Comparing the Occurrence of Healthcare-Associated Infections in Patients with and without COVID-19 Hospitalized during the Pandemic: A 16-Month Retrospective Cohort Study in a Hospital Intensive Care Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
3.2. Occurrence and Characteristics of HAIs
3.3. Risk Factors for HAI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Guidance for Health System Contingency Planning during Widespread Transmission of SARS-CoV-2 with High Impact on Healthcare Services; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- McCabe, R.; Schmit, N.; Christen, P.; D’Aeth, J.C.; Løchen, A.; Rizmie, D.; Nayagam, S.; Miraldo, M.; Aylin, P.; Bottle, A.; et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020, 18, 329. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Strenghtening the Health System Response to COVID-19. Maintaining the Delivery of Essential Health Care Services While Mobilizing the Health Workforce for the COVID-19 Response; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2020. [Google Scholar]
- De Filippis, G.; Cavazzana, L.; Gimigliano, A.; Piacenza, M.; Vimercati, S. Covid-19 pandemic: A frontline hospital reorganization to cope with therapeutic and diagnostic emergency. Pharmacol. Res. 2020, 161, 105160. [Google Scholar] [CrossRef]
- Rana, S.; Hughes, L.A.; Rana, S.; Adam, L.A. The Effects of ICU Crisis Reorganization on Outcomes in Patients Not Infected with Coronavirus Disease 2019 During the Initial Surge of the Coronavirus Disease 2019 Pandemic. Crit. Care Explor. 2021, 3, e0333. [Google Scholar] [CrossRef]
- Tan, E.; Song, J.; Deane, A.M.; Plummer, M.P. Global Impact of Coronavirus Disease 2019 Infection Requiring Admission to the ICU: A Systematic Review and Meta-analysis. Chest 2021, 159, 524–536. [Google Scholar] [CrossRef]
- Marckmann, G.; Neitzke, G.; Schildmann, J.; Michalsen, A.; Dutzmann, J.; Hartog, C.; Jöbges, S.; Knochel, K.; Michels, G.; Pin, M.; et al. Decisions on the allocation of intensive care resources in the context of the COVID-19 pandemic. Med. Klin. Intensivmed. Notfmed. 2020, 115, 115–122. [Google Scholar] [CrossRef]
- Despotovic, A.; Milosevic, B.; Cirkovic, A.; Vujovic, A.; Cucanic, K.; Cucanic, T.; Stevanovic, G. The impact of covid-19 on the profile of hospital-acquired infections in adult intensive care units. Antibiotics 2021, 10, 1146. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Wu, J.; Li, Y.; Zhou, X.; Li, X.; Chen, H.; Guo, M.; Chen, S.; Sun, F.; et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 1958–1964. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef]
- Stevens, M.P.; Doll, M.; Pryor, R.; Godbout, E.; Cooper, K.; Bearman, G. Impact of COVID-19 on Traditional Healthcare Associated Infection Prevention Efforts. Infect. Control Hosp. Epidemiol. 2020, 41, 946–947. [Google Scholar] [CrossRef] [Green Version]
- Billings, J.; Ching, B.C.F.; Gkofa, V.; Greene, T.; Bloomfield, M. Experiences of frontline healthcare workers and their views about support during COVID-19 and previous pandemics: A systematic review and qualitative meta-synthesis. BMC Health Serv. Res. 2021, 21, 923. [Google Scholar] [CrossRef]
- Unoki, T.; Tamoto, M.; Ouchi, A.; Sakuramoto, H.; Nakayama, A.; Katayama, Y.; Miyazaki, S.; Yamada, T.; Fujitani, S.; Nishida, O.; et al. Personal protective equipment use by health-care workers in intensive care units during the COVID-19 pandemic in Japan: Comparative analysis with the PPE-SAFE survey. Acute Med. Surg. 2020, 7, e584. [Google Scholar] [CrossRef]
- NHS England and NHS Improvement with Health Education England. Advice on Acute Sector Workforce Models during COVID-19; National Health System England: London, UK, 2020. [Google Scholar]
- Bardi, T.; Pintado, V.; Gomez-rojo, M.; Escudero-sanchez, R.; Lopez, A.A. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and metaanalysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Saini, V.; Jain, C.; Singh, N.P.; Alsulimani, A.; Gupta, C.; Dar, S.A.; Haque, S.; Das, S. Paradigm shift in antimicrobial resistance pattern of bacterial isolates during the covid-19 pandemic. Antibiotics 2021, 10, 954. [Google Scholar] [CrossRef]
- Rodríguez-Acelas, A.L.; de Abreu Almeida, M.; Engelman, B.; Cañon-Montañez, W. Risk factors for health care–associated infection in hospitalized adults: Systematic review and meta-analysis. Am. J. Infect. Control 2017, 45, e149–e156. [Google Scholar] [CrossRef] [Green Version]
- Zee-Cheng, J.E.; McCluskey, C.K.; Klein, M.J.; Scanlon, M.C.; Rotta, A.T.; Shein, S.L.; Pineda, J.A.; Remy, K.E.; Carroll, C.L. Changes in Pediatric ICU Utilization and Clinical Trends During the Coronavirus Pandemic. Chest 2021, 160, 529–537. [Google Scholar] [CrossRef]
- Tyrrell, C.S.B.; Mytton, O.T.; Gentry, S.V.; Thomas-Meyer, M.; Allen, J.L.Y.; Narula, A.A.; McGrath, B.; Lupton, M.; Broadbent, J.; Ahmed, A.; et al. Managing intensive care admissions when there are not enough beds during the COVID-19 pandemic: A systematic review. Thorax 2021, 76, 302–312. [Google Scholar] [CrossRef]
- Lesieur, O.; Quenot, J.P.; Cohen-Solal, Z.; David, R.; De Saint Blanquat, L.; Elbaz, M.; Gaillard Le Roux, B.; Goulenok, C.; Lavoué, S.; Lemiale, V.; et al. Admission criteria and management of critical care patients in a pandemic context: Position of the Ethics Commission of the French Intensive Care Society, update of April 2021. Ann. Intensive Care 2021, 11, 10–12. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.C.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 87. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 25. [Google Scholar] [CrossRef]
- Advani, S.D.; Sickbert-Bennett, E.; Dodds Ashley, E.; Cromer, A.; Lokhnygina, Y.; Nelson, A.; Akinboyo, I.; DiBiase, L.; Weber, D.J.; Anderson, D.J. Impact of COVID-19 Pandemic on Healthcare-associated Infections (HAIs) in a Large Network of Hospitals. Open Forum Infect. Dis. 2021, 8, 103–104. [Google Scholar] [CrossRef]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of COVID-19 on Healthcare-Associated Infections. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Jabarpour, M.; Dehghan, M.; Afsharipour, G.; Hajipour Abaee, E.; Mangolian Shahrbabaki, P.; Ahmadinejad, M.; Maazallahi, M. The Impact of COVID-19 Outbreak on Nosocomial Infection Rate: A Case of Iran. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6650920. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, Z.; Zhao, X.; Peng, H.; Hong, Y.; Huang, L.; Huang, J.; Yan, X.; Wu, S.; Bai, Z. Changes in prevalence of nosocomial infection pre- and post-COVID-19 pandemic from a tertiary Hospital in China. BMC Infect. Dis. 2021, 21, 693. [Google Scholar] [CrossRef]
- Ong, C.C.H.; Farhanah, S.; Linn, K.Z.; Tang, Y.W.; Poon, C.Y.; Lim, A.Y.; Tan, H.R.; Binte Hamed, N.H.; Huan, X.; Puah, S.H.; et al. Nosocomial infections among COVID-19 patients: An analysis of intensive care unit surveillance data. Antimicrob. Resist. Infect. Control 2021, 10, 119. [Google Scholar] [CrossRef]
- Migliara, G.; Di Paolo, C.; Barbato, D.; Baccolini, V.; Salerno, C.; Nardi, A.; Alessandri, F.; Giordano, A.; Tufi, D.; Marinelli, L.; et al. Multimodal surveillance of healthcare associated infections in an intensive care unit of a large teaching hospital. Ann. Ig. 2019, 31, 399–413. [Google Scholar]
- Center for Disease and Prevention Control. National Healthcare Safety Network (NHSN) Overview: Patient Safety Component Manual; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. [Google Scholar]
- European Centre for Disease Prevention and Control. European Surveillance of Healthcare-Associated Infections in Intensive Care Units—HAI-Net ICU Protocol, Version 1.02; ECDC: Stockholm, Sweden, 2015; ISBN 9789291936274. [Google Scholar]
- Prentice, R.L.; Williams, B.J.; Peterson, A.V. On the regression analysis of multivariate failure time data. Biometrika 1981, 68, 373–379. [Google Scholar] [CrossRef]
- Graham, J.W.; Olchowski, A.E.; Gilreath, T.D. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev. Sci. 2007, 8, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Sturdy, A.; Basarab, M.; Cotter, M.; Hager, K.; Shakespeare, D.; Shah, N.; Randall, P.; Spray, D.; Arnold, A. Severe COVID-19 and healthcare-associated infections on the ICU: Time to remember the basics? J. Hosp. Infect. 2020, 105, 593–595. [Google Scholar] [CrossRef]
- Mcmullen, K.; Smith, B.; Rebmann, T. Impact of SARS-CoV-2 on hospital acquired infection rates in the United States: Predictions and early results. Am. J. Infect. Control 2020, 48, 1409–1411. [Google Scholar] [CrossRef]
- Cawcutt, K.A.; Starlin, R.; Rupp, M.E. Fighting fear in healthcare workers during the COVID-19 pandemic. Infect. Control Hosp. Epidemiol. 2020, 41, 1192–1193. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.G.; Gardner, A.; Stone, P.W.; Hall, L.; Pogorzelska-Maziarz, M. Hospital Staffing and Health Care–Associated Infections: A Systematic Review of the Literature. Jt. Comm. J. Qual. Patient Saf. 2018, 44, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, G.; Riccardo, F.; Marziano, V.; Poletti, P.; Trentini, F.; Bella, A.; Andrianou, X.; Del Manso, M.; Fabiani, M.; Bellino, S.; et al. Impact of a nationwide lockdown on SARS-CoV-2 transmissibility, Italy. Emerg. Infect. Dis. 2021, 27, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Marzianoa, V.; Guzzettaa, G.; Rondinone, B.M.; Boccuni, F.; Riccardo, F.; Bella, A.; Poletti, P.; Trentini, F.; Pezzotti, P.; Brusaferro, S.; et al. Retrospective analysis of the Italian exit strategy from COVID-19 lockdown. Proc. Natl. Acad. Sci. USA 2021, 118, e2019617118. [Google Scholar] [CrossRef]
- Bontempi, E. The europe second wave of COVID-19 infection and the Italy “strange” situation. Environ. Res. 2021, 193, 110476. [Google Scholar] [CrossRef]
- Hajure, M.; Tariku, M.; Bekele, F.; Abdu, Z.; Dule, A.; Mohammedhussein, M.; Tsegaye, T. Attitude towards covid-19 vaccination among healthcare workers: A systematic review. Infect. Drug Resist. 2021, 14, 3883–3897. [Google Scholar] [CrossRef]
- Despotovic, A.; Milosevic, B.; Milosevic, I.; Mitrovic, N.; Cirkovic, A.; Jovanovic, S.; Stevanovic, G. Hospital-acquired infections in the adult intensive care unit—Epidemiology, antimicrobial resistance patterns, and risk factors for acquisition and mortality. Am. J. Infect. Control 2020, 48, 1211–1215. [Google Scholar] [CrossRef] [Green Version]
- Askarian, M.; Yadollahi, M.; Assadian, O. Point prevalence and risk factors of hospital acquired infections in a cluster of university-affiliated hospitals in Shiraz, Iran. J. Infect. Public Health 2012, 5, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Adams, A.; Hererra, M.; Rojas, E.R.; Singh, V.; Sakhuja, A.; Meersman, M.; Dalton, D.; Kethireddy, S.; Nanchal, R.; et al. Predictors and outcomes of healthcare-associated infections in COVID-19 patients. Int. J. Infect. Dis. 2021, 104, 287–292. [Google Scholar] [CrossRef]
- Kubin, C.J.; McConville, T.H.; Dietz, D.; Zucker, J.; May, M.; Nelson, B.; Istorico, E.; Bartram, L.; Small-Saunders, J.; Sobieszczyk, M.E.; et al. Characterization of Bacterial and Fungal Infections in Hospitalized Patients with Coronavirus Disease 2019 and Factors Associated with Health Care-Associated Infections. Open Forum Infect. Dis. 2021, 8, ofab201. [Google Scholar] [CrossRef]
- Baghdadi, J.D.; Coffey, K.C.; Adediran, T.; Goodman, K.E.; Pineles, L.; Magder, L.S.; Pineles, B.L.; Nadimpalli, G.; Morgan, D.J.; Harris, A.D. Antibiotic Use and Bacterial Infection Among Inpatients in the First Wave of COVID-19: A Retrospective Cohort Study of 64,691 Patients. Antimicrob. Agents Chemother. 2021, 65, e01341-21. [Google Scholar] [CrossRef] [PubMed]
- Migliara, G.; Baccolini, V.; Isonne, C.; Cianfanelli, S.; Di Paolo, C.; Mele, A.; Lia, L.; Nardi, A.; Salerno, C.; Caminada, S.; et al. Prior antibiotic therapy and the onset of healthcare-associated infections sustained by multidrug-resistant klebsiella pneumoniae in intensive care unit patients: A nested case–control study. Antibiotics 2021, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Alessandri, F.; Oliva, A.; Borrazzo, C.; Dell’Isola, S.; Ialungo, A.M.; Rastrelli, E.; Pelli, M.; Raponi, G.; Turriziani, O.; et al. The role of teicoplanin in the treatment of SARS-CoV-2 infection: A retrospective study in critically ill COVID-19 patients (Tei-COVID study). J. Med. Virol. 2021, 93, 4319–4325. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.; Al-Naimi, M.; Lungnier, C.; Al-Gareeb, A. Macrolides and COVID-19: An optimum premise. Biomed. Biotechnol. Res. J. 2020, 4, 189–192. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Core Elements of Hospital Antibiotic Stewardship Programs; U.S. Department of Health & Human Services, CDC: Washington, DC, USA, 2019; Volume 59, pp. S97–S100. [Google Scholar]
- François, B.; Laterre, P.F.; Luyt, C.E.; Chastre, J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit. Care 2020, 24, 289. [Google Scholar] [CrossRef]
- Rouyer, M.; Strazzulla, A.; Youbong, T.; Tarteret, P.; Pitsch, A.; De Pontfarcy, A.; Cassard, B.; Vignier, N.; Pourcine, F.; Jochmans, S.; et al. Ventilator-associated pneumonia in covid-19 patients: A retrospective cohort study. Antibiotics 2021, 10, 988. [Google Scholar] [CrossRef]
- Bonnet, N.; Martin, O.; Boubaya, M.; Levy, V.; Ebstein, N.; Karoubi, P.; Tandjaoui-Lambiotte, Y.; Van Der Meersch, G.; Oziel, J.; Soulie, M.; et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: A retrospective study. Ann. Intensive Care 2021, 11, 37. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: CPAP reduces need for invasive mechanical ventilation in patients requiring oxygen, study finds. BMJ 2021, 374, n1950. [Google Scholar] [CrossRef]
- Klopfenstein, T.; Zayet, S.; Lohse, A.; Selles, P. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID-19 patients. Int. J. Infect. Dis. 2020, 99, 491–495. [Google Scholar] [CrossRef]
- Blonz, G.; Kouatchet, A.; Chudeau, N.; Pontis, E.; Lorber, J.; Lemeur, A.; Planche, L.; Lascarrou, J.B.; Colin, G. Epidemiology and microbiology of ventilator-associated pneumonia in COVID-19 patients: A multicenter retrospective study in 188 patients in an un-inundated French region. Crit. Care 2021, 25, 72. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.F.; Bruno-Murtha, L.A.; Dick, B. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Crit. Care Med. 2010, 38, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarivet, B.; Grau, D.; Jumas-Bilak, E.; Jean-Pierre, H.; Pantel, A.; Parer, S.; Lotthé, A. Persisting transmission of carbapenemase-producing Klebsiella pneumoniae due to an environmental reservoir in a university hospital, France, 2012 to 2014. Eurosurveillance 2016, 21, 30213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venier, A.G.; Leroyer, C.; Slekovec, C.; Talon, D.; Bertrand, X.; Parer, S.; Alfandari, S.; Guerin, J.M.; Megarbane, B.; Lawrence, C.; et al. Risk factors for Pseudomonas aeruginosa acquisition in intensive care units: A prospective multicentre study. J. Hosp. Infect. 2014, 88, 103–108. [Google Scholar] [CrossRef]
- Barbato, D.; Castellani, F.; Angelozzi, A.; Isonne, C.; Baccolini, V.; Migliara, G.; Marzuillo, C.; De Vito, C.; Villari, P.; Romano, F.; et al. Prevalence survey of healthcare-associated infections in a large teaching hospital. Ann. Ig. Med. Prev. E Comunita 2019, 31, 423–435. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control 2007, 35, S165–S193. [Google Scholar] [CrossRef]
- Baccolini, V.; D’Egidio, V.; De Soccio, P.; Migliara, G.; Massimi, A.; Alessandri, F.; Tellan, G.; Marzuillo, C.; De Vito, C.; Ranieri, M.V.; et al. Effectiveness over time of a multimodal intervention to improve compliance with standard hygiene precautions in an intensive care unit of a large teaching hospital. Antimicrob. Resist. Infect. Control 2019, 8, 92. [Google Scholar] [CrossRef]
- Angelozzi, A.; Caminada, S.; Dorelli, B.; Sindoni, A.; Baccolini, V.; di Paolo, C.; Mele, A.; Salvatori, L.M.; Alessandri, F.; Marzuillo, C.; et al. Knowledge, attitude, barriers, professional behaviour and possible interventions: A survey on healthcareassociated infections among the healthcare workers of an intensive care unit in a large teaching hospital in Rome. Ann. Ig. Med. Prev. E Comunita 2021, 33, 628–643. [Google Scholar] [CrossRef]

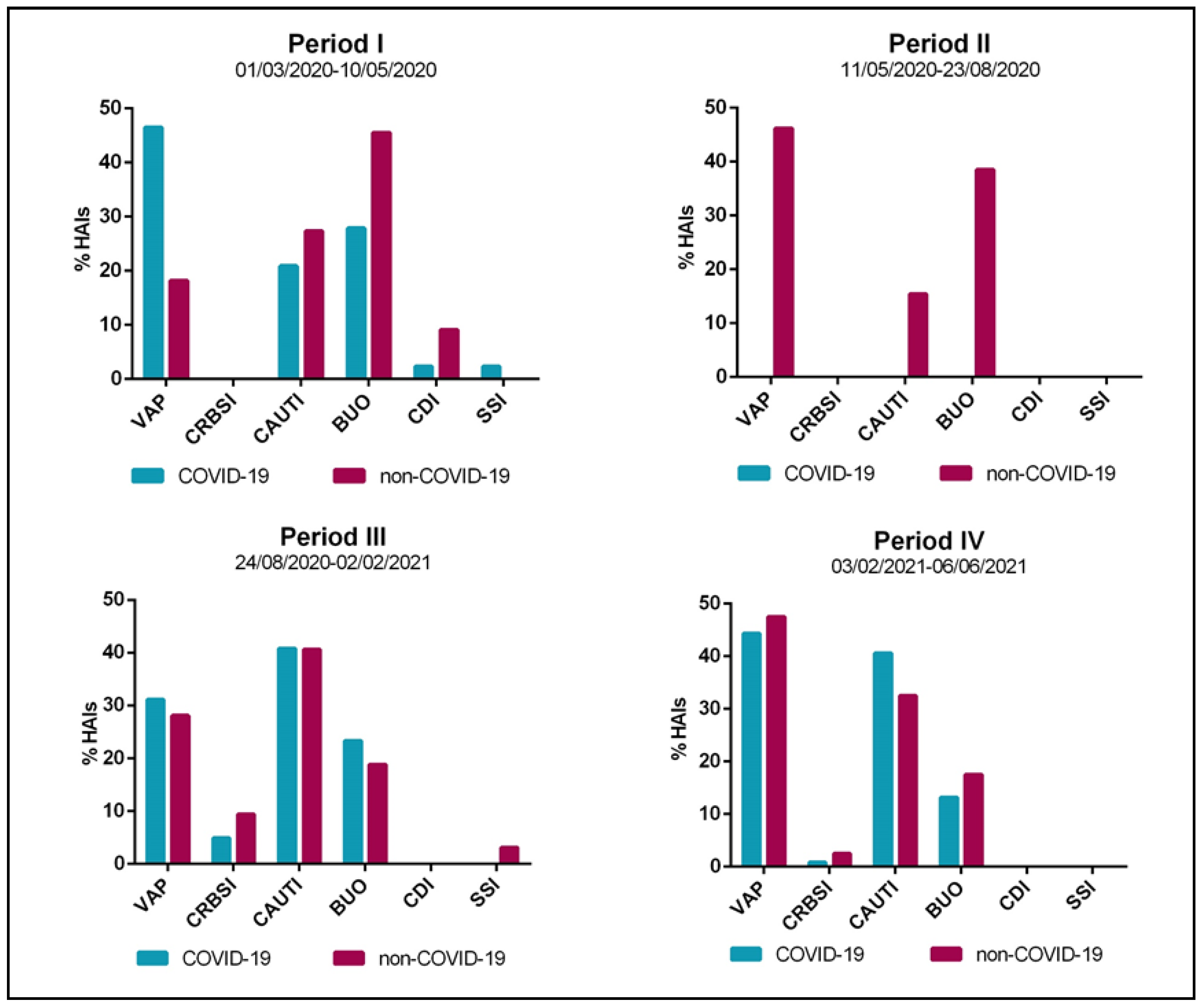
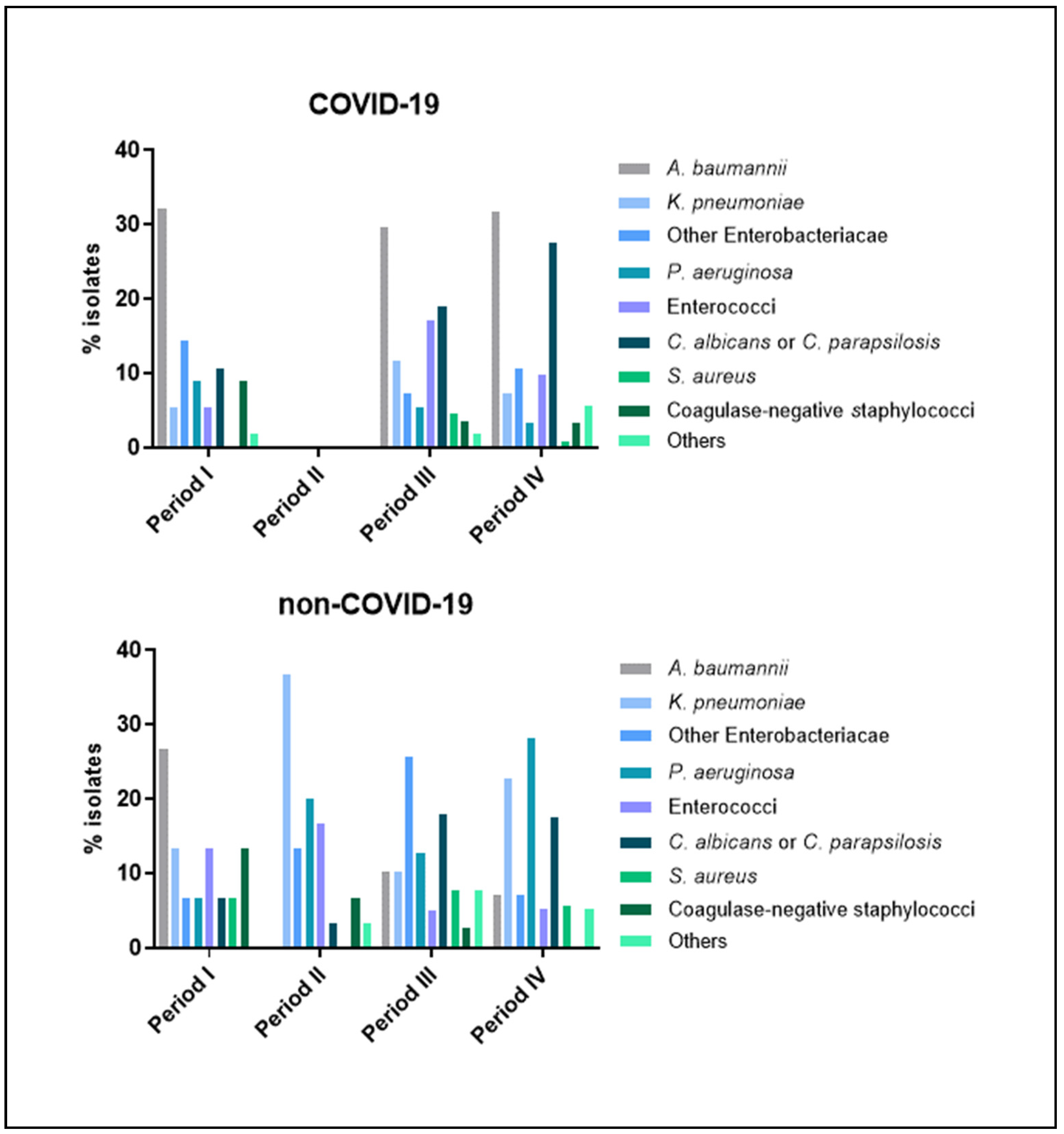
| Period I | Period II | Period III | Period IV | |||||
|---|---|---|---|---|---|---|---|---|
| 1 March 2020 to 10 May 2020 | 11 May 2020 to 23 August 2020 | 24 August 2020 to 2 February 2021 | 3 February 2021 to 6 June 2021 | |||||
| With | Without | With | Without | With | Without | With | Without | |
| COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | COVID-19 | |
| Patients | 47 | 18 | 4 | 45 | 130 | 33 | 171 | 34 |
| Observation time, person-days | 800 | 301 | 61 | 932 | 2179 | 894 | 2734 | 720 |
| Gender (female) | 16 (34.0) | 7 (38.9) | 2 (50.0) | 22 (48.9) | 41 (31.5) | 12 (36.4) | 59 (34.5) | 17 (50.0) |
| Age, years | 69 (13.0) | 65 (16.0) | 72.3 (17.0) | 61.5 (16.2) | 61.2 (13.1) | 64.3 (18.2) | 59.2 (13.9) | 68.2 (13.9) |
| Admission to the ICU | ||||||||
| Ward | 22 (46.9) | 12 (66.7) | 1 (25.0) | 12 (26.7) | 42 (33.0) | 13 (39.4) | 36 (21.1) | 17 (50.0) |
| Other hospital | 4 (8.5) | 0 (0.0) | 0 (0.0) | 2 (4.4) | 0 (0.0) | 1 (3.0) | 26 (15.3) | 1 (2.9) |
| Emergency Department | 21 (44.7) | 6 (33.3) | 3 (75.0) | 31 (68.9) | 68 (52.3) | 15 (45.5) | 109 (63.8) | 16 (47.1) |
| SAPS II Score (N = 341) | 37.3 (9.6) | 36.6 (20.8) | 33.3 (11.1) | 38.7 (15.9) | 34.6 (11.4) | 45.4 (13.7) | 35.8 (10.7) | 50 (14.0) |
| Coexisting conditions | ||||||||
| Hypertension | 24 (51.1) | 5 (27.8) | 0 (0.0) | 12 (26.7) | 54 (41.5) | 10 (30.3) | 66 (38.6) | 14 (41.2) |
| Diabetes mellitus | 7 (14.9) | 2 (11.1) | 0 (0.0) | 5 (11.1) | 26 (20.0) | 3 (9.1) | 29 (17.0) | 6 (17.6) |
| Obesity (BMI ≥ 30) | 3 (6.4) | 1 (5.6) | 0 (0.0) | 2 (4.4) | 18 (13.9) | 2 (6.1) | 31 (18.1) | 5 (14.7) |
| COPD | 2 (4.3) | 3 (16.7) | 0 (0.0) | 5 (11.1) | 16 (12.3) | 4 (12.1) | 5 (2.9) | 2 (5.9) |
| Asthma | 3 (6.4) | 1 (5.6) | 0 (0.0) | 1 (2.2) | 4 (3.1) | 0 (0.0) | 3 (1.8) | 0 (0.0) |
| Coronary heart disease | 5 (10.6) | 2 (11.1) | 0 (0.0) | 1 (2.2) | 12 (9.2) | 1 (3.0) | 13 (7.6) | 2 (5.9) |
| Chronic kidney disease | 2 (4.3) | 1 (5.6) | 0 (0.0) | 2 (4.4) | 10 (7.7) | 1 (3.0) | 5 (2.9) | 2 (5.9) |
| Chronic liver disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (8.9) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 1 (2.9) |
| Active cancer | 6 (12.8) | 0 (0.0) | 0 (0.0) | 2 (4.4) | 14 (10.8) | 4 (12.1) | 15 (8.8) | 4 (11.8) |
| Immunodeficiency | 1 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ICU deaths | 30 (63.8) | 6 (33.3) | 3 (75.0) | 10 (22.0) | 80 (61.5) | 9 (27.3) | 74 (43.3) | 9 (26.5) |
| Mortality rate (95% CI) per 1000 patient-days | 0.04 (0.03–0.05) | 0.02 (0.01–0.04) | 0.05 (0.02–0.15) | 0.01 (0.01–0.02) | 0.04 (0.02–0.04) | 0.01 (0.01–0.02) | 0.03 (0.02–0.03) | 0.01 (0.01–0.02) |
| Length of ICU stay, days | 17.0 (13.5) | 16.7 (25.6) | 15.3 (10.4) | 20.7 (15.2) | 16.8 (11.9) | 27.1 (39.0) | 16.0 (14.7) | 21.2 (16.5) |
| Central venous catheter, days | 15.4 (14.4) | 14.6 (26.3) | 11.5 (11.8) | 19.0 (16.4) | 10.0 (11.4) | 17.2 (22.2) | 11.3 (13.2) | 19.7 (15.7) |
| Urinary catheter, days | 16.0 (14.2) | 15.2 (26.2) | 15.3 (10.4) | 20.0 (15.0) | 16.2 (12.0) | 20.0 (23.5) | 15.7 (13.6) | 19.7 (16.7) |
| Invasive ventilation, days | 12.8 (8.9) | 10.9 (12.5) | 19.5 (13.4) | 23.7 (29.8) | 18.4 (35.4) | 22.0 (26.1) | 16.1 (14.7) | 18.1 (16.3) |
| Patients with invasive ventilation | 40 (85.1) | 13 (72.2) | 2 (50.0) | 40 (88.9) | 109 (83.8) | 26 (78.8) | 99 (57.9) | 32 (94.1) |
| Period I | Period III | Period IV | ||||
|---|---|---|---|---|---|---|
| 1 March 2020–10 May 2020 | 24 August 2020–2 February 2021 | 3 February 2021–6 June 2021 | ||||
| aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | |
| COVID-19 | 1.19 (0.25–5.67) | 0.823 | 2.43 (1.26–4.67) | 0.008 | 0.84 (0.48–1.46) | 0.531 |
| Age (years) | 0.99 (0.94–1.03) | 0.553 | 1.03 (1.01–1.04) | 0.001 | 1.01 (0.99–1.03) | 0.317 |
| Sex (male) | 2.50 (0.88–7.10) | 0.085 | 0.93 (0.65–1.35) | 0.717 | 1.16 (0.78–1.71) | 0.460 |
| SAPS II | 0.98 (0.93–1.03) | 0.410 | 1.00 (0.98–1.03) | 0.772 | 1.01 (0.99–1.04) | 0.334 |
| Hypertension | 0.82 (0.30–2.22) | 0.696 | 0.93 (0.62–1.39) | 0.719 | 1.01 (0.66–1.54) | 0.967 |
| Diabetes mellitus | 0.87 (0.22–3.50) | 0.845 | 0.32 (0.10–1.06) | 0.061 | 1.29 (0.82–2.03) | 0.272 |
| Invasive ventilation, days | 0.86 (0.81–0.92) | <0.001 | 1.00 (1.00–1.01) | 0.546 | 0.94 (0.92–0.96) | <0.001 |
| Carbapenems | 0.42 (0.17–1.08) | 0.073 | 0.54 (0.35–0.84) | 0.006 | 0.60 (0.39–0.93) | 0.024 |
| Extended-spectrum cephalosporins | 0.40 (0.12–1.32) | 0.133 | 0.63 (0.34–1.16) | 0.136 | 0.89 (0.58–1.37) | 0.598 |
| Glycopeptides | 0.36 (0.12–1.03) | 0.057 | 0.20 (0.08–0.49) | <0.001 | 0.67 (0.43–1.04) | 0.077 |
| Penicillins | 1.13 (0.24–5.38) | 0.880 | 0.50 (0.31–0.81) | 0.005 | 0.53 (0.34–0.84) | 0.007 |
| Polymixins | 0.79 (0.35–1.80) | 0.576 | 0.64 (0.38–1.08) | 0.097 | 0.68 (0.44–1.06) | 0.088 |
| Macrolides | 0.65 (0.26–1.63) | 0.356 | 0.80 (0.49–1.31) | 0.381 | 0.56 (0.35–0.91) | 0.018 |
| Age * time | 1.00 (1.00–1.01) | 0.030 | ||||
| Glycopeptides * time | 1.08 (1.03–1.12) | <0.001 | ||||
| Diabetes mellitus * time | 1.13 (1.04–1.22) | 0.002 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isonne, C.; Baccolini, V.; Migliara, G.; Ceparano, M.; Alessandri, F.; Ceccarelli, G.; Tellan, G.; Pugliese, F.; De Giusti, M.; De Vito, C.; et al. Comparing the Occurrence of Healthcare-Associated Infections in Patients with and without COVID-19 Hospitalized during the Pandemic: A 16-Month Retrospective Cohort Study in a Hospital Intensive Care Unit. J. Clin. Med. 2022, 11, 1446. https://doi.org/10.3390/jcm11051446
Isonne C, Baccolini V, Migliara G, Ceparano M, Alessandri F, Ceccarelli G, Tellan G, Pugliese F, De Giusti M, De Vito C, et al. Comparing the Occurrence of Healthcare-Associated Infections in Patients with and without COVID-19 Hospitalized during the Pandemic: A 16-Month Retrospective Cohort Study in a Hospital Intensive Care Unit. Journal of Clinical Medicine. 2022; 11(5):1446. https://doi.org/10.3390/jcm11051446
Chicago/Turabian StyleIsonne, Claudia, Valentina Baccolini, Giuseppe Migliara, Mariateresa Ceparano, Francesco Alessandri, Giancarlo Ceccarelli, Guglielmo Tellan, Francesco Pugliese, Maria De Giusti, Corrado De Vito, and et al. 2022. "Comparing the Occurrence of Healthcare-Associated Infections in Patients with and without COVID-19 Hospitalized during the Pandemic: A 16-Month Retrospective Cohort Study in a Hospital Intensive Care Unit" Journal of Clinical Medicine 11, no. 5: 1446. https://doi.org/10.3390/jcm11051446
APA StyleIsonne, C., Baccolini, V., Migliara, G., Ceparano, M., Alessandri, F., Ceccarelli, G., Tellan, G., Pugliese, F., De Giusti, M., De Vito, C., Marzuillo, C., Villari, P., Barone, L. C., Giannini, D., Marotta, D., Marte, M., Mazzalai, E., Germani, I., Bellini, A., ... Zanni, S. (2022). Comparing the Occurrence of Healthcare-Associated Infections in Patients with and without COVID-19 Hospitalized during the Pandemic: A 16-Month Retrospective Cohort Study in a Hospital Intensive Care Unit. Journal of Clinical Medicine, 11(5), 1446. https://doi.org/10.3390/jcm11051446








