A Bayesian Network Meta-Analysis of First-Line Treatments for Non-Small Cell Lung Cancer with High Programmed Death Ligand-1 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Searching Strategy
2.2. Selection Criteria
2.3. Definition of High PD-L1 Expression
2.4. Data Extraction
2.5. Data Analysis
3. Results
3.1. Literature Search and Study Characteristics
3.2. Characteristics of the Included Studies
3.3. Network Analysis Diagrams
3.4. Risk of Bias Assessment
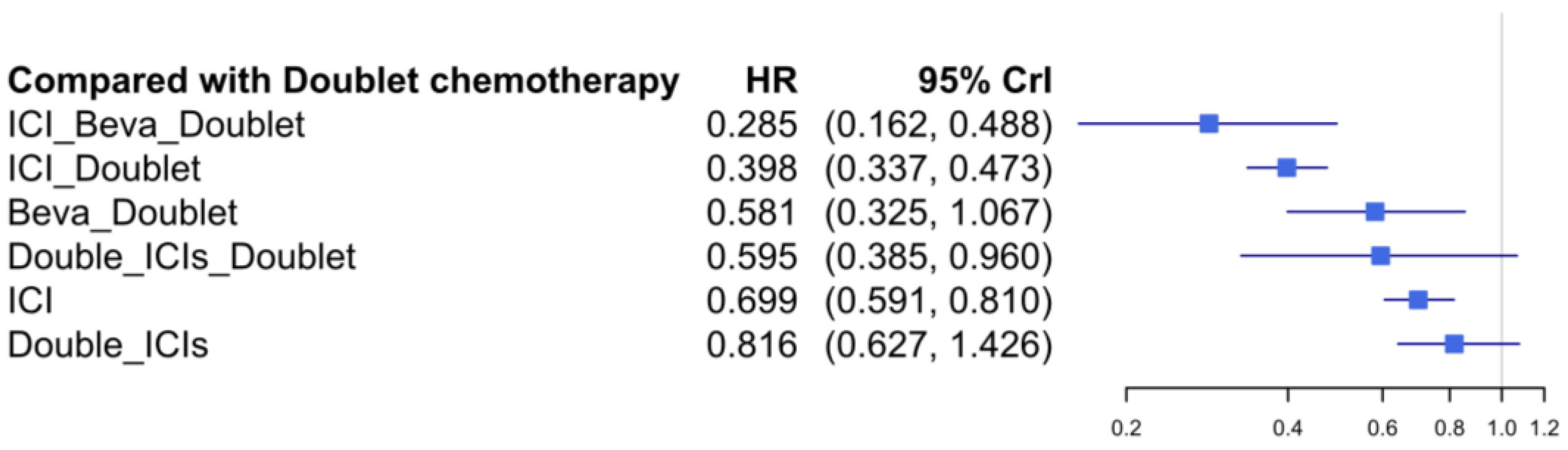
3.5. Progression-Free Survival
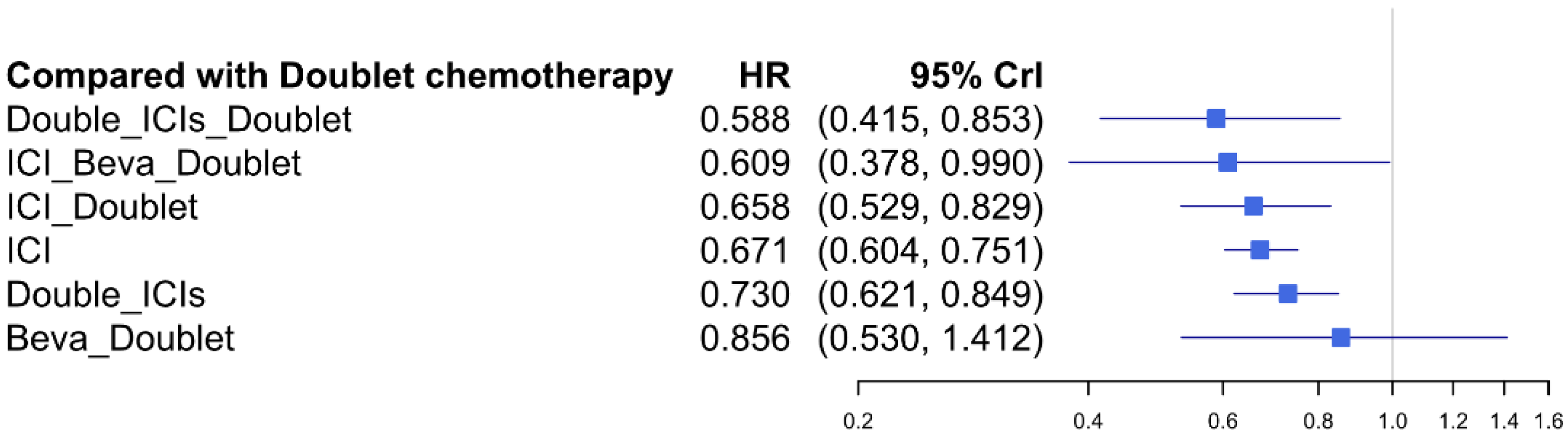
3.6. Overall Survival
3.7. Safety Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: A review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M. Immune checkpoint inhibitors for nonsmall cell lung cancer treatment. J. Chin. Med. Assoc. 2017, 80, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, R.; Jassem, J.; Abogunrin, S.; James, D.; McCool, R.; Belleli, R.; Giaccone, G.; De Marinis, F. A Network Meta-Analysis of Cancer Immunotherapies Versus Chemotherapy for First-Line Treatment of Patients With Non-Small Cell Lung Cancer and High Programmed Death-Ligand 1 Expression. Front. Oncol. 2021, 11, 676732. [Google Scholar] [CrossRef]
- Korman, A.J.; Peggs, K.S.; Allison, J.P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 2006, 90, 297–339. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodriguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazieres, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodriguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients with Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Awad, M.M.; Gadgeel, S.M.; Borghaei, H.; Patnaik, A.; Yang, J.C.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Altan, M.; et al. Long-Term Overall Survival From KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin With or Without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 162–168. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodriguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Barlesi, F.; West, H.; Ball, S.; Bordoni, R.; Cobo, M.; Longeras, P.D.; Goldschmidt, J., Jr.; Novello, S.; Orlandi, F.; et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J. Thorac. Oncol. 2021, 16, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; Hu, Y.; et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir. Med. 2021, 9, 305–314. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gumus, M.; Bondarenko, I.; Ozguroglu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab With or Without Tremelimumab vs. Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, S.; Lee, J.S.; Kang, J.H.; Kim, H.R.; Inui, N.; Hida, T.; Lee, K.H.; Yoshida, T.; Tanaka, H.; Yang, C.T.; et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 2021, 32, 1137–1147. [Google Scholar] [CrossRef]
- Rodriguez-Abreu, D.; Powell, S.F.; Hochmair, M.J.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; De Angelis, F.; Domine, M.; Cheng, S.Y.; et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 2021, 32, 881–895. [Google Scholar] [CrossRef]
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Wang, Z.; Fang, J.; Yu, Q.; Han, B.; Cang, S.; Chen, G.; Mei, X.; Yang, Z.; et al. Updated Overall Survival Data and Predictive Biomarkers of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC in the Phase 3 ORIENT-11 Study. J. Thorac. Oncol. 2021, 16, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Yu, X.; Hu, Y.; Sun, Y.; Wang, Z.; Zhao, J.; Yu, Y.; Hu, C.; Yang, K.; et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Yu, Y.; Yu, X.; Hu, Y.; Ai, X.; Ma, Z.; Li, X.; Zhuang, W.; Liu, Y.; et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J. Thorac. Oncol. 2021, 16, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, L.; Fan, Y.; Wang, Z.; Liu, L.; Chen, G.; Zhang, L.; Huang, D.; Cang, S.; Yang, Z.; et al. Sintilimab Plus Platinum and Gemcitabine as First-Line Treatment for Advanced or Metastatic Squamous NSCLC: Results From a Randomized, Double-Blind, Phase 3 Trial (ORIENT-12). J. Thorac. Oncol. 2021, 16, 1501–1511. [Google Scholar] [CrossRef]
- Liu, L.; Bai, H.; Wang, C.; Seery, S.; Wang, Z.; Duan, J.; Li, S.; Xue, P.; Wang, G.; Sun, Y.; et al. Efficacy and Safety of First-Line Immunotherapy Combinations for Advanced NSCLC: A Systematic Review and Network Meta-Analysis. J. Thorac. Oncol. 2021, 16, 1099–1117. [Google Scholar] [CrossRef]
- Li, X.; Yan, S.; Yang, J.; Wang, Y.; Lv, C.; Li, S.; Zhao, J.; Yang, Y.; Zhuo, M.; Wu, N. Efficacy and Safety of PD-1/PD-L1 Inhibitors Plus Chemotherapy Versus PD-1/PD-L1 Inhibitors in Advanced Non-Small Cell Lung Cancer: A Network Analysis of Randomized Controlled Trials. Front. Oncol. 2020, 10, 574752. [Google Scholar] [CrossRef]
- Tonin, F.S.; Rotta, I.; Mendes, A.M.; Pontarolo, R. Network meta-analysis: A technique to gather evidence from direct and indirect comparisons. Pharm. Pract. 2017, 15, 943. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.M.; Jackson, D.; Wei, Y.; Thompson, S.G.; Higgins, J.P. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat. Med. 2015, 34, 984–998. [Google Scholar] [CrossRef] [Green Version]
- Woods, B.S.; Hawkins, N.; Scott, D.A. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: A tutorial. BMC Med. Res. Methodol. 2010, 10, 54. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.P.; Gelman, A. General Methods for Monitoring Convergence of Iterative Simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar] [CrossRef] [Green Version]
- Paz-Ares, L.G.; Ramalingam, S.S.; Ciuleanu, T.E.; Lee, J.S.; Urban, L.; Caro, R.B.; Park, K.; Sakai, H.; Ohe, Y.; Nishio, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J. Thorac. Oncol. 2021, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Laurie, S.A.; Goss, G.D.; Hughes, B.G.M.; Stockler, M.; Tsao, M.S.; Hwang, D.M.; Joubert, P.; Kulkarni, S.; Blais, N.; et al. CCTG BR34: A randomized phase II trial of durvalumab and tremelimumab +/- platinum-based chemotherapy in patients with metastatic non-small cell lung cancer. J. Thorac. Oncol. 2021, 17, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Sendur, M.A.N.; Rodriguez-Abreu, D.; Park, K.; Lee, D.H.; Cicin, I.; Yumuk, P.F.; Orlandi, F.J.; Leal, T.A.; Molinier, O.; et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score >/= 50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J. Clin. Oncol. 2021, 39, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ladoire, S.; Coukos, G.; Ghiringhelli, F. Combining immunotherapy and anticancer agents: The right path to achieve cancer cure? Ann. Oncol. 2015, 26, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Smyth, M.J.; Kroemer, G. Mechanism of action of conventional and targeted anticancer therapies: Reinstating immunosurveillance. Immunity 2013, 39, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ando, K.; Kishino, Y.; Homma, T.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; Ohnishi, T.; Sagara, H. Nivolumab plus ipilimumab versus existing immunotherapies in patients with PD-1-positive advanced non-small cell lung cancer: A systematic review and network meta-analysis. Cancers 2020, 12, 1905. [Google Scholar] [CrossRef]


| Study [Ref] | Sample Size | Histology | * PD-L1 Status: n (%) | Intervention Arm | Control Arm | OS | PFS |
|---|---|---|---|---|---|---|---|
| KEYNOTE-024 [13] | 305 | NSCLC | ≥50%: 305 (100) | Pembrolizumab | Doublet chemotherapy | 0.62 (0.48–0.81) | 0.50 (0.39–0.65) |
| KEYNOTE-042 [15] | 1274 | NSCLC | ≥50%: 599 (47) | Pembrolizumab | Doublet chemotherapy | 0.68 (0.57–0.82) | 0.85 (0.72–1.02) |
| KEYNOTE-189 [29] | 616 | Nonsquamous | ≥50%: 202 (33) | Pembrolizumab + Doublet chemotherapy | Doublet chemotherapy | 0.59 (0.40–0.86) | 0.35 (0.25–0.49) |
| KEYNOTE-407 [16] | 559 | Squamous | ≥50%: 146 (26) | Pembrolizumab + Doublet chemotherapy | Doublet chemotherapy | 0.79 (0.52–1.21) | 0.37 (0.24–0.58) |
| KEYNOTE-598 [43] | 568 | NSCLC | ≥50%: 568 (100) | Pembrolizumab + ipilimumab | Pembrolizumab | 1.08 (0.85–1.37) | 1.06 (0.86–1.30) |
| IMpower110 [21] | 554 | NSCLC | TC3 or IC3: 205 (37) | Atezolizumab | Doublet chemotherapy | 0.59 (0.40–0.89) | 0.63 (0.45–0.88) |
| IMpower130 [19] | 724 | Nonsquamous | TC3 or IC3: 134 (19) | Atezolizumab + Doublet chemotherapy | Doublet chemotherapy | 0.84 (0.51–1.39) | 0.51 (0.34–0.77) |
| IMpower131 [20] | 1021 | Squamous | TC3 or IC3: 154 (15) | Atezolizumab + Doublet chemotherapy | Doublet chemotherapy | 0.48 (0.29–0.81) | 0.41 (0.25–0.68) |
| IMpower132 [22] | 578 | Nonsquamous | TC3 or IC3: 45 (8) | Atezolizumab + Doublet chemotherapy | Doublet chemotherapy | 0.73 (0.31–1.73) | 0.46 (0.22–0.96) |
| IMpower150 [30] | 1047 | Nonsquamous | ≥50%: 206 (24) | 1. Atezolizumab + Bevacizumab + Doublet chemotherapy | Bevacizumab + Doublet chemotherapy | 0.70 (0.46–1.08) | 0.42 (0.28–0.63) |
| 2. Atezolizumab + Doublet chemotherapy | 0.76 (0.49–1.17) | 0.62 (0.3–0.89) | |||||
| CheckMate 026 [17] | 541 | NSCLC | ≥50%: 214 (40) | Nivolumab | Doublet chemotherapy | 0.90 (0.63–1.29) | 1.07 (0.77–1.49) |
| CheckMate 9LA [26] | 719 | NSCLC | ≥50%: 174 (26) | Nivolumab + Ipilimumab + Doublet chemotherapy | Doublet chemotherapy | 0.66 (0.44–0.99) | 0.61 (0.42–0.89) |
| CheckMate 227 [41] | 1189 | NSCLC | ≥50%: 611 (51) | 1. Nivolumab + Ipilimumab 2. Nivolumab | Doublet chemotherapy | 0.70 (0.55–0.90) | - |
| MYSTIC [27] | 1118 | NSCLC | ≥50%: 333 (30) | 1. Durvalumab + Tremelimumab | Doublet chemotherapy | 0.77 (0.56–1.07) | 1.05 (0.72–1.53) |
| 2. Durvalumab | 0.76 (0.55–1.04) | 0.87 (0.59–1.29) | |||||
| CameL [23] | 412 | Nonsquamous | ≥50%: 50 (24) | Camrelizumab + Doublet chemotherapy | Doublet chemotherapy | - | 0.39 (0.14–0.99) |
| CCTG BR 34 [42] | 301 | NSCLC | ≥50%: 57 (19) | Durvalumab + Tremelimumab + Doublet chemotherapy | Durvalumab + Tremelimumab | 0.56 (0.27–1.17) | 0.62 (0.32–1.19) |
| RATIONALE 304 [33] | 334 | Nonsquamous | ≥50%: 110 (33) | Tislelizumab + Doublet chemotherapy | Doublet chemotherapy | - | 0.308 (0.167–0.567) |
| RATIONALE 307 [32] | 360 | Squamous | ≥50%: 125 (35 | Tislelizumab + Doublet chemotherapy | Doublet chemotherapy | - | 0.46 (0.31–0.70) |
| ORIENT-11 [31] | 397 | Nonsquamous | ≥50%: 168 (42) | Sintilimab + Doublet chemotherapy | Doublet chemotherapy | - | 0.310 (0.197–0.489) |
| ORIENT-12 [34] | 357 | Squamous | ≥50%: 121 (34) | Sintilimab + Doublet chemotherapy | Doublet chemotherapy | - | 0.458 (0.302–0.695) |
| EMPOWER-Lung 1 [24] | 710 | NSCLC | ≥50%: 563 (79) | Cemiplimab | Doublet chemotherapy | 0.57 (0.42–0.77) | 0.54 (0.43–0.68) |
| TASUKI-52 [28] | 550 | Nonsquamous | ≥50%: 147 (27) | Nivolumab + Bevacizumab + Doublet chemotherapy | Bevacizumab + Doublet chemotherapy | - | 0.55 (0.36–0.83) |
| PFS | |||||||
|---|---|---|---|---|---|---|---|
| 0.285 (0.163, 0.493) | 0.398 (0.336, 0.473) | 0.581 (0.399, 0.854) | 0.595 (0.327, 1.068) | 0.699 (0.605, 0.815) | 0.816 (0.641, 1.078) | SUCRA 1.8% Doublet | |
| 0.347 (0.188, 0.632) | 0.487 (0.352, 0.652) | 0.712 (0.465, 1.059) | 0.725 (0.379, 1.367) | 0.856 (0.665, 1.073) | SUCRA 20.2% Double_ICIs | SUCRA 79.1% Double_ICIs_Doublet | |
| 0.407 (0.229, 0.716) | 0.571 (0.454, 0.709) | 0.832 (0.561, 1.235) | 0.850 (0.462, 1.546) | SUCRA 39.7% ICI | SUCRA 73.4% ICI_Beva_Doublet | 0.970 (0.533, 1.748) | |
| 0.478 (0.339, 0.678) | 0.671 (0.382, 1.19) | 0.977 (0.492, 2.002) | SUCRA 51.7% Beva_Doublet | SUCRA 64.9% ICI_Doublet | 0.923 (0.61, 1.442) | 0.892 (0.598, 1.385) | |
| 0.489 (0.249, 0.949) | 0.686 (0.45, 1.032) | SUCRA 55.7% Double_ICIs_Doublet | SUCRA 61.8% ICI | 0.978 (0.771, 1.259) | 0.907 (0.555, 1.502) | 0.877 (0.61, 1.294) | |
| 0.714 (0.423, 1.203) | SUCRA 82.9% ICI_Doublet | SUCRA 41.7% Double_ICIs | 0.920 (0.793, 1.089) | 0.902 (0.691, 1.201) | 0.840 (0.51, 1.381) | 0.808 (0.564, 1.188) | |
| SUCRA 98.1% ICI_Beva_Doublet | SUCRA 24.2% Beva_Doublet | 0.849 (0.493, 1.427) | 0.783 (0.466, 1.28) | 0.768 (0.487, 1.172) | 0.709 (0.455, 1.118) | 0.694 (0.372, 1.245) | |
| SUCRA 4.9% Doublet | 0.856 (0.53, 1.412) | 0.730 (0.621, 0.849) | 0.671 (0.604, 0.751) | 0.658 (0.529, 0.829) | 0.609 (0.378, 0.99) | 0.588 (0.415, 0.853) | |
| OS | |||||||
| AE of Grade 3–5 | |||||||
|---|---|---|---|---|---|---|---|
| 0.156 (0.092, 0.265) | 0.32 (0.181, 0.558) | 0.48 (0.294, 0.783) | 0.669 (0.351, 1.246) | 0.715 (0.452, 1.126) | 0.816 (0.572, 1.167) | ICI_Beva_Doublet SUCRA 4.8% | |
| 0.191 (0.112, 0.321) | 0.391 (0.222, 0.672) | 0.588 (0.36, 0.951) | 0.817 (0.429, 1.531) | 0.877 (0.552, 1.374) | Beva_Doublet SUCRA 23.5% | ICI SUCRA 99.7% | |
| 0.218 (0.167, 0.284) | 0.448 (0.322, 0.613) | 0.672 (0.564, 0.8) | 0.932 (0.598, 1.439) | ICI_Doublet SUCRA 34.0% | Double_ICIs SUCRA 83.0% | 0.544 (0.313, 0.919) | |
| 0.234 (0.152, 0.363) | 0.48 (0.312, 0.732) | 0.72 (0.484, 1.084) | Double_ICIs_Doublet SUCRA 39.0% | Doublet SUCRA 62.40% | 0.465 (0.286, 0.797) | 0.253 (0.18, 0.372) | |
| 0.325 (0.266, 0.397) | 0.667 (0.508, 0.862) | Doublet SUCRA 65.5% | Double_ICIs_Doublet SUCRA 46.0% | 0.676 (0.285, 1.735) | 0.317 (0.12, 0.918) | 0.171 (0.068, 0.471) | |
| 0.487 (0.369, 0.652) | Double_ICIs SUCRA 83.2% | ICI_Doublet SUCRA 28.5% | 0.637 (0.218, 1.755) | 0.432 (0.251, 0.728) | 0.202 (0.096, 0.423) | 0.11 (0.058, 0.208) | |
| ICI SUCRA 100% | ICI_Beva_Doublet SUCRA 27.3% | 0.865 (0.256, 2.972) | 0.547 (0.109, 2.719) | 0.37 (0.098, 1.435) | 0.173 (0.042, 0.755) | 0.094 (0.024, 0.382) | |
| Beva_Doublet SUCRA 3.5% | 0.414 (0.108, 1.386) | 0.354 (0.085, 1.403) | 0.223 (0.037, 1.208) | 0.152 (0.033, 0.663) | 0.071 (0.014, 0.345) | 0.039 (0.008, 0.177) | |
| AE of All Grades | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Jeong, S.Y.; Lee, J.-J.; Park, S.T.; Kim, H.S. A Bayesian Network Meta-Analysis of First-Line Treatments for Non-Small Cell Lung Cancer with High Programmed Death Ligand-1 Expression. J. Clin. Med. 2022, 11, 1492. https://doi.org/10.3390/jcm11061492
Kim JH, Jeong SY, Lee J-J, Park ST, Kim HS. A Bayesian Network Meta-Analysis of First-Line Treatments for Non-Small Cell Lung Cancer with High Programmed Death Ligand-1 Expression. Journal of Clinical Medicine. 2022; 11(6):1492. https://doi.org/10.3390/jcm11061492
Chicago/Turabian StyleKim, Jung Han, Soo Young Jeong, Jae-Jun Lee, Sung Taek Park, and Hyeong Su Kim. 2022. "A Bayesian Network Meta-Analysis of First-Line Treatments for Non-Small Cell Lung Cancer with High Programmed Death Ligand-1 Expression" Journal of Clinical Medicine 11, no. 6: 1492. https://doi.org/10.3390/jcm11061492
APA StyleKim, J. H., Jeong, S. Y., Lee, J.-J., Park, S. T., & Kim, H. S. (2022). A Bayesian Network Meta-Analysis of First-Line Treatments for Non-Small Cell Lung Cancer with High Programmed Death Ligand-1 Expression. Journal of Clinical Medicine, 11(6), 1492. https://doi.org/10.3390/jcm11061492






