The Future of Adhesion Prophylaxis Trials in Abdominal Surgery: An Expert Global Consensus †
Abstract
1. Introduction
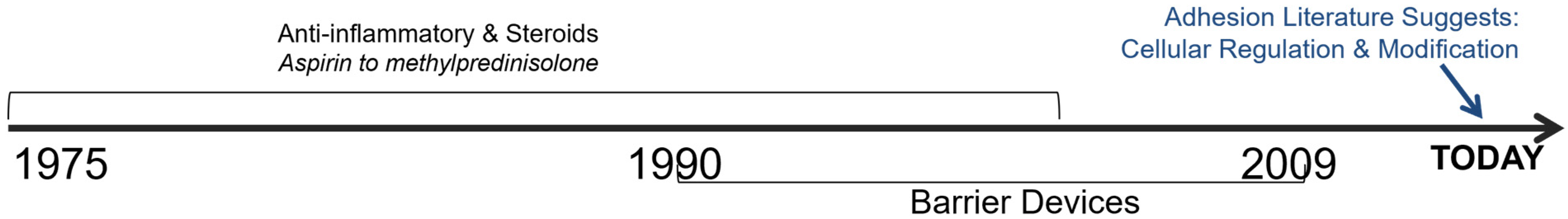
2. History of Adhesion Treatments
3. From Anti-Adhesion Barriers to Drugs
4. Guidance for Clinical Research Design in Anti-Adhesion Research
5. Assessment and Diagnosis of Adhesions
6. Conclusions
7. Further Outlooks Brought Up by The Global Consensus Meeting
- Adhesions are a common surgical sequela and health problem. Lack of awareness and acceptance of these problems needs to be rectified. Patients’ voices concerning the burden of adhesions on their daily quality of life need to be heard. Patients need to be informed about the risks that are associated with postoperative adhesions, as part of surgical informed consent.
- Surgeons face medicolegal implications related to the morbidities associated with postoperative adhesions and should be informed of this risk.
- The cost of adhesions is considerable. Health care authorities, insurance providers, scientific societies, and governments should think in longer time frames when tackling adhesion-related disease.
- Secondary endpoints such as fertility, pain, bowel obstruction, and quality of life are important, but difficult to scientifically study and evaluate.
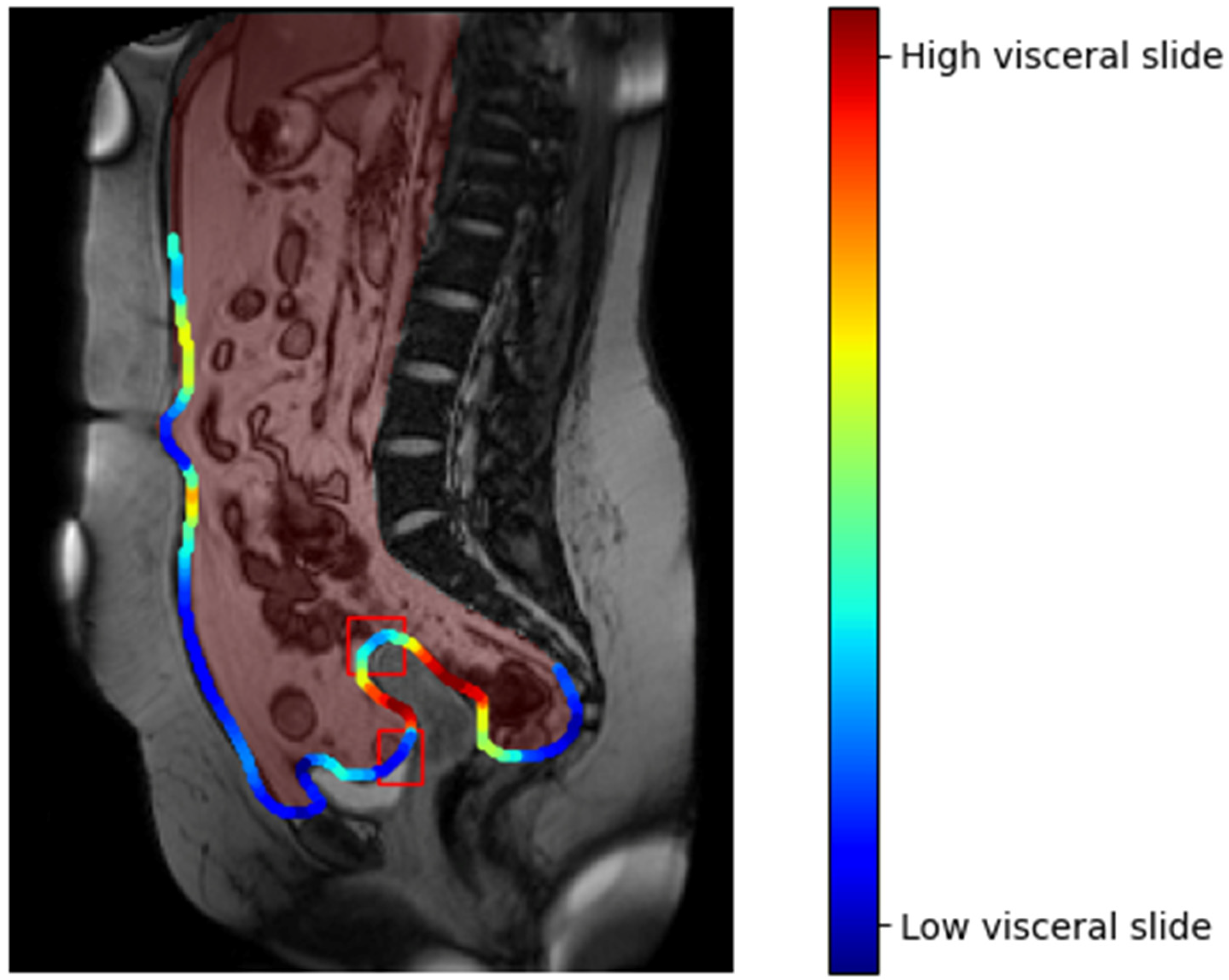
- Second-look laparoscopy has remained the gold standard for adhesion assessment and diagnosis, until now. New diagnostic tools such as CineMRI and US require further clinical evaluation.
- A patient with no adhesions assumes no risk of the related complications: pursuit of a zero-adhesion state should be the goal.
- More research regarding new antiadhesion options is necessary, by means of prospective, randomized and blinded designs, when possible.
- A surgery- and disease-related risk score should be constructed, as long as a genetic biobank evaluation is not established to clearly define adhesion-prone populations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okabayashi, K.; Ashrafian, H.; Zacharakis, E.; Hasegawa, H.; Kitagawa, Y.; Athanasiou, T.; Darzi, A. Adhesions after abdominal surgery: A systematic review of the incidence, distribution and severity. Surg. Today 2014, 44, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Stommel, M.W.J.; Ten Broek, R.P.G.; Strik, C.; Slooter, G.D.; Verhoef, C.; Grünhagen, D.J.; van Duijvendijk, P.; Bemelmans, M.H.A.; den Dulk, M.; Sietses, C.; et al. Multicenter Observational Study of Adhesion Formation After Open-and Laparoscopic Surgery for Colorectal Cancer. Ann. Surg. 2018, 267, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Krielen, P.; Stommel, M.W.J.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; van Goor, H.; Ten Broek, R.P.G. Adhesion-related readmissions after open and laparoscopic surgery: A retrospective cohort study (SCAR update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef]
- Ahmad, G.; Kim, K.; Thompson, M.; Agarwal, P.; O’Flynn, H.; Hindocha, A.; Watson, A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst. Rev. 2020, 3, CD000475. [Google Scholar] [CrossRef] [PubMed]
- DeWilde, R.L.; Trew, G. Postoperative abdominal adhesions and their prevention in gynaecological surgery. Expert consensus position. Part 2 Steps to Reduce Adhesions. Gynecol. Surg. 2007, 4, 243–253. [Google Scholar] [CrossRef]
- Lang, J.; Ma, D.; Xiang, Y.; Hua, K.; Liu, K.; Pan, L.; Wang, P.; Yao, S.; Zhao, F.; Cheng, W.; et al. Chinese expert consensus on the prevention of abdominal pelvic adhesions after gynecological tumor surgeries. Ann. Transl. Med. 2020, 8, 79. [Google Scholar] [CrossRef]
- Akiyama, Y.; Luo, Y.; Hanno, P.M.; Maeda, D.; Homma, Y. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int. J. Urol. 2020, 27, 491–503. [Google Scholar] [CrossRef]
- Vipond, M.N.; Whawell, S.A.; Thompson, J.N.; Dudley, H.A. Peritoneal fibrinolytic activity and intra-abdominal adhesions. Lancet 1990, 335, 1120–1122. [Google Scholar] [CrossRef]
- Cheong, Y.C.; Bajekal, N.; Li, T.C. Peritoneal closure—To close or not to close. Hum. Reprod. 2001, 16, 1548–1552. [Google Scholar] [CrossRef][Green Version]
- Franklin, R.R. Reduction of ovarian adhesions by the use of Interceed. Ovarian Adhesion Study Group. Obstet. Gynecol. 1995, 86, 335–340. [Google Scholar] [CrossRef]
- Li, T.C.; Cooke, I.D. The value of an absorbable adhesion barrier, Interceed, in the prevention of adhesion reformation following microsurgical adhesiolysis. Br. J. Obstet. Gynaecol. 1994, 101, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D.; Pascual, M.H.; Walz, M.K.; Duron, J.J.; Tonelli, F.; Crowe, A.; Knight, A.; Registry, A. Use of icodextrin 4% solution in the prevention of adhesion formation following general surgery: From the multicentre ARIEL Registry. Ann. R. Coll. Surg. Engl. 2006, 88, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Hosie, K.; Gilbert, J.A.; Kerr, D.; Brown, C.B.; Peers, E.M. Fluid dynamics in man of an intraperitoneal drug delivery solution: 4% icodextrin. Drug Deliv. 2001, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Di Zerega, G.S.; Verco, S.J.; Young, P.; Kettel, M.; Kobak, W.; Martin, D.; Sanfilippo, J.; Peers, E.M.; Scrimgeour, A.; Brown, C.B. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum. Reprod. 2002, 17, 1031–1038. [Google Scholar] [CrossRef]
- Sutton, C.; Minelli, L.; Garcia, E.; Korell, M.; Pouly, J.L.; Pados, G.; Crowe, A.M.; Osborne, L.W.J.; Knight, A.D. Use of icodextrin 4% solution in the reduction of adhesion formation after gynaecological surgery. Gynecol. Surg. 2005, 2, 10. [Google Scholar] [CrossRef]
- Korell, M.; Ziegler, N.; De Wilde, R.L. Use of Modified Polysaccharide 4DryField PH for Adhesion Prevention and Hemostasis in Gynecological Surgery: A Two-Center Observational Study by Second-Look Laparoscopy. BioMed Res. Int. 2016, 2016, 3029264. [Google Scholar] [CrossRef]
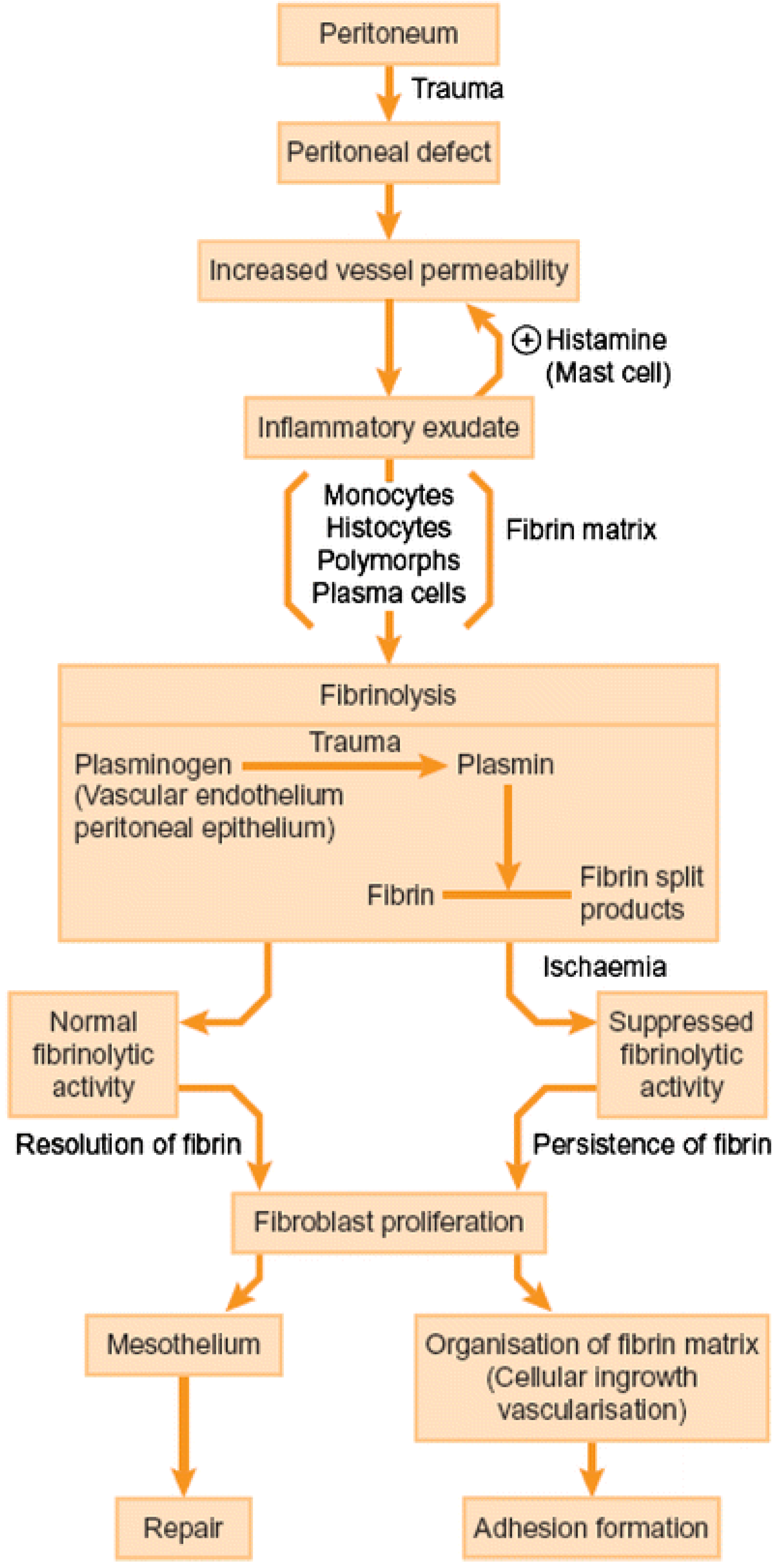
- Duron, J.J. Postoperative intraperitoneal adhesion pathophysiology. Colorectal. Dis. 2007, 9 (Suppl. 2), 14–24. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: A committee opinion. Fertil. Steril. 2013, 99, 1550–1555. [Google Scholar] [CrossRef]
- Awonuga, A.O.; Belotte, J.; Abuanzeh, S.; Fletcher, N.M.; Diamond, M.P.; Saed, G.M. Advances in the Pathogenesis of Adhesion Development: The Role of Oxidative Stress. Reprod. Sci. 2014, 21, 823–836. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Awonuga, A.O.; Neubauer, B.R.; Abusamaan, M.S.; Saed, M.G.; Diamond, M.P.; Saed, G.M. Shifting anaerobic to aerobic metabolism stimulates apoptosis through modulation of redox balance: Potential intervention in the pathogenesis of postoperative adhesions. Fertil. Steril. 2015, 104, 1022–1029. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Gomel, V.; Ussia, A.; Adamyan, L. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil. Steril. 2016, 106, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.M.; Fletcher, N.M.; Diamond, M.P.; Saed, G.M. Evitar (l-Alanyl-l-Glutamine) Regulates Key Signaling Molecules in the Pathogenesis of Postoperative Tissue Fibrosis. Reprod. Sci. 2019, 26, 724–733. [Google Scholar] [CrossRef] [PubMed]
- van den Beukel, B.A.W.; Stommel, M.W.J.; van Leuven, S.; Strik, C.; Ijsseldijk, M.A.; Joosten, F.; van Goor, H.; Ten Broek, R.P.G. A Shared Decision Approach to Chronic Abdominal Pain Based on Cine-MRI: A Prospective Cohort Study. Am. J. Gastroenterol. 2018, 113, 1229–1237. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lee, R.K.; Hwu, Y.M.; Lin, M.H. Reproducibility of the revised American Fertility Society classification of endometriosis using laparoscopy or laparotomy. Int. J. Gynaecol. Obstet. 1998, 60, 265–269. [Google Scholar] [CrossRef]
- Lier, E.J.; van den Beukel, B.A.W.; Gawria, L.; van der Wees, P.J.; van den Hil, L.; Bouvy, N.D.; Cheong, Y.; de Wilde, R.L.; van Goor, H.; Stommel, M.W.J.; et al. Clinical adhesion score (CLAS): Development of a novel clinical score for adhesion-related complications in abdominal and pelvic surgery. Surg. Endosc. 2020, 35, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Wexner, S.D.; di Zereg, G.S.; Korell, M.; Zmora, O.; Van Goor, H.; Kamar, M. Adhesion prevention and reduction: Current status and future recommendations of a multinational interdisciplinary consensus conference. Surg. Innov. 2010, 17, 183–188. [Google Scholar] [CrossRef] [PubMed]
| Product Category | Product | Mechanism or Strategies | Stage of Development |
|---|---|---|---|
| Medical Device-Mechanical Barriers (Fabric, film, gels, polymers, liquids) | Seprafilm® (Baxter, Deerfield, IL, USA), Interceed® (Ethicon, Somerville, NJ, USA), Adept® (Ethicon, Somerville, NJ, USA), SprayShield® 8Coviden; Dublin, Irland), Hyalobarrier® (Nordic Pharma, Paris, France), Repel-CV® ((SyntheMed Inc, Iselin, NJ, USA), Adcon®, (Leader Biomedical, Amsterdam, The Netherlands), Coseal® (Baxter Healthcare Inc, Deerfield, IL, USA), etc. | Physical separation of tissues Site specific | FDA, CE mark, approved. |
| Anti-adhesive Agents | Ibuprofen, celecoxib, resveratrol or pirfenidone, myomycin C, heparin. | ECM Fibrinolytic Inflammation Cell proliferation Anticoagulant | Serious side effects, delivery problems and/or moderate to low efficacy. |
| Gene Therapy | tPA gene siRNA HGF gene | Promote fibrinolysis HIF1a PAI-1 Mesothelial regeneration | Serious side effects, low efficacy, expensive. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Wilde, R.L.; Devassy, R.; Broek, R.P.G.t.; Miller, C.E.; Adlan, A.; Aquino, P.; Becker, S.; Darmawan, F.; Gergolet, M.; Habana, M.A.E.; et al. The Future of Adhesion Prophylaxis Trials in Abdominal Surgery: An Expert Global Consensus. J. Clin. Med. 2022, 11, 1476. https://doi.org/10.3390/jcm11061476
De Wilde RL, Devassy R, Broek RPGt, Miller CE, Adlan A, Aquino P, Becker S, Darmawan F, Gergolet M, Habana MAE, et al. The Future of Adhesion Prophylaxis Trials in Abdominal Surgery: An Expert Global Consensus. Journal of Clinical Medicine. 2022; 11(6):1476. https://doi.org/10.3390/jcm11061476
Chicago/Turabian StyleDe Wilde, Rudy Leon, Rajesh Devassy, Richard P. G. ten Broek, Charles E. Miller, Aizura Adlan, Prudence Aquino, Sven Becker, Ferry Darmawan, Marco Gergolet, Maria Antonia E. Habana, and et al. 2022. "The Future of Adhesion Prophylaxis Trials in Abdominal Surgery: An Expert Global Consensus" Journal of Clinical Medicine 11, no. 6: 1476. https://doi.org/10.3390/jcm11061476
APA StyleDe Wilde, R. L., Devassy, R., Broek, R. P. G. t., Miller, C. E., Adlan, A., Aquino, P., Becker, S., Darmawan, F., Gergolet, M., Habana, M. A. E., Khoo, C. K., Koninckx, P. R., Korell, M., Krentel, H., Musigavong, O., Pistofidis, G., Puntambekar, S., Rachman, I. A., Sendag, F., ... Torres-de la Roche, L. A. (2022). The Future of Adhesion Prophylaxis Trials in Abdominal Surgery: An Expert Global Consensus. Journal of Clinical Medicine, 11(6), 1476. https://doi.org/10.3390/jcm11061476










