11.63 | 47.47 ± 9.54 | 0.086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Choi, M.; Yun, C.; Kim, S.-W. Retinal Vessel Density in Age-Related Macular Degeneration Patients with Geographic Atrophy. J. Clin. Med. 2022, 11, 1501. https://doi.org/10.3390/jcm11061501
Hong S, Choi M, Yun C, Kim S-W. Retinal Vessel Density in Age-Related Macular Degeneration Patients with Geographic Atrophy. Journal of Clinical Medicine. 2022; 11(6):1501. https://doi.org/10.3390/jcm11061501
Chicago/Turabian StyleHong, Suji, Mihyun Choi, Cheolmin Yun, and Seong-Woo Kim. 2022. "Retinal Vessel Density in Age-Related Macular Degeneration Patients with Geographic Atrophy" Journal of Clinical Medicine 11, no. 6: 1501. https://doi.org/10.3390/jcm11061501
APA StyleHong, S., Choi, M., Yun, C., & Kim, S.-W. (2022). Retinal Vessel Density in Age-Related Macular Degeneration Patients with Geographic Atrophy. Journal of Clinical Medicine, 11(6), 1501. https://doi.org/10.3390/jcm11061501






