Blood Pressure Monitoring and Perinatal Outcomes in Normotensive Women with Gestational Diabetes Mellitus
Abstract
:1. Introduction
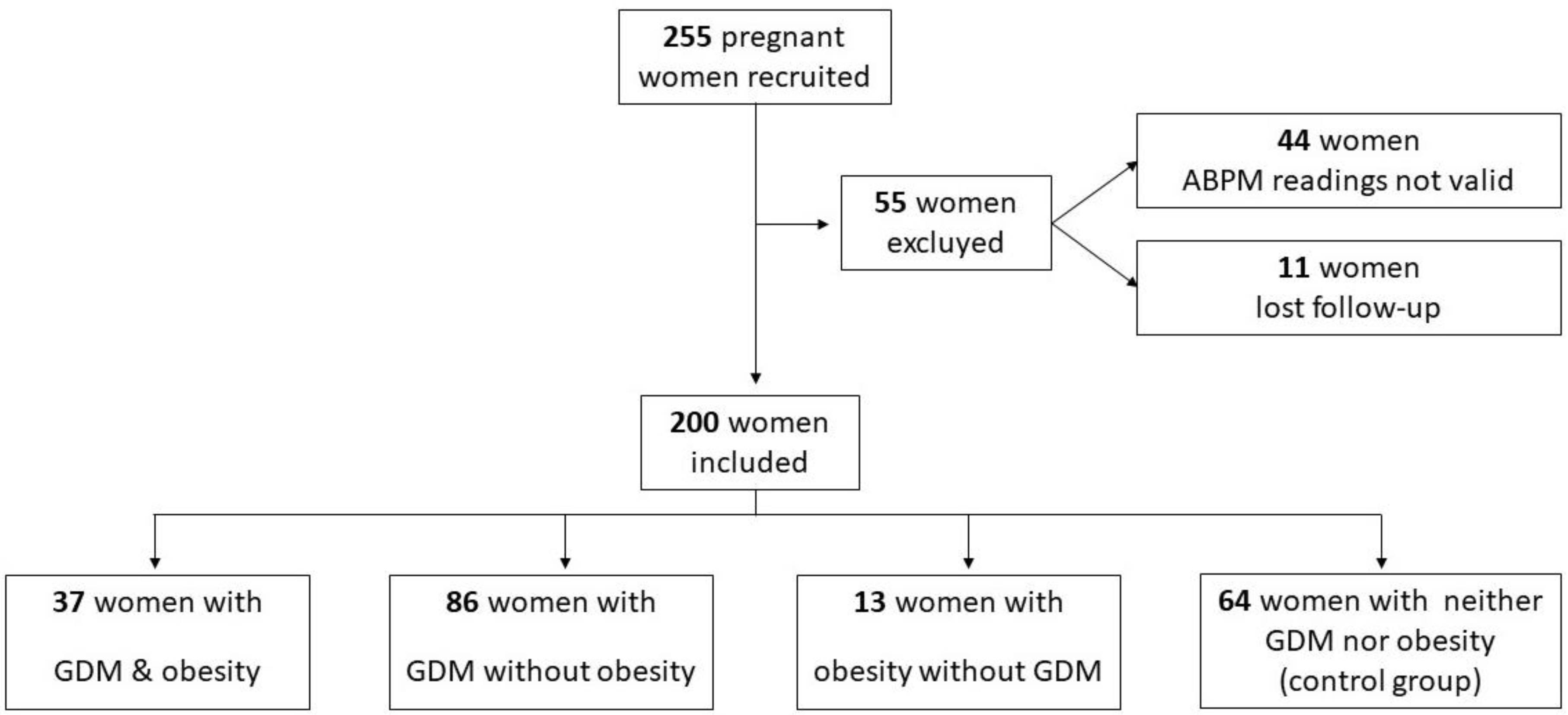
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Statistical Analysis
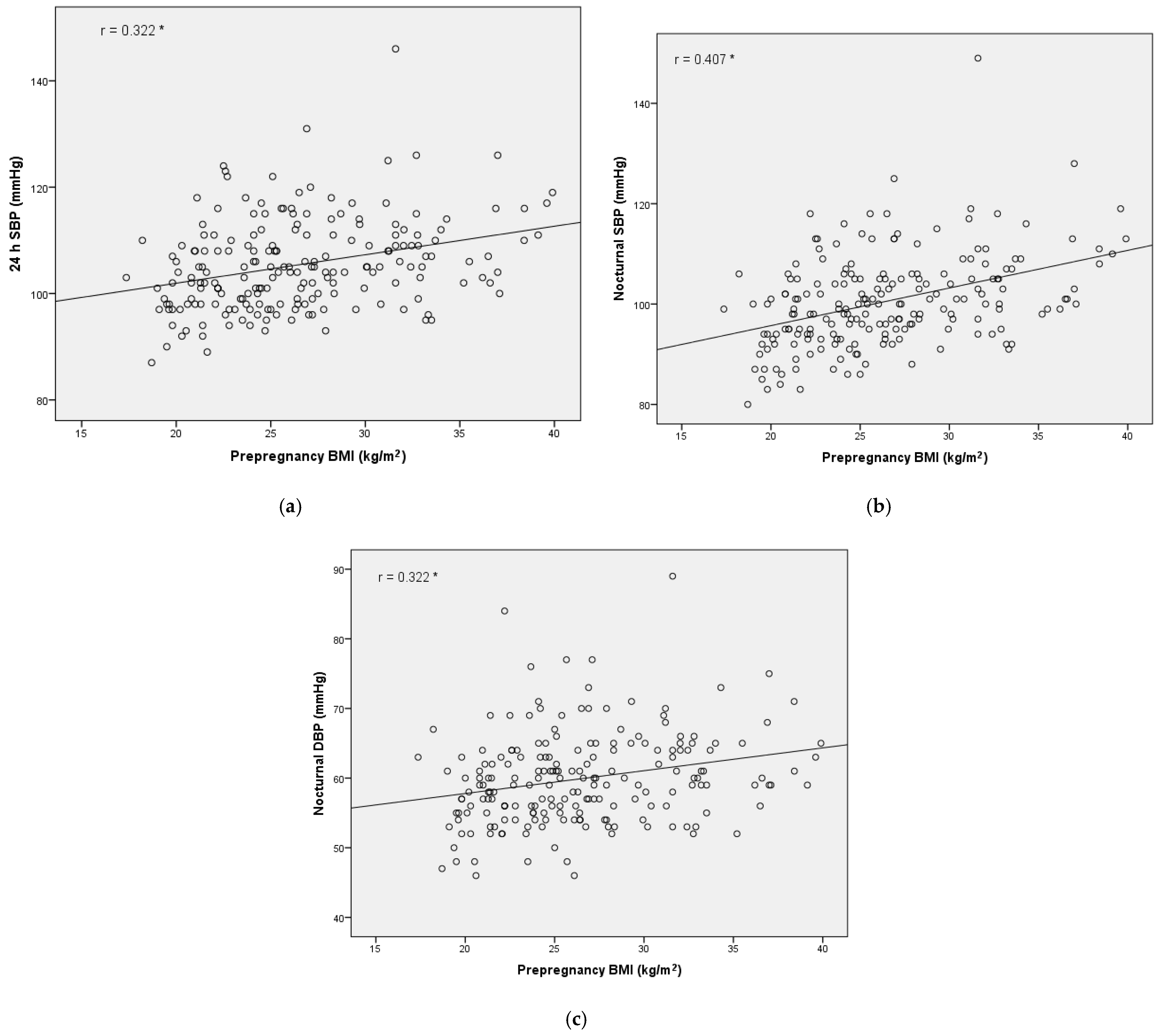
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ananth, C.V.; Basso, O. Impact of pregnancy-induced hypertension on stillbirth and neonatal mortality. Epidemiology 2010, 21, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, W.; Lin, J.; Liu, H.; Yang, Z.; Teng, Y.; Huang, J.; Peng, Q.; Lin, X.; Zhang, J.; et al. Hypertensive disorders of pregnancy and risks of adverse pregnancy outcomes: A retrospective cohort study of 2368 patients. J. Hum. Hypertens. 2021, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Khedagi, A.M.; Bello, N.A. Hypertensive Disorders of Pregnancy. Cardiol. Clin. 2021, 39, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Roberts, C.L.; Ford, J.B.; Algert, C.S.; Antonsen, S.; Chalmers, J.; Cnattingius, S.; Gokhale, M.; Kotelchuck, M.; Melve, K.K.; Langridge, A.; et al. Population-based trends in pregnancy hypertension and pre-eclampsia: An international comparative study. BMJ Open 2014, 1, e000101. [Google Scholar] [CrossRef] [Green Version]
- Sathyapalan, T.; Mellor, D.; Atkin, S.L. Obesity and gestational diabetes. Semin. Fetal Neonatal Med. 2010, 15, 89–93. [Google Scholar] [CrossRef]
- Gorostidi, M.; Banegas, J.R.; de la Sierra, A.; Vinyoles, E.; Segura, J.; Ruilope, L.M. Ambulatory blood pressure monitoring in daily clinical practice—The Spanish ABPM Registry experience. Eur. J. Clin. Investig. 2016, 46, 92–98. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; De La Sierra, A.; De Leeuw, P.; Dolan, E.; et al. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Bhide, A.; Sankaran, S.; Moore, J.; Khalil, A.; Furneaux, E. Ambulatory blood pressure measurements in mid-pregnancy and development of hypertensive pregnancy disorders. Hypertens. Pregnancy 2014, 33, 159–167. [Google Scholar] [CrossRef]
- Saremi, A.T.; Shafiee, M.-A.; Montazeri, M.; Rashidi, N.; Montazeri, M. Blunted Overnight Blood Pressure Dipping in Second Trimester; A Strong Predictor of Gestational Hypertension and Preeclampsia. Curr. Hypertens. Rev. 2018, 15, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.R.; Espeche, W.G.; Leiva Sisnieguez, C.E.; Leiva Sisnieguez, B.C.; Balbín, E.; Stavile, R.N.; March, C.; Olano, R.D.; Soria, A.; Yoma, O.; et al. Nocturnal hypertension in high-risk mid-pregnancies predict the development of preeclampsia/eclampsia. J. Hypertens. 2019, 37, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.R.; Espeche, W.G.; Sisnieguez, B.C.L.; Balbín, E.; Sisnieguez, C.E.L.; Stavile, R.N.; March, C.E.; Grassi, F.; Santillan, C.; Cor, S.; et al. Significance ofmasked and nocturnal hypertension in normotensive women coursing a high-risk pregnancy. J. Hypertens. 2016, 34, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lechuga, B.; Lara-Barea, A.; Córdoba-Doña, J.A.; Montero Galván, A.; Abal Cruz, A.; Aguilar-Diosdado, M.; López-Tinoco, C. Usefulness of blood pressure monitoring in patients with gestational diabetes mellitus. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2018, 65, 394–401. [Google Scholar] [CrossRef]
- Brown, M.A.; Davis, G.K.; McHugh, L. The prevalence and clinical significance of nocturnal hypertension in pregnancy. J. Hypertens. 2001, 19, 1437–1444. [Google Scholar] [CrossRef]
- Ilic, A.; Ilic, D.J.; Tadic, S.; Stefanovic, M.; Stojsic-Milosavljevic, A.; Pavlovic, K.; Redzek, A.; Velicki, L. Influence of non-dipping pattern of blood pressure in gestational hypertension on maternal cardiac function, hemodynamics and intrauterine growth restriction. Pregnancy Hypertens. 2017, 10, 34–41. [Google Scholar] [CrossRef]
- Liro, M.; Gąsowski, J.; Wydra, D.; Grodzicki, T.; Emerich, J.; Narkiewicz, K. Twenty-four-hour and conventional blood pressure components and risk of preterm delivery or neonatal complications in gestational hypertension. Blood Press. 2009, 18, 36–43. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Giannubilo, S.R. Blood pressure is elevated in normotensive pregnant women with intrauterine growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 45–48. [Google Scholar] [CrossRef]
- Prefumo, F.; Muiesan, M.L.; Perini, R.; Paini, A.; Bonzi, B.; Lojacono, A.; Agabiti-Rosei, E.; Frusca, T. Maternal cardiovascular function in pregnancies complicated by intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2008, 31, 65–71. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Hypertension in Pregnancy: The Management of Hypertensive Disorders during Pregnancy; RCOG Press: London, UK, 2010. [Google Scholar]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Benedetto, A.; D’anna, R.; Cannata, M.L.; Giordano, D.; Interdonato, M.L.; Corrado, F. Effects of prepregnancy body mass index and weight gain during pregnancy on perinatal outcome in glucose-tolerant women. Diabetes Metab. 2012, 38, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Huet, J.; Beucher, G.; Rod, A.; Morello, R.; Dreyfus, M. Joint impact of gestational diabetes and obesity on perinatal outcomes. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.S.; Rebarber, A.; Fox, N.S.; Klauser, C.K.; Istwan, N.; Rhea, D.; Saltzman, D. The effect of maternal obesity on pregnancy outcomes in women with gestational diabetes. J. Matern. Neonatal Med. 2011, 24, 723–727. [Google Scholar] [CrossRef]
- Ricart, W.; López, J.; Mozas, J.; Pericot, A.; Sancho, M.A.; González, N.; Balsells, M.; Luna, R.; Cortázar, A.; Navarro, P.; et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia 2005, 48, 1736–1742. [Google Scholar] [CrossRef] [Green Version]
- Callaway, L.K.; Prins, J.B.; Chang, A.M.; Mcintyre, H.D. Australian obstetric population. Med. J. Aust. 2006, 184, 56–59. [Google Scholar] [CrossRef]
- Athukorala, C.; Rumbold, A.R.; Willson, K.J.; Crowther, C.A. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth 2010, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Scifres, C.; Feghali, M.; Althouse, A.D.; Caritis, S.; Catov, J. Adverse Outcomes and Potential Targets for Intervention in Gestational Diabetes and Obesity. Obstet. Gynecol. 2015, 126, 316–325. [Google Scholar] [CrossRef]
- Wahabi, H.A.; Fayed, A.A.; Alzeidan, R.A.; Mandil, A.A. The independent effects of maternal obesity and gestational diabetes on the pregnancy outcomes. BMC Endocr. Disord. 2014, 14, 47. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Brown, M.A. Is there a role for ambulatory blood pressure monitoring in pregnancy? Clin. Exp. Pharmacol. Physiol. 2014, 41, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Murmu, S.; Dwivedi, J. Second-Trimester Maternal Serum Beta-Human Chorionic Gonadotropin and Lipid Profile as a Predictor of Gestational Hypertension, Preeclampsia, and Eclampsia: A Prospective Observational Study. Int. J. Appl. Basic Med. Res. 2020, 10, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Adank, M.C.; Benschop, L.; Peterbroers, K.R.; Smak Gregoor, A.M.; Kors, A.W.; Mulder, M.T.; Schalekamp-Timmermans, S.; Roeters Van Lennep, J.E.; Steegers, E.A.P. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am. J. Obstet. Gynecol. 2019, 221, 150.e1–150.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateo-Gavira, I.; Vílchez-López, F.J.; García-Palacios, M.V.; Carral-San Laureano, F.; Visiedo-García, F.M.; Aguilar-Diosdado, M. Early blood pressure alterations are associated with pro-inflammatory markers in type 1 diabetes mellitus. J. Hum. Hypertens. 2017, 31, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Zhang, W. Joint and independent associations of gestational weight gain and pre-pregnancy body mass index with outcomes of pregnancy in Chinese women: A retrospective cohort study. PLoS ONE 2015, 10, e0136850. [Google Scholar] [CrossRef] [PubMed]
- Soydinc, H.E.; Davutoglu, V.; Sak, M.E.; Ercan, S.; Evsen, M.S.; Kaya, H.; Oylumlu, M.; Buyukaslan, H.; Sari, I. Circadian variation of blood pressure is impaired in normotensive pregnant women with gestational diabetes mellitus. Clin. Exp. Hypertens. 2013, 35, 128–133. [Google Scholar] [CrossRef]
- Zhong, L.; Deng, W.; Zheng, W.; Yu, S.; Huang, X.; Wen, Y.; Chiu, P.C.N.; Lee, C.L. The relationship between circadian blood pressure variability and maternal/perinatal outcomes in women with preeclampsia with severe features. Hypertens. Pregnancy 2020, 39, 405–410. [Google Scholar] [CrossRef]
- Lv, L.J.; Ji, W.J.; Wu, L.L.; Miao, J.; Wen, J.Y.; Lei, Q.; Duan, D.M.; Chen, H.; Hirst, J.E.; Henry, A.; et al. Thresholds for Ambulatory Blood Pressure Monitoring Based on Maternal and Neonatal Outcomes in Late Pregnancy in a Southern Chinese Population. J. Am. Heart Assoc. 2019, 8, e012027. [Google Scholar] [CrossRef] [Green Version]
| Variables | GDM and Obesity (n = 37) | GDM without Obesity (n = 86) | Obesity without GDM (n = 13) | Control (n = 64) | p-Value |
|---|---|---|---|---|---|
| Maternal age (y) | 33.97 ± 3.956 | 34.96 ± 4.182 | 30.85 ± 5.414 | 33.36 ± 4.876 | 0.009 a |
| Prepregnancy BMI (kg/m2) | 33.81 ± 2.51 | 24.27 ± 3.02 | 32.97 ± 3.15 | 23.80 ± 2.79 | <0.001 b |
| Family history DM | 19 (51.45%) | 38 (44.2%) | 6 (46.2%) | 22 (34.4%) | 0.4 |
| Family history AHT | 16 (43.2%) | 38 (44.2%) | 5 (38.5%) | 23 (35.9%) | 0.8 |
| Parity | 0.5 | ||||
| Nulliparous | 16 (43.2%) | 33 (38.4%) | 7 (53.8%) | 28 (43.8%) | |
| Multiparous | 21 (56.8%) | 53 (61.6%) | 6 (46.2%) | 36 (56.2%) | |
| Previous history of GDM | 9 (24.3%) | 21 (24.4%) | 0 | 5 (7.8%) | 0.01 |
| Office SBP (mmHg) | 114.7 ± 12.2 | 107.6 ± 15.6 | 116.2 ± 12.4 | 110.6 ± 11.3 | 0.02 |
| Office DBP (mmHg) | 70.9 ± 8.8 | 65.2 ± 8.6 | 67.6 ± 7.7 | 65.2 ± 8.5 | 0.005 c |
| Total cholesterol (mmol/L) | 6.13 ± 1.21 | 6.47 ± 1.22 | 6.27 ± 1.33 | 6.46 ± 0.99 | 0.5 |
| LDL-cholesterol (mmol/L) | 3.75 ± 1.48 | 3.77 ± 1.28 | 3.29 ± 1.15 | 3.46 ± 0.97 | 0.5 |
| HDL-cholesterol (mmol/L) | 1.91 ± 0.55 | 1.92 ± 0.43 | 1.89 ± 0.47 | 1.99 ± 0.54 | 0.9 |
| Triglycerides (mmol/L) | 2.55 ± 1.15 | 2.16 ± 0.72 | 2.46 ± 0.88 | 2.01 ± 0.7 | 0.012 d |
| HbA1c (mmol/mol) | 33.3 ± 2.62 | 30.4 ± 2.03 | 29.3 ± 2.03 | 29.2 ± 1.87 | <0.001 e |
| 24 h SBP (mmHg) | 109.19 ± 9.61 | 104.27 ± 8.46 | 108.15 ± 7.58 | 104.19 ± 7.45 | 0.009 f |
| 24 h DBP (mmHg) | 66.73 ± 5.45 | 64.53 ± 6.66 | 64.38 ± 4.42 | 64.69 ± 5.45 | 0.3 |
| Daytime SBP (mmHg) | 110.89 ± 9.51 | 106.64 ± 9.15 | 109.15 ± 8.57 | 106.55 ± 8.04 | 0.06 |
| Daytime DBP (mmHg) | 68.54 ± 5.53 | 66.97 ± 6.90 | 65.77 ± 5.31 | 66.97 ± 5.86 | 0.46 |
| Nocturnal SBP (mmHg) | 105.84 ± 10.90 | 99.15 ± 8.68 | 105.31 ± 7.67 | 98.39 ± 7.60 | <0.001 g |
| Nocturnal DBP (mmHg) | 62.70 ± 7.04 | 59.28 ± 7.08 | 60.38 ± 5.63 | 59.03 ± 5.49 | 0.034 h |
| Non-dipper pattern | 22 (59.5%) | 42 (48.8%) | 9 (69.2%) | 20 (31.3%) | 0.01 |
| Variables | GDM and Obesity (n = 37) | GDM without Obesity (n = 86) | Obesity without GDM (n = 13) | Control (n = 64) | p-Value |
|---|---|---|---|---|---|
| GDM treatment | - | - | 0.05 | ||
| Diet only | 15 (40.5%) | 50 (58.1%) | |||
| Diet + insulin | 22 (59.5%) | 36 (41.9%) | |||
| Total doses insulin (UI) | 20.95 ± 17.06 | 19.92 ± 14.21 | 0.8 | ||
| ASA prophylaxis | 11 (29.7%) | 7 (8.1%) | 3 (23.1%) | 3 (4.7%) | 0.001 |
| Development of HDPs | 6 (16.2%) | 5 (5.8%) | 3 (23.1%) | 2 (3.1%) | 0.02 |
| Preeclampsia | 2 (5.4%) | 2(2.3%) | 0 | 1 (1.6%) | 0.6 |
| Gestational hypertension | 6 (16.2%) | 5 (5.8%) | 3 (23.1%) | 1 (1.6%) | 0.007 |
| Gestational age at delivery (week) | 38.47 ± 1.30 | 36.16 ± 1.37 | 40.12 ± 0.92 | 39.54 ± 1.23 | <0.001 a |
| Preterm delivery < 37 week | 4 (10.8%) | 6 (7.0%) | 0 | 0 | 0.06 |
| Weight gain (kg) | 7.30 ± 4.32 | 8.40 ± 4.17 | 9.15 ± 5.05 | 10.86 ± 4.29 | 0.001 b |
| Instrumental delivery | 10 (27%) | 25 (29.1%) | 1 (7.7%) | 12 (18.8%) | 0.1 |
| Cesarean section | 14 (37.8%) | 19 (22.1%) | 6 (46.2%) | 16 (25%) | 0.1 |
| Childbirth complications | 25 (67.6%) | 39 (45.3%) | 6 (46.2%) | 22 (34.4%) | 0.015 |
| Birthweight (g) | 3212 ± 615 | 3188 ± 465 | 3391 ± 421 | 3313 ± 475 | 0.3 |
| FGR | 3 (8.1%) | 5 (5.8%) | 1 (7.7%) | 5 (7.8%) | 0.9 |
| SGA | 7 (18.9%) | 9 (10.5%) | 2 (15.4%) | 8 (12.5%) | 0.6 |
| LGA | 5 (13.5%) | 8 (9.3%) | 0 | 8 (12.5%) | 0.5 |
| Neonatal adverse outcomes | 8 (21.6%) | 12 (14.2%) | 2 (15.4%) | 5 (7.8%) | 0.3 |
| Variables | Development of HDPs | Preterm Delivery | Neonatal Adverse Outcomes | |||
|---|---|---|---|---|---|---|
| Yes (n = 16) | No (n = 184) | Yes (n = 10) | No (n = 190) | Yes (n = 27) | No (n = 173) | |
| 24 h SBP (mmHg) | 113.1 ± 13.5 * | 104.7 ± 7.6 * | 114.5 ± 14.5 * | 104.9 ± 7.8 * | 107.9 ± 11.1 | 105 ± 7.9 |
| 24 h DBP (mmHg) | 68.8 ± 8.9 | 64.6 ± 5.5 | 72.1 ± 8.1 * | 64.6 ± 5.6 * | 67.0 ± 7.9 | 64.6 ± 5.5 |
| Daytime SBP (mmHg) | 115.6 ± 12.5 * | 106.9 ± 8.2 * | 115.9 ± 13.6 | 107.1 ± 8.4 | 109.7 ± 10.8 | 107.2 ± 8.5 |
| Daytime DBP (mmHg) | 71.1 ± 8.1 * | 66.8 ± 5.9 * | 73.6 ± 7.8 * | 66.8 ± 5.9 * | 68.9 ± 8.0 | 66.9 ± 5.9 |
| Nocturnal SBP (mmHg) | 107.8 ± 16.7 * | 99.9 ± 7.9 * | 112.0 ± 17.4 * | 99.9 ± 8.1 * | 103.8 ± 13.2 * | 100 ± 8.3 * |
| Nocturnal DBP (mmHg) | 62.9 ± 11.4 | 59.6 ± 5.9 | 68.9 ± 9.8 * | 59.4 ± 6.1 * | 62.7 ± 8.6 * | 59.4 ± 6.1 * |
| Non-dipper pattern | 7 (43.8%) | 86 (46.7%) | 9 (90.0%) * | 84 (44.2%) * | 15 (55.6%) | 78 (45.1%) |
| Variables | B | p-Value | Exp (B) | 95% CI for Exp (B) |
|---|---|---|---|---|
| Nocturnal SBP | 0.074 | 0.015 | 1.077 | 1.015–1.143 |
| Prepregnancy BMI | 0.123 | 0.035 | 1.131 | 1.009–1.268 |
| Dipper pattern | 0.990 | 0.112 | 2.691 | 0.793–9.130 |
| GDM | 0.077 | 0.902 | 1.080 | 0.316–3.685 |
| Age | −0.033 | 0.601 | 0.967 | 0.854–1.096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Barea, A.; Sánchez-Lechuga, B.; Vidal-Suárez, Á.; Arroba, A.I.; Bugatto, F.; López-Tinoco, C. Blood Pressure Monitoring and Perinatal Outcomes in Normotensive Women with Gestational Diabetes Mellitus. J. Clin. Med. 2022, 11, 1435. https://doi.org/10.3390/jcm11051435
Lara-Barea A, Sánchez-Lechuga B, Vidal-Suárez Á, Arroba AI, Bugatto F, López-Tinoco C. Blood Pressure Monitoring and Perinatal Outcomes in Normotensive Women with Gestational Diabetes Mellitus. Journal of Clinical Medicine. 2022; 11(5):1435. https://doi.org/10.3390/jcm11051435
Chicago/Turabian StyleLara-Barea, Almudena, Begoña Sánchez-Lechuga, Álvaro Vidal-Suárez, Ana I. Arroba, Fernando Bugatto, and Cristina López-Tinoco. 2022. "Blood Pressure Monitoring and Perinatal Outcomes in Normotensive Women with Gestational Diabetes Mellitus" Journal of Clinical Medicine 11, no. 5: 1435. https://doi.org/10.3390/jcm11051435
APA StyleLara-Barea, A., Sánchez-Lechuga, B., Vidal-Suárez, Á., Arroba, A. I., Bugatto, F., & López-Tinoco, C. (2022). Blood Pressure Monitoring and Perinatal Outcomes in Normotensive Women with Gestational Diabetes Mellitus. Journal of Clinical Medicine, 11(5), 1435. https://doi.org/10.3390/jcm11051435






