Postoperative Thromboembolism According to the Type of Surgery: A Nationwide Study in the Republic of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patients
2.3. Clinical Endpoints
2.4. Statistical Analysis
3. Results
3.1. Incidence of TE within 6 Months from the Date of Surgery
3.2. Proportion of Patients Who Underwent Prophylactic Anticoagulant Treatment and Its Effect on the Incidence of TE Events
3.3. Type of TE within 6 Months from the Date of Surgery
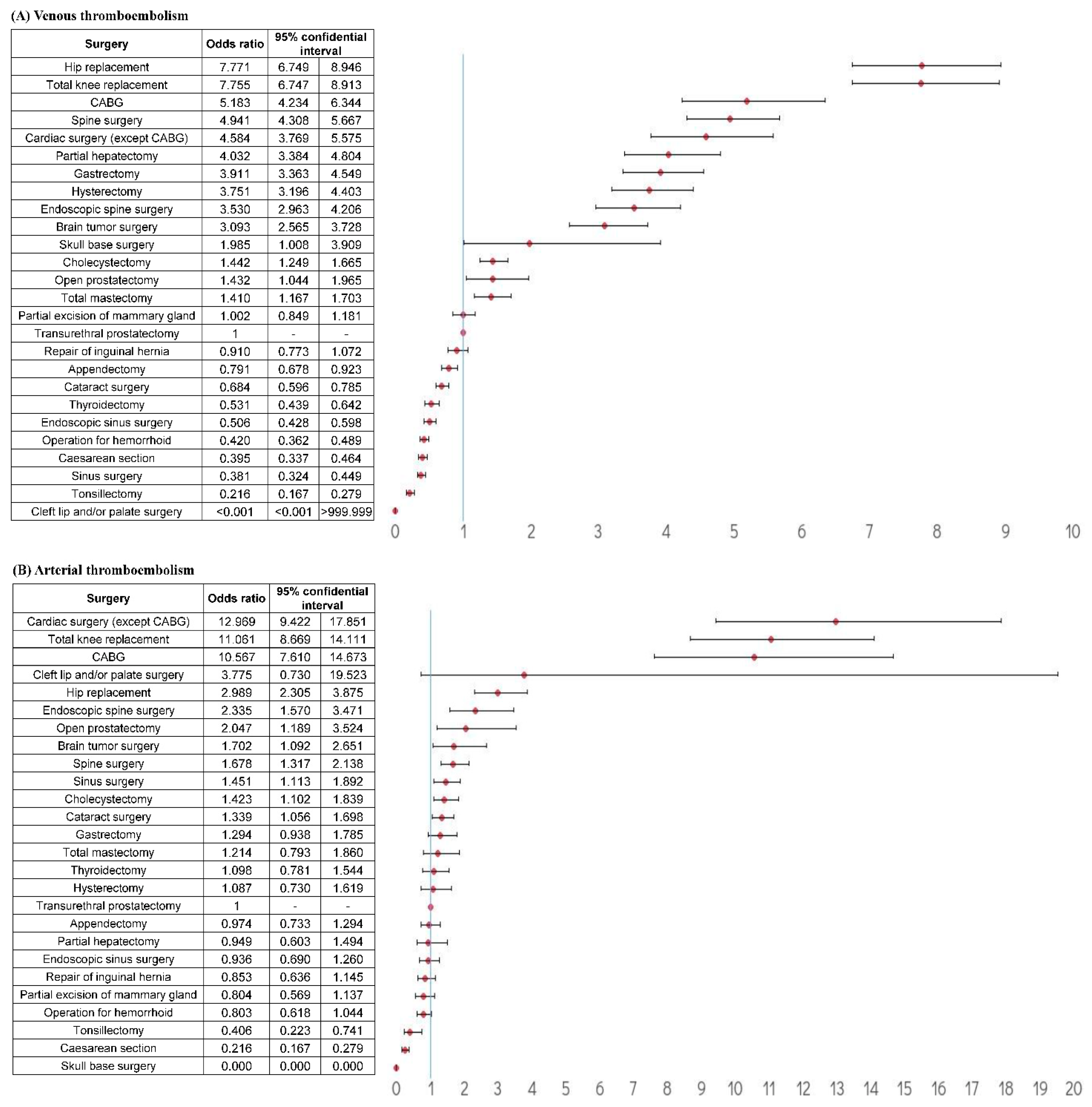
3.4. Relative risk of TE According to Surgery Type
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jerjes-Sanchez, C. Venous and arterial thrombosis: A continuous spectrum of the same disease? Eur. Heart J. 2004, 26, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, L.; Minami, C.; Yang, A. Preventing venous thromboembolism after surgery. JAMA 2016, 315, 2136. [Google Scholar] [CrossRef] [PubMed]
- D’Astous, J.; Liederman, Z.; Douketis, J.D. Venous thromboembolism prophylaxis in high-risk orthopedic and cancer surgery. Postgrad. Med. 2021, 133, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Douketis, J.D. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012, 120, 2954–2962. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef]
- Kyrle, P.A.; Eichinger, S. Deep vein thrombosis. Lancet 2005, 365, 1163–1174. [Google Scholar] [CrossRef]
- van Veen, J.J.; Makris, M. Management of peri-operative anti-thrombotic therapy. Anaesthesia 2015, 70, 58-e23. [Google Scholar] [CrossRef]
- Falck-Ytter, Y.; Francis, C.W.; Johanson, N.A.; Curley, C.; Dahl, O.E.; Schulman, S.; Ortel, T.L.; Pauker, S.G.; Colwell, C.W., Jr. Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST 2012, 141, e278S–e325S. [Google Scholar] [CrossRef]
- Gee, E. The National VTE Exemplar Centres Network response to implementation of updated NICE guidance: Venous thromboembolism in over 16s: Reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism (NG89). Br. J. Haematol. 2019, 186, 792–793. [Google Scholar] [CrossRef]
- Cronin, M.; Dengler, N.; Krauss, E.S.; Segal, A.; Wei, N.; Daly, M.; Mota, F.; Caprini, J.A. Completion of the Updated Caprini Risk Assessment Model (2013 Version). Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619838052. [Google Scholar] [CrossRef]
- Rogers, S.O., Jr.; Kilaru, R.K.; Hosokawa, P.; Henderson, W.G.; Zinner, M.J.; Khuri, S.F. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: Results from the patient safety in surgery study. J. Am. Coll. Surg. 2007, 204, 1211–1221. [Google Scholar] [CrossRef]
- Afshari, A.; Ageno, W.; Ahmed, A.; Duranteau, J.; Faraoni, D.; Kozek-Langenecker, S.; Llau, J.; Nizard, J.; Solca, M.; Stensballe, J.; et al. European guidelines on perioperative venous thromboembolism prophylaxis: Executive summary. Eur. J. Anaesthesiol. 2018, 35, 77–83. [Google Scholar] [CrossRef]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST 2012, 141, e227S–e277S. [Google Scholar] [CrossRef]
- Caprini, J.A. Thrombosis risk assessment as a guide to quality patient care. Dis. Mon. 2005, 51, 70–78. [Google Scholar] [CrossRef]
- Bahl, V.; Hu, H.M.; Henke, P.K.; Wakefield, T.W.; Campbell, D.A., Jr.; Caprini, J.A. A validation study of a retrospective venous thromboembolism risk scoring method. Ann. Surg. 2010, 251, 344–350. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data resource profile: The national health information database of the national health insurance service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef]
- Geerts, W.H.; Bergqvist, D.; Pineo, G.F.; Heit, J.A.; Samama, C.M.; Lassen, M.R.; Colwell, C.W. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). CHEST 2008, 133, 381s–453s. [Google Scholar] [CrossRef]
- Geerts, W.H.; Pineo, G.F.; Heit, J.A.; Bergqvist, D.; Lassen, M.R.; Colwell, C.W.; Ray, J.G. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. CHEST 2004, 126, 338s–400s. [Google Scholar] [CrossRef]
- Kepler, C.K.; McKenzie, J.; Kreitz, T.; Vaccaro, A. Venous thromboembolism prophylaxis in spine surgery. J. Am. Acad. Orthop. Surg. 2018, 26, 489–500. [Google Scholar] [CrossRef]
- Liew, N.C.; Gul, Y.; Moissinac, K. Postoperative venous thromboembolism in Asia: A critical appraisal of its incidence. Asian J. Surg. 2003, 26, 154–158. [Google Scholar] [CrossRef]
- Do, J.G.; Kim, D.H.; Sung, D.H. Incidence of deep vein thrombosis after spinal cord injury in Korean patients at acute rehabilitation unit. J. Korean Med. Sci. 2013, 28, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Leizorovicz, A.; Turpie, A.G.; Cohen, A.T.; Wong, L.; Yoo, M.C.; Dans, A. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J. Thromb. Haemost. 2005, 3, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.B.; Weaver, K.J.; Neal, D.W.; Jacob, R.P.; Hoh, D.J. Decreased incidence of venous thromboembolism after spine surgery with early multimodal prophylaxis: Clinical article. J. Neurosurg. Spine 2014, 21, 677–684. [Google Scholar] [CrossRef]
- Jung, Y.J.; Seo, H.S.; Park, C.H.; Jeon, H.M.; Kim, J.I.; Yim, H.W.; Song, K.Y. Venous thromboembolism incidence and prophylaxis use after gastrectomy among Korean patients with gastric adenocarcinoma: The PROTECTOR randomized clinical trial. JAMA Surg. 2018, 153, 939–946. [Google Scholar] [CrossRef]
- Emoto, S.; Nozawa, H.; Kawai, K.; Hata, K.; Tanaka, T.; Shuno, Y.; Nishikawa, T.; Sasaki, K.; Kaneko, M.; Hiyoshi, M.; et al. Venous thromboembolism in colorectal surgery: Incidence, risk factors, and prophylaxis. Asian J. Surg. 2019, 42, 863–873. [Google Scholar] [CrossRef]
- Manoucheri, R.; Fallahi, M.J. Adherence to venous thromboprophylaxis guidelines for medical and surgical inpatients of teaching hospitals, Shiraz-Iran. Tanaffos 2015, 14, 17–26. [Google Scholar]
- Geahchan, N.; Basile, M.; Tohmeh, M.; on behalf of the DIONYS registry. Venous thromboembolism prophylaxis in patients undergoing abdominal and pelvic cancer surgery: Adherence and compliance to ACCP guidelines in DIONYS registry. SpringerPlus 2016, 5, 1541. [Google Scholar] [CrossRef]
- Galante, M.; Languasco, A.; Gotta, D.; Bell, S.; Lancelotti, T.; Knaze, V.; Saubidet, C.L.; Grand, B.; Milberg, M. Venous thromboprophylaxis in general surgery ward admissions: Strategies for improvement. Int. J. Qual. Health Care 2012, 24, 649–656. [Google Scholar] [CrossRef][Green Version]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- Kanchanabat, B.; Stapanavatr, W.; Meknavin, S.; Soorapanth, C.; Sumanasrethakul, C.; Kanchanasuttirak, P. Systematic review and meta-analysis on the rate of postoperative venous thromboembolism in orthopaedic surgery in Asian patients without thromboprophylaxis. Br. J. Surg. 2011, 98, 1356–1364. [Google Scholar] [CrossRef]
- Yhim, H.Y.; Jang, M.J.; Bang, S.M.; Kim, K.H.; Kim, Y.K.; Nam, S.H.; Bae, S.H.; Kim, S.H.; Mun, Y.C.; Kim, I.; et al. Incidence of venous thromboembolism following major surgery in Korea: From the Health Insurance Review and Assessment Service database. J. Thromb. Haemost. 2014, 12, 1035–1043. [Google Scholar] [CrossRef]
- Lewis-Lloyd, C.A.; Pettitt, E.M.; Adiamah, A.; Crooks, C.J.; Humes, D.J. Risk of postoperative venous thromboembolism after surgery for colorectal malignancy: A systematic review and meta-analysis. Dis. Colon Rectum 2021, 64, 484–496. [Google Scholar] [CrossRef]
- Moubayed, S.P.; Eskander, A.; Mourad, M.W.; Most, S.P. Systematic review and meta-analysis of venous thromboembolism in otolaryngology-head and neck surgery. Head Neck 2017, 39, 1249–1258. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, W.; Cheng, X.; Fang, K.; Zhang, X.; Gu, Y.; Leng, B.; Dong, Q. Risk factors and stroke characteristic in patients with postoperative strokes. J. Stroke Cerebrovasc. Dis. 2017, 26, 1635–1640. [Google Scholar] [CrossRef]
- Blacker, D.J. In-hospital stroke. Lancet Neurol. 2003, 2, 741–746. [Google Scholar] [CrossRef]
- Sellers, D.; Srinivas, C.; Djaiani, G. Cardiovascular complications after non-cardiac surgery. Anaesthesia 2018, 73 (Suppl. S1), 34–42. [Google Scholar] [CrossRef]
- Sahara, K.; Ishibe, A.; Yabuno, T.; Kondo, H.; Nakayama, G.; Yasuda, S.; Nishida, T.; Watanabe, J.; Uranaka, Y.; Akiyama, H.; et al. Acute iliac arterial thrombosis during laparoscopic abdominoperineal resection. J. Surg. Case Rep. 2019, 2019, rjz020. [Google Scholar] [CrossRef]
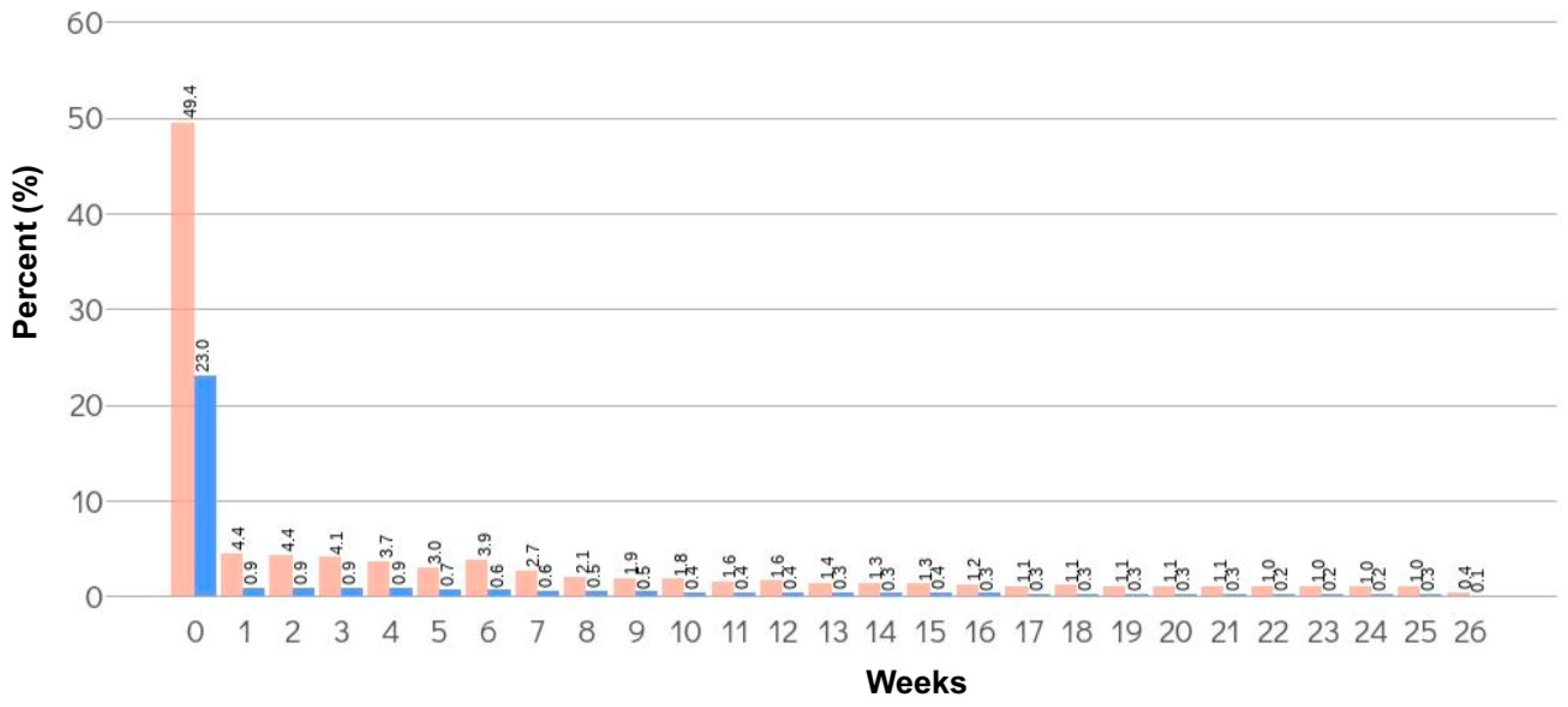

| Surgery | Total Patients, n | Thromboembolism within 6 Months from the Date of Surgery | ||
|---|---|---|---|---|
| A: Total Claimed Thromboembolism, n (%) | B: Thromboembolism with Anticoagulant Treatment, n (%) | B/A (%) * | ||
| Total knee replacement | 108,111 | 13,811 (12.77) | 7995 (7.40) | 57.89 |
| Hip replacement | 43,415 | 4975 (11.46) | 3124 (7.20) | 62.79 |
| Spine surgery | 265,317 | 15,857 (5.98) | 3336 (1.26) | 21.04 |
| Coronary artery bypass graft | 6591 | 343 (5.20) | 251 (3.81) | 73.18 |
| Partial hepatectomy | 9322 | 429 (4.60) | 331 (3.55) | 77.16 |
| Cardiac surgery (except coronary artery bypass graft) | 10,296 | 443 (4.30) | 386 (3.75) | 87.13 |
| Gastrectomy | 27,203 | 1114 (4.10) | 302 (1.11) | 27.11 |
| Hysterectomy | 20,831 | 714 (3.43) | 540 (2.59) | 75.63 |
| Endoscopic spine surgery | 12,157 | 392 (3.22) | 23 (0.19) | 5.87 |
| Brain tumor surgery | 11,516 | 302 (2.62) | 173 (1.50) | 57.28 |
| Open prostatectomy | 3387 | 81 (2.39) | 23 (0.68) | 28.40 |
| Cholecystectomy | 129,081 | 2511 (1.95) | 828 (0.64) | 32.97 |
| Transurethral prostatectomy | 18,547 | 342 (1.84) | 85 (0.46) | 24.85 |
| Skull base surgery | 674 | 10 (1.48) | 4 (0.59) | 40.00 |
| Cataract surgery | 907,397 | 12,819 (1.41) | 2489 (0.27) | 19.42 |
| Total mastectomy | 22,091 | 280 (1.27) | 98 (0.44) | 35.00 |
| Repair of inguinal hernia | 56,698 | 671 (1.18) | 153 (0.27) | 22.80 |
| Partial excision of mammary gland | 63,033 | 586 (0.93) | 130 (0.21) | 22.18 |
| Appendectomy | 137,344 | 1024 (0.75) | 263 (0.19) | 25.68 |
| Thyroidectomy | 51,506 | 351 (0.68) | 80 (0.16) | 22.79 |
| Sinus surgery | 158,677 | 867 (0.55) | 82 (0.05) | 9.46 |
| Endoscopic sinus surgery | 109,454 | 578 (0.53) | 115 (0.11) | 19.90 |
| Operation for hemorrhoid | 303,456 | 1401 (0.46) | 158 (0.05) | 11.28 |
| Cesarean section | 255,600 | 684 (0.27) | 84 (0.03) | 12.28 |
| Cleft lip and/or palate surgery | 1282 | 2 (0.16) | 1 (0.08) | 50.00 |
| Tonsillectomy | 66,307 | 96 (0.14) | 9 (0.01) | 9.38 |
| Total | 2,799,293 | 60,683 (2.17) | 21,063 (0.75) | 34.71 |
| Surgery | Total Patients, n | Patients Who Underwent Prophylactic Anticoagulant Treatment, n (%) | Total Claimed Thromboembolism, n (%) | Thromboembolism with Anticoagulant Treatment, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| With Prophylactic Anticoagulant Treatment | Without Prophylactic Anticoagulant Treatment | p Value | With Prophylactic Anticoagulant Treatment | Without Prophylactic Anticoagulant Treatment | p Value | |||
| Total knee replacement | 108,111 | 56,887 (52.62) | 1717 (1.59) | 12,094 (11.19) | <0.001 | 551 (0.51) | 7444 (6.89) | <0.001 |
| Hip replacement | 43,415 | 17,876 (41.17) | 684 (1.58) | 4291 (9.88) | <0.001 | 350 (0.81) | 2774 (6.39) | <0.001 |
| Spine surgery | 265,317 | 24,029 (9.06) | 1131 (0.43) | 14,726 (5.55) | <0.001 | 551 (0.21) | 2785 (1.05) | <0.001 |
| Coronary artery bypass graft | 6591 | 6378 (96.77) | 162 (2.46) | 181 (2.75) | 0.305 | 70 (1.06) | 181 (2.75) | <0.001 |
| Partial hepatectomy | 9322 | 3801 (40.77) | 134 (1.44) | 295 (3.16) | <0.001 | 94 (1.01) | 237 (2.54) | <0.001 |
| Cardiac surgery (except coronary artery bypass graft) | 10,296 | 9799 (95.17) | 216 (2.10) | 227 (2.20) | 0.601 | 161 (1.56) | 225 (2.19) | 0.001 |
| Gastrectomy | 27,203 | 7123 (26.18) | 164 (0.60) | 950 (3.49) | <0.001 | 79 (0.29) | 223 (0.82) | <0.001 |
| Hysterectomy | 20,831 | 3949 (18.96) | 178 (0.85) | 536 (2.57) | <0.001 | 126 (0.60) | 414 (1.99) | <0.001 |
| Endoscopic spine surgery | 12,157 | 86 (0.71) | 3 (0.02) | 389 (3.20) | <0.001 | 1 (0.01) | 22 (0.18) | <0.001 |
| Brain tumor surgery | 11,516 | 4888 (42.45) | 126 (1.09) | 176 (1.53) | 0.004 | 75 (0.65) | 98 (0.85) | 0.0804 |
| Open prostatectomy | 3387 | 1708 (50.43) | 44 (1.30) | 37 (1.09) | 0.437 | 9 (0.27) | 14 (0.41) | 0.2971 |
| Cholecystectomy | 129,081 | 10,164 (7.87) | 356 (0.28) | 2155 (1.67) | <0.001 | 202 (0.16) | 626 (0.48) | <0.001 |
| Transurethral prostatectomy | 18,547 | 678 (3.66) | 41 (0.22) | 301 (1.62) | <0.001 | 24 (0.13) | 61 (0.33) | <0.001 |
| Skull base surgery | 674 | 258 (38.28) | 6 (0.89) | 4 (0.59) | 0.527 | 2 (0.30) | 2 (0.30) | 1.000 |
| Cataract surgery | 907,397 | 2432 (0.27) | 760 (0.08) | 12,059 (1.33) | <0.001 | 460 (0.05) | 2029 (0.22) | <0.001 |
| Total mastectomy | 22,091 | 2278 (10.31) | 66 (0.30) | 214 (0.97) | <0.001 | 33 (0.15) | 65 (0.29) | 0.001 |
| Repair of inguinal hernia | 56,698 | 559 (0.99) | 54 (0.10) | 617 (1.09) | <0.001 | 25 (0.04) | 128 (0.23) | <0.001 |
| Partial excision of mammary gland | 63,033 | 1341 (2.13) | 68 (0.11) | 518 (0.82) | <0.001 | 37 (0.06) | 93 (0.15) | <0.001 |
| Appendectomy | 137,344 | 1291 (0.94) | 88 (0.06) | 936 (0.68) | <0.001 | 58 (0.04) | 205 (0.15) | <0.001 |
| Thyroidectomy | 51,506 | 1372 (2.66) | 40 (0.08) | 311 (0.60) | <0.001 | 22 (0.04) | 58 (0.11) | <0.001 |
| Sinus surgery | 158,677 | 275 (0.17) | 39 (0.02) | 828 (0.52) | <0.001 | 17 (0.01) | 98 (0.06) | <0.001 |
| Endoscopic sinus surgery | 109,454 | 801 (0.73) | 32 (0.03) | 546 (0.50) | <0.001 | 14 (0.01) | 68 (0.06) | <0.001 |
| Operation for hemorrhoid | 303,456 | 179 (0.06) | 34 (0.01) | 1367 (0.45) | <0.001 | 18 (0.01) | 140 (0.05) | <0.001 |
| Cesarean section | 255,600 | 690 (0.27) | 16 (0.01) | 668 (0.26) | <0.001 | 10 (0.003) | 74 (0.03) | <0.001 |
| Cleft lip and/or palate surgery | 1282 | 5 (0.39) | - | 2 (0.16) | - | 0 (0.00) | 1 (0.08) | - |
| Tonsillectomy | 66,307 | 140 (0.21) | 7 (0.01) | 89 (0.13) | <0.001 | 4 (0.01) | 5 (0.01) | 0.739 |
| Total | 2,799,293 | 158,987 (5.68) | 6166 (0.22) | 4517 (1.95) | <0.001 | 2993 (0.11) | 18,070 (0.65) | <0.001 |
| Type of TE | No. | Details | Total Claimed TE (n = 60,683) | TE with Anticoagulant Treatment (n = 21,603) | ||
|---|---|---|---|---|---|---|
| Number of Patients, n | Events (%) | Number of Patients, n | Events (%) | |||
| Venous TE | 1 | I26 Pulmonary embolism | 2 | 0.003% | 2 | 0.009% |
| 2 | I80 Phlebitis and thrombophlebitis | 5 | 0.008% | - | 0.000% | |
| 3 | I80.0 Phlebitis and thrombophlebitis of superficial vessels of lower extremities | 884 | 1.457% | 147 | 0.698% | |
| 4 | I80.1 Phlebitis and thrombophlebitis of femoral vein | 87 | 0.143% | 31 | 0.147% | |
| 5 | I80.2 Phlebitis and thrombophlebitis of other deep vessels of lower extremities | 13,931 | 22.957% | 7717 | 36.638% | |
| 6 | I80.3 Phlebitis and thrombophlebitis of lower extremities, unspecified | 3788 | 6.242% | 1299 | 6.167% | |
| 7 | I80.8 Phlebitis and thrombophlebitis of other sites | 2811 | 4.632% | 350 | 1.662% | |
| 8 | I80.9 Phlebitis and thrombophlebitis of unspecified site | 6977 | 11.497% | 1505 | 7.145% | |
| 9 | I81 Portal vein thrombosis | 389 | 0.641% | 174 | 0.826% | |
| 10 | I82 Other venous embolism and thrombosis | 8 | 0.013% | 1 | 0.005% | |
| 11 | I82.0 Budd–Chiari syndrome | 55 | 0.091% | 12 | 0.057% | |
| 12 | I82.1 Thrombophlebitis migrans | 11 | 0.018% | 2 | 0.009% | |
| 13 | I82.2 Embolism and thrombosis of vena cava | 63 | 0.104% | 38 | 0.180% | |
| 14 | I82.3 Embolism and thrombosis of renal vein | 84 | 0.138% | 22 | 0.104% | |
| 15 | I82.8 Embolism and thrombosis of other specified veins | 2867 | 4.725% | 1538 | 7.302% | |
| 16 | I82.9 Embolism and thrombosis of unspecified vein | 15,327 | 25.257% | 6067 | 28.804% | |
| 17 | I63.6 Cerebral infarction due to cerebral venous thrombosis, nonpyogenic | 66 | 0.109% | 16 | 0.076% | |
| 18 | I67.6 Nonpyogenic thrombosis of intracranial venous system | 50 | 0.082% | 12 | 0.057% | |
| 19 | O22.2 Superficial thrombophlebitis in pregnancy | 3 | 0.005% | - | 0.000% | |
| 20 | O22.3 Deep phlebothrombosis in pregnancy | 106 | 0.175% | 2 | 0.009% | |
| 21 | O22.5 Cerebral venous thrombosis in pregnancy | 3 | 0.005% | - | 0.000% | |
| 22 | O87.1 Deep phlebothrombosis in the puerperium | 126 | 0.208% | 11 | 0.052% | |
| 23 | O87.3 Cerebral venous thrombosis in the peurperium | 3 | 0.005% | 2 | 0.009% | |
| 24 | G08 Intracranial and intraspinal phlebitis and thrombophlebitis | 69 | 0.114% | 20 | 0.095% | |
| 25 | G95 Other diseases of spinal cord | 0 | 0.000% | 0 | 0.000% | |
| 26 | K55.0 Acute vascular disorders of intestine | 519 | 0.855% | 223 | 1.059% | |
| 27 | K55.1 Chronic vascular disorders of intestine | 178 | 0.293% | 33 | 0.157% | |
| Total | 48,412 | 79.779% | 19,224 | 91.269% | ||
| Arterial TE | 28 | I21, I22, I24 Myocardial infarction and other acute ischemic heart disease | 14 | 0.023% | 2 | 0.009% |
| 29 | I63 Cerebral infarction | 54 | 0.089% | 1 | 0.005% | |
| 30 | I74.0 Embolism and thrombosis of abdominal aorta | 145 | 0.239% | 44 | 0.209% | |
| 31 | I74.1 Embolism and thrombosis of other and unspecified parts of aorta | 159 | 0.262% | 51 | 0.242% | |
| 32 | I74.2 Embolism and thrombosis of arteries of upper extremities | 163 | 0.269% | 61 | 0.290% | |
| 33 | I74.3 Embolism and thrombosis of arteries of lower extremities | 3 | 0.005% | - | 0.000% | |
| 34 | I74.4 Embolism and thrombosis of arteries of extremities, unspecified | 1614 | 2.660% | 402 | 1.909% | |
| 35 | I74.5 Embolism and thrombosis of iliac artery | 203 | 0.335% | 74 | 0.351% | |
| 36 | I74.8 Embolism and thrombosis of other arteries | 2757 | 4.543% | 484 | 2.298% | |
| 37 | I74.9 Embolism and thrombosis of unspecified artery | 6686 | 11.018% | 478 | 2.269% | |
| 38 | N28.0 Ischemia and infarction of kidney | 473 | 0.779% | 242 | 1.149% | |
| Total | 12,271 | 20.221% | 1839 | 8.731% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.-W.; Lee, J.Y.; Lee, B.-H.; Jeon, M.J.; Yu, E.S.; Kim, D.S.; Lee, S.R.; Choi, C.W.; Park, Y.; Sung, H.J.; et al. Postoperative Thromboembolism According to the Type of Surgery: A Nationwide Study in the Republic of Korea. J. Clin. Med. 2022, 11, 1477. https://doi.org/10.3390/jcm11061477
Kang K-W, Lee JY, Lee B-H, Jeon MJ, Yu ES, Kim DS, Lee SR, Choi CW, Park Y, Sung HJ, et al. Postoperative Thromboembolism According to the Type of Surgery: A Nationwide Study in the Republic of Korea. Journal of Clinical Medicine. 2022; 11(6):1477. https://doi.org/10.3390/jcm11061477
Chicago/Turabian StyleKang, Ka-Won, Ji Yoon Lee, Byung-Hyun Lee, Min Ji Jeon, Eun Sang Yu, Dae Sik Kim, Se Ryeon Lee, Chul Won Choi, Yong Park, Hwa Jung Sung, and et al. 2022. "Postoperative Thromboembolism According to the Type of Surgery: A Nationwide Study in the Republic of Korea" Journal of Clinical Medicine 11, no. 6: 1477. https://doi.org/10.3390/jcm11061477
APA StyleKang, K.-W., Lee, J. Y., Lee, B.-H., Jeon, M. J., Yu, E. S., Kim, D. S., Lee, S. R., Choi, C. W., Park, Y., Sung, H. J., & Kim, B. S. (2022). Postoperative Thromboembolism According to the Type of Surgery: A Nationwide Study in the Republic of Korea. Journal of Clinical Medicine, 11(6), 1477. https://doi.org/10.3390/jcm11061477






