Sleep Health Assessment and Treatment in Children and Adolescents with Chronic Pain: State of the Art and Future Directions
Abstract
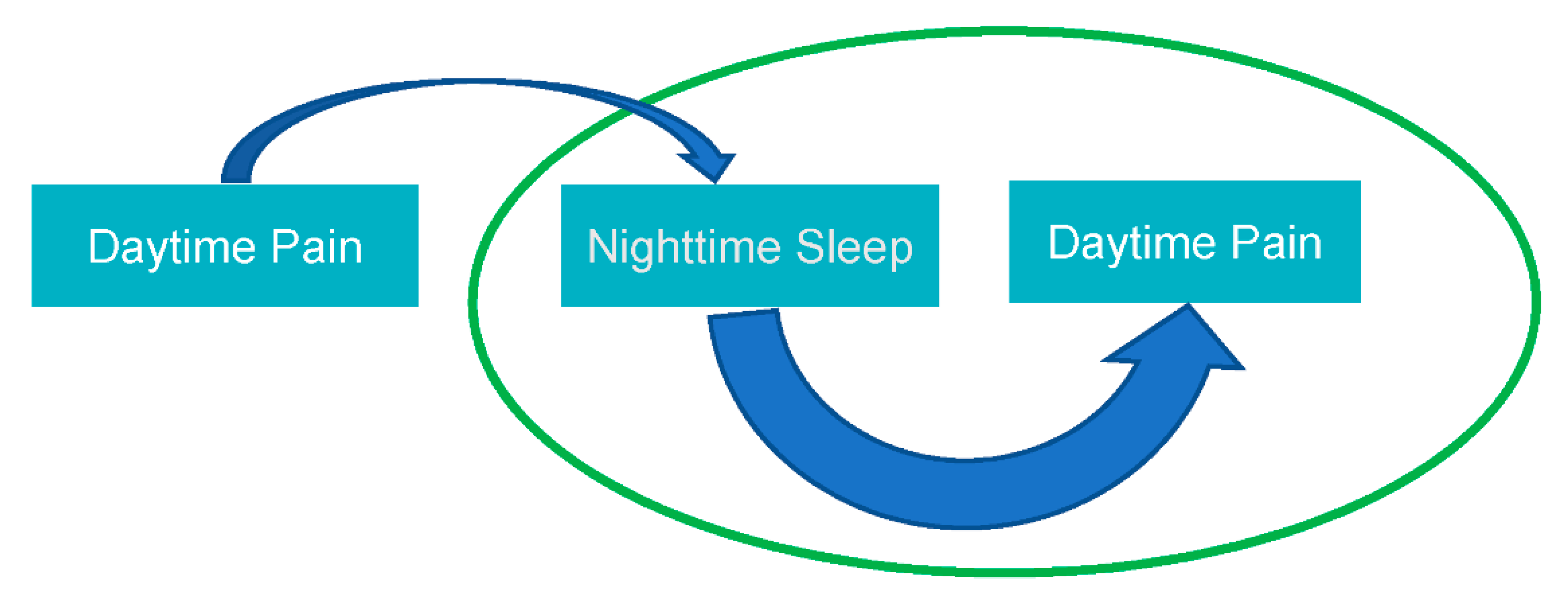
:1. Introduction
2. Approaches for Sleep Health Assessment in Pediatric Pain Populations
| Measure Name | Domain | Age Range | Reporter | Items/Subscales | Primary Citation |
|---|---|---|---|---|---|
| Adolescent Sleep Wake Scale (ASWS) | Sleep quality | 12–18 years | Youth | 28 items, yields a total score and 5 subscale scores (Going to Bed, FallingAsleep, Maintaining Sleep, Reinitiating Sleep, Returning to Wakefulness) | LeBourgeois et al., [38] |
| Adolescent Sleep Wake Scale Short Form (ASWS-SF) | Sleep quality | 12–18 years | Youth | 10 items, yields a total score and 3 subscale scores (Falling Asleep and Reinitiating Sleep, Returning to Wakefulness, Going to Bed) | Essner et al., [40] |
| Adolescent Sleep Hygiene Scale (ASHS) | Sleep habits | 12–18 years | Youth | 28 items, yields a total score and 9 subscale scores (Physiological, Cognitive, Emotional, Sleep Environment, Daytime Sleep, Substances, Bedtime Routine, Sleep Stability, Bed/Bedroom Sharing) | LeBourgeois et al., [38] |
| Children’s Sleep Habits Questionnaire (CSHQ) | Sleep disorders screen | 4–10 years | Parent | 45 items, yields a total score and 8 subscale scores (Bedtime Resistance, Parasomnia, Sleep Onset Delay, Sleep Duration, Sleep Anxiety, Night Wakings, Sleep-Disordered Breathing, Daytime Sleepiness) | Owens et al., [39] |
| Adolescent Insomnia Questionnaire (AIQ) | Insomnia screen | 11–18 years | Youth | 13 items, yields a total score and 3 subscale scores (Sleep Onset, Sleep Maintenance, Sleep Dissatisfaction and Impairments) | Bromberg et al., [44] |
3. Interventions to Improve Sleep Health in Pediatric Pain Populations: Cognitive-Behavioral Therapy for Pain Management, Cognitive-Behavioral Therapy for Insomnia, and Sleep Hygiene Education
4. Future Directions for Clinical Practice
5. Future Directions for Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobina, I.; Villberg, J.; Valimaa, R.; Tynjala, J.; Whitehead, R.; Cosma, A.; Brooks, F.; Cavallo, F.; Ng, K.; de Matos, M.G.; et al. Prevalence of self-reported chronic pain among adolescents: Evidence from 42 countries and regions. Eur. J. Pain 2019, 23, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.S.; Henschke, N.; Kamper, S.J.; Gobina, I.; Ottova-Jordan, V.; Maher, C.G. An international survey of pain in adolescents. BMC Public Health 2014, 14, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, J.I.; Mahrer, N.E.; Yee, J.; Palermo, T.M. Pain, fatigue, and health-related quality of life in children and adolescents with chronic pain. Clin. J. Pain 2009, 25, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashikar-Zuck, S.; Goldschneider, K.R.; Powers, S.W.; Vaught, M.H.; Hershey, A.D. Depression and functional disability in chronic pediatric pain. Clin. J. Pain 2001, 17, 341–349. [Google Scholar] [CrossRef]
- Lewandowski, A.S.; Palermo, T.M.; Peterson, C.C. Age-dependent relationships among pain, depressive symptoms, and functional disability in youth with recurrent headaches. Headache 2006, 46, 656–662. [Google Scholar] [CrossRef]
- Palermo, T.M.; Putnam, J.; Armstrong, G.; Daily, S. Adolescent autonomy and family functioning are associated with headache-related disability. Clin. J. Pain 2007, 23, 458–465. [Google Scholar] [CrossRef]
- Hoff, A.L.; Palermo, T.M.; Schluchter, M.; Zebracki, K.; Drotar, D. Longitudinal relationships of depressive symptoms to pain intensity and functional disability among children with disease-related pain. J. Pediatr. Psychol. 2006, 31, 1046–1056. [Google Scholar] [CrossRef] [Green Version]
- Fales, J.L.; Murphy, L.K.; Rights, J.D.; Palermo, T.M. Daily Peer Victimization Experiences of Adolescents With and Without Chronic Pain: Associations With Mood, Sleep, Pain, and Activity Limitations. J. Pain 2020, 21, 97–107. [Google Scholar] [CrossRef]
- Arruda, M.A.; Arruda, R.; Guidetti, V.; Bigal, M.E. Psychosocial adjustment of children with migraine and tension-type headache—A nationwide study. Headache 2015, 55 (Suppl. 1), 39–50. [Google Scholar] [CrossRef]
- Logan, D.E.; Simons, L.E.; Kaczynski, K.J. School functioning in adolescents with chronic pain: The role of depressive symptoms in school impairment. J. Pediatr. Psychol. 2009, 34, 882–892. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.B.; Groenewald, C.B.; de la Vega, R.; Palermo, T.M. Long-term impact of adolescent chronic pain on young adult educational, vocational, and social outcomes. Pain 2020, 161, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Cunningham, N.; Peugh, J.; Black, W.R.; Nelson, S.; Lynch-Jordan, A.M.; Pfeiffer, M.; Tran, S.T.; Ting, T.V.; Arnold, L.M.; et al. Long-term outcomes of adolescents with juvenile-onset fibromyalgia into adulthood and impact of depressive symptoms on functioning over time. Pain 2019, 160, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Cunningham, N.; Sil, S.; Bromberg, M.H.; Lynch-Jordan, A.M.; Strotman, D.; Peugh, J.; Noll, J.; Ting, T.V.; Powers, S.W.; et al. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics 2014, 133, e592–e600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.S.; Dengler-Crish, C.M.; Rippel, S.; Bruehl, S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain 2010, 150, 568–572. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.S.; Sherman, A.L.; Bruehl, S.; Garber, J.; Smith, C.A. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012, 153, 1798–1806. [Google Scholar] [CrossRef] [Green Version]
- Horst, S.; Shelby, G.; Anderson, J.; Acra, S.; Polk, D.B.; Saville, B.R.; Garber, J.; Walker, L.S. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clin. Gastroenterol. Hepatol. 2014, 12, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Palermo, T.M. Pain prevention and management must begin in childhood: The key role of psychological interventions. Pain 2020, 161 (Suppl. 1), S114–S121. [Google Scholar] [CrossRef]
- Valrie, C.R.; Bromberg, M.H.; Palermo, T.; Schanberg, L.E. A systematic review of sleep in pediatric pain populations. J. Dev. Behav. Pediatr. 2013, 34, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Palermo, T.M.; Wilson, A.C.; Lewandowski, A.S.; Toliver-Sokol, M.; Murray, C.B. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain 2011, 152, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Butbul Aviel, Y.; Stremler, R.; Benseler, S.M.; Cameron, B.; Laxer, R.M.; Ota, S.; Schneider, R.; Spiegel, L.; Stinson, J.N.; Tse, S.M.; et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology 2011, 50, 2051–2060. [Google Scholar] [CrossRef] [Green Version]
- LaPlant, M.M.; Adams, B.S.; Haftel, H.M.; Chervin, R.D. Insomnia and quality of life in children referred for limb pain. J. Rheumatol. 2007, 34, 2486–2490. [Google Scholar] [PubMed]
- Meltzer, L.J.; Logan, D.E.; Mindell, J.A. Sleep patterns in female adolescents with chronic musculoskeletal pain. Behav. Sleep. Med. 2005, 3, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, A.S.; Palermo, T.M.; Motte, S.D.l.; Fu, R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain 2010, 151, 220–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Vega, R.; Miro, J. The assessment of sleep in pediatric chronic pain sufferers. Sleep Med. Rev. 2012, 17, 185–192. [Google Scholar] [CrossRef]
- Galland, B.; Meredith-Jones, K.; Terrill, P.; Taylor, R. Challenges and Emerging Technologies within the Field of Pediatric Actigraphy. Front. Psychiatry 2014, 5, 99. [Google Scholar] [CrossRef]
- Ward, T.M.; Brandt, P.; Archbold, K.; Lentz, M.; Ringold, S.; Wallace, C.A.; Landis, C.A. Polysomnography and self-reported sleep, pain, fatigue, and anxiety in children with active and inactive juvenile rheumatoid arthritis. J. Pediatr. Psychol. 2008, 33, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Ward, T.M.; Chen, M.L.; Landis, C.A.; Ringold, S.; Beebe, D.W.; Pike, K.C.; Wallace, C.A. Congruence between polysomnography obstructive sleep apnea and the pediatric sleep questionnaire: Fatigue and health-related quality of life in juvenile idiopathic arthritis. Qual. Life Res. 2017, 26, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Acebo, C. Using and Scoring Actigraphy; E.P. Bradley Hospital Sleep Research Lab, Brown Medical School: Providence, RI, USA, 2006. [Google Scholar]
- Sadeh, A.; Acebo, C. The role of actigraphy in sleep medicine. Sleep Med. Rev. 2002, 6, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, L.J.; Montgomery-Downs, H.E.; Insana, S.P.; Walsh, C.M. Use of actigraphy for assessment in pediatric sleep research. Sleep Med. Rev. 2012, 16, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, T.M.; Toliver-Sokol, M.; Fonareva, I.; Koh, J.L. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin. J. Pain 2007, 23, 812–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlova, M.; Kopala-Sibley, D.C.; Nania, C.; Mychasiuk, R.; Christensen, J.; McPeak, A.; Tomfohr-Madsen, L.; Katz, J.; Palermo, T.M.; Noel, M. Sleep disturbance underlies the co-occurrence of trauma and pediatric chronic pain: A longitudinal examination. Pain 2020, 161, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.Y.; Labyak, S.E.; Richardson, L.P.; Lentz, M.J.; Brandt, P.A.; Ward, T.M.; Landis, C.A. Actigraphic sleep and daytime naps in adolescent girls with chronic musculoskeletal pain. J. Pediatric. Psychol. 2008, 33, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, L.J.; Westin, A.M. A comparison of actigraphy scoring rules used in pediatric research. Sleep Med. 2011, 12, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Galland, B.C.; Short, M.A.; Terrill, P.; Rigney, G.; Haszard, J.J.; Coussens, S.; Foster-Owens, M.; Biggs, S.N. Establishing normal values for pediatric nighttime sleep measured by actigraphy: A systematic review and meta-analysis. Sleep 2018, 41, zsy017. [Google Scholar] [CrossRef]
- LeBourgeois, M.K.; Giannotti, F.; Cortesi, F.; Wolfson, A.R.; Harsh, J. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics 2005, 115, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef]
- Essner, B.; Noel, M.; Myrvik, M.; Palermo, T. Examination of the Factor Structure of the Adolescent Sleep-Wake Scale (ASWS). Behav. Sleep Med. 2015, 13, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Palermo, T.M.; Beals-Erickson, S.; Bromberg, M.; Law, E.; Chen, M. A Single Arm Pilot Trial of Brief Cognitive Behavioral Therapy for Insomnia in Adolescents with Physical and Psychiatric Comorbidities. J. Clin. Sleep Med. 2017, 13, 401–410. [Google Scholar] [CrossRef]
- Law, E.F.; Tham, S.W.; Aaron, R.V.; Dudeney, J.; Palermo, T.M. Hybrid cognitive-behavioral therapy intervention for adolescents with co-occurring migraine and insomnia: A single-arm pilot trial. Headache 2018, 58, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Olufsen, I.S.; Sorensen, M.E.; Bjorvatn, B. New diagnostic criteria for insomnia and the association between insomnia, anxiety and depression. Tidsskr. Nor. Laegeforen. 2020, 140. [Google Scholar] [CrossRef]
- Bromberg, M.H.; de la Vega, R.; Law, E.F.; Zhou, C.; Palermo, T.M. Development and Validation of the Adolescent Insomnia Questionnaire. J. Pediatr. Psychol. 2020, 45, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, L.J.; Wainer, A.; Engstrom, E.; Pepa, L.; Mindell, J.A. Seeing the Whole Elephant: A scoping review of behavioral treatments for pediatric insomnia. Sleep Med. Rev. 2021, 56, 101410. [Google Scholar] [CrossRef]
- Maski, K.; Owens, J. Pediatric Sleep Disorders. Continuum 2018, 24, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, L.J.; Plaufcan, M.R.; Thomas, J.H.; Mindell, J.A. Sleep problems and sleep disorders in pediatric primary care: Treatment recommendations, persistence, and health care utilization. J. Clin. Sleep Med. 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Owens, J.A.; Rosen, C.L.; Mindell, J.A.; Kirchner, H.L. Use of pharmacotherapy for insomnia in child psychiatry practice: A national survey. Sleep Med. 2010, 11, 692–700. [Google Scholar] [CrossRef]
- Meltzer, L.J.; Mindell, J.A. Systematic Review and Meta-Analysis of Behavioral Interventions for Pediatric Insomnia. J. Pediatr. Psychol. 2014, 39, 932–948. [Google Scholar] [CrossRef] [Green Version]
- Fisher, E.; Law, E.; Dudeney, J.; Palermo, T.M.; Stewart, G.; Eccleston, C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2018, 9, CD003968. [Google Scholar]
- Palermo, T.M. Cognitive-Behavioral Therapy for Chronic Pain in Children and Adolescents; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Palermo, T.M.; Walco, G.A.; Paladhi, U.R.; Birnie, K.A.; Crombez, G.; de la Vega, R.; Eccleston, C.; Kashikar-Zuck, S.; Stone, A.L. Core outcome set for pediatric chronic pain clinical trials: Results from a Delphi poll and consensus meeting. Pain 2021, 162, 2539–2547. [Google Scholar] [CrossRef]
- Law, E.F.; Beals-Erickson, S.E.; Fisher, E.; Lang, E.A.; Palermo, T.M. Components of Effective Cognitive-Behavioral Therapy for Pediatric Headache: A Mixed Methods Approach. Clin. Pract. Pediatric Psychol. 2017, 5, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Klausen, S.H.; Ronde, G.; Tornoe, B.; Bjerregaard, L. Nonpharmacological Interventions Addressing Pain, Sleep, and Quality of Life in Children and Adolescents with Primary Headache: A Systematic Review. J. Pain Res. 2019, 12, 3437–3459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, E.F.; Beals-Erickson, S.E.; Noel, M.; Claar, R.; Palermo, T.M. Pilot randomized controlled trial of internet-delivered cognitive-behavioral treatment for pediatric headache. Headache 2015, 55, 1410–1425. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Law, E.F.; Fales, J.; Bromberg, M.H.; Jessen-Fiddick, T.; Tai, G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: A randomized controlled multicenter trial. Pain 2016, 157, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, J.; Pham, C.; Nagappa, M.; Peng, P.W.H.; Englesakis, M.; Espie, C.A.; Morin, C.M.; Chung, F. Cognitive behavioral therapy for insomnia in patients with chronic pain—A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2021, 60, 101460. [Google Scholar] [CrossRef]
- McCurry, S.M.; Zhu, W.; Von Korff, M.; Wellman, R.; Morin, C.M.; Thakral, M.; Yeung, K.; Vitiello, M.V. Effect of Telephone Cognitive Behavioral Therapy for Insomnia in Older Adults With Osteoarthritis Pain: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 530–538. [Google Scholar] [CrossRef]
- Vitiello, M.V.; McCurry, S.M.; Shortreed, S.M.; Baker, L.D.; Rybarczyk, B.D.; Keefe, F.J.; Von Korff, M. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain 2014, 155, 1547–1554. [Google Scholar] [CrossRef] [Green Version]
- Bruni, O.; Galli, F.; Guidetti, V. Sleep hygiene and migraine in children and adolescents. Cephalalgia 1999, 19 (Suppl. 25), 57–59. [Google Scholar] [CrossRef]
- Aslund, L.; Lekander, M.; Wicksell, R.K.; Henje, E.; Jernelov, S. Cognitive-behavioral therapy for insomnia in adolescents with comorbid psychiatric disorders: A clinical pilot study. Clin. Child. Psychol. Psychiatry 2020, 25, 958–971. [Google Scholar] [CrossRef]
- Babiloni, A.H.; Beetz, G.; Tang, N.K.Y.; Heinzer, R.; Nijs, J.; Martel, M.O.; Lavigne, G.J. Towards the endotyping of the sleep-pain interaction: A topical review on multitarget strategies based on phenotypic vulnerabilities and putative pathways. Pain 2021, 162, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Buenaver, L.F.; Coryell, V.T.; Smith, M.T. Cognitive-Behavioral Therapy for Comorbid Insomnia and Chronic Pain. Sleep Med. Clin. 2014, 9, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, N.K.; Lereya, S.T.; Boulton, H.; Miller, M.A.; Wolke, D.; Cappuccio, F.P. Nonpharmacological Treatments of Insomnia for Long-Term Painful Conditions: A Systematic Review and Meta-analysis of Patient-Reported Outcomes in Randomized Controlled Trials. Sleep 2015, 38, 1751–1764. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-X.; Zhang, L.-F.; Ai, Y.-Q.; Li, Z.-S. Efficacy of Internet-delivered cognitive-behavioral therapy for the management of chronic pain in children and adolescents: A systematic review and meta-analysis. Medicine 2018, 97, e12061. [Google Scholar] [CrossRef]
- Malfliet, A.; Bilterys, T.; Van Looveren, E.; Meeus, M.; Danneels, L.; Ickmans, K.; Cagnie, B.; Mairesse, O.; Neu, D.; Moens, M.; et al. The added value of cognitive behavioral therapy for insomnia to current best evidence physical therapy for chronic spinal pain: Protocol of a randomized controlled clinical trial. Braz. J. Phys. Ther. 2019, 23, 62–70. [Google Scholar] [CrossRef]
- Zachariae, R.; Lyby, M.S.; Ritterband, L.M.; O’Toole, M.S. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2016, 30, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Carmona, N.E.; Usyatynsky, A.; Kutana, S.; Corkum, P.; Henderson, J.; McShane, K.; Shapiro, C.; Sidani, S.; Stinson, J.; Carney, C.E. A Transdiagnostic Self-management Web-Based App for Sleep Disturbance in Adolescents and Young Adults: Feasibility and Acceptability Study. JMIR Form. Res. 2021, 5, e25392. [Google Scholar] [CrossRef]
- Peersmann, S.H.M.; van Straten, A.; Kaspers, G.J.L.; Thano, A.; van den Bergh, E.; Grootenhuis, M.A.; van Litsenburg, R.R.L. Does the guided online cognitive behavioral therapy for insomnia i-“Sleep youth” improve sleep of adolescents and young adults with insomnia after childhood cancer? (MICADO-study): Study protocol of a randomized controlled trial. Trials 2021, 22, 307. [Google Scholar] [CrossRef]
| Intervention | Target Population | Level of Evidence | Setting of Care Delivery | Provider Discipline |
|---|---|---|---|---|
| Sleep hygiene education | Youth with chronic pain | Promising | Tertiary care clinic (e.g., Pain Medicine Clinic, Sleep Clinic), Digital health technology | Psychologist, Behavioral Sleep Specialist, Pain medicine specialist |
| Cognitive-Behavioral Therapy for Insomnia (CBT-I) | Youth with comorbid chronic pain, insomnia, and mental health conditions | Promising | Tertiary care clinic (e.g., Pain Medicine Clinic, Sleep Clinic), Self-guided digital health technology | Psychologist, Behavioral sleep specialist |
| 1. | Integrate screening for sleep disturbances into the assessment of all children and adolescents presenting with chronic pain. |
| 2. | Provide sleep health interventions to target sleep hygiene and insomnia in youth presenting with sleep disturbances. |
| 3. | Where available, use technology to deliver sleep health treatments, e.g., via telehealth and digital health technologies. |
| 4. | Disseminate evidence-based sleep interventions. |
| 5. | Before considering approaches to dissemination and implementation, further work is needed to understand safety and efficacy of CBT-I and Hybrid CBT-I/CBT-Pain interventions for youth with chronic pain via controlled trials. |
| 1. | Comprehensively characterize the impact of pain treatments on sleep health in youth with chronic pain. |
| 2. | Evaluate the safety and efficacy of CBT-I and Hybrid CBT-I/CBT-Pain interventions for youth with chronic pain and co-occurring sleep disturbances. |
| 3. | Conduct research to understand optimal sequencing of pain and sleep interventions, in particular to understand whether children and adolescents may benefit synergistically from improvements in sleep prior to beginning pain self-management interventions. |
| 4. | Conduct longitudinal studies to identify the causal relationship between sleep health and chronic pain over time to uncover mechanisms and identify key vulnerability periods. |
| 5. | Characterize resiliency in sleep health and how this can be enhanced among youth with chronic pain. |
| 6. | Understand sociodemographic influences on sleep health among youth with chronic pain. |
| 7. | Understand how sleep health influences motivation and self-efficacy among youth with chronic pain. |
| 8. | Identify shared biopsychosocial mechanisms that underlie treatment benefits of pain and sleep interventions for individuals with co-occurring conditions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, E.F.; Kim, A.; Ickmans, K.; Palermo, T.M. Sleep Health Assessment and Treatment in Children and Adolescents with Chronic Pain: State of the Art and Future Directions. J. Clin. Med. 2022, 11, 1491. https://doi.org/10.3390/jcm11061491
Law EF, Kim A, Ickmans K, Palermo TM. Sleep Health Assessment and Treatment in Children and Adolescents with Chronic Pain: State of the Art and Future Directions. Journal of Clinical Medicine. 2022; 11(6):1491. https://doi.org/10.3390/jcm11061491
Chicago/Turabian StyleLaw, Emily F., Agnes Kim, Kelly Ickmans, and Tonya M. Palermo. 2022. "Sleep Health Assessment and Treatment in Children and Adolescents with Chronic Pain: State of the Art and Future Directions" Journal of Clinical Medicine 11, no. 6: 1491. https://doi.org/10.3390/jcm11061491
APA StyleLaw, E. F., Kim, A., Ickmans, K., & Palermo, T. M. (2022). Sleep Health Assessment and Treatment in Children and Adolescents with Chronic Pain: State of the Art and Future Directions. Journal of Clinical Medicine, 11(6), 1491. https://doi.org/10.3390/jcm11061491






