Raman Spectroscopic Study of Amyloid Deposits in Gelatinous Drop-like Corneal Dystrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Clinical Data
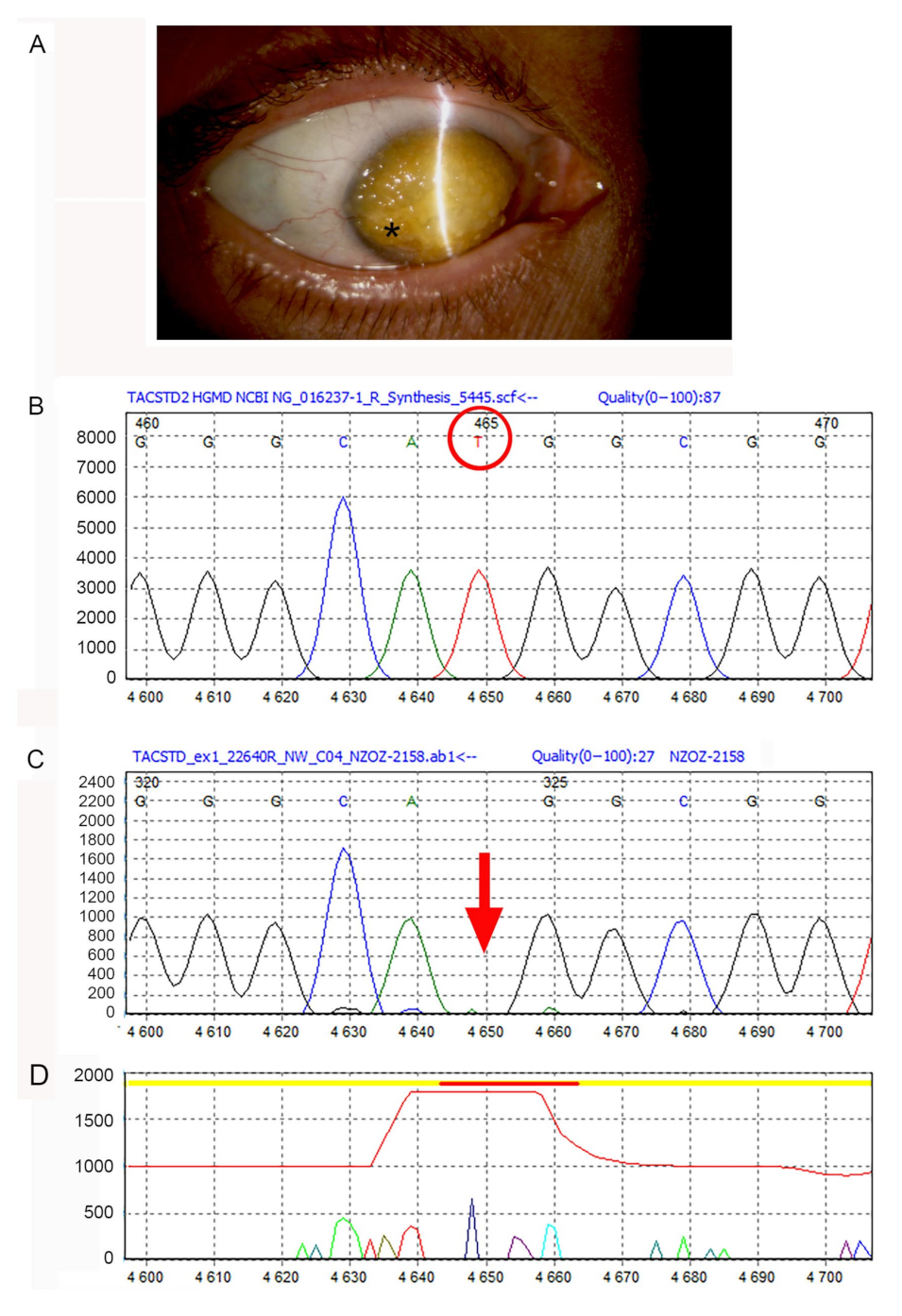
2.3. Genetic Analysis
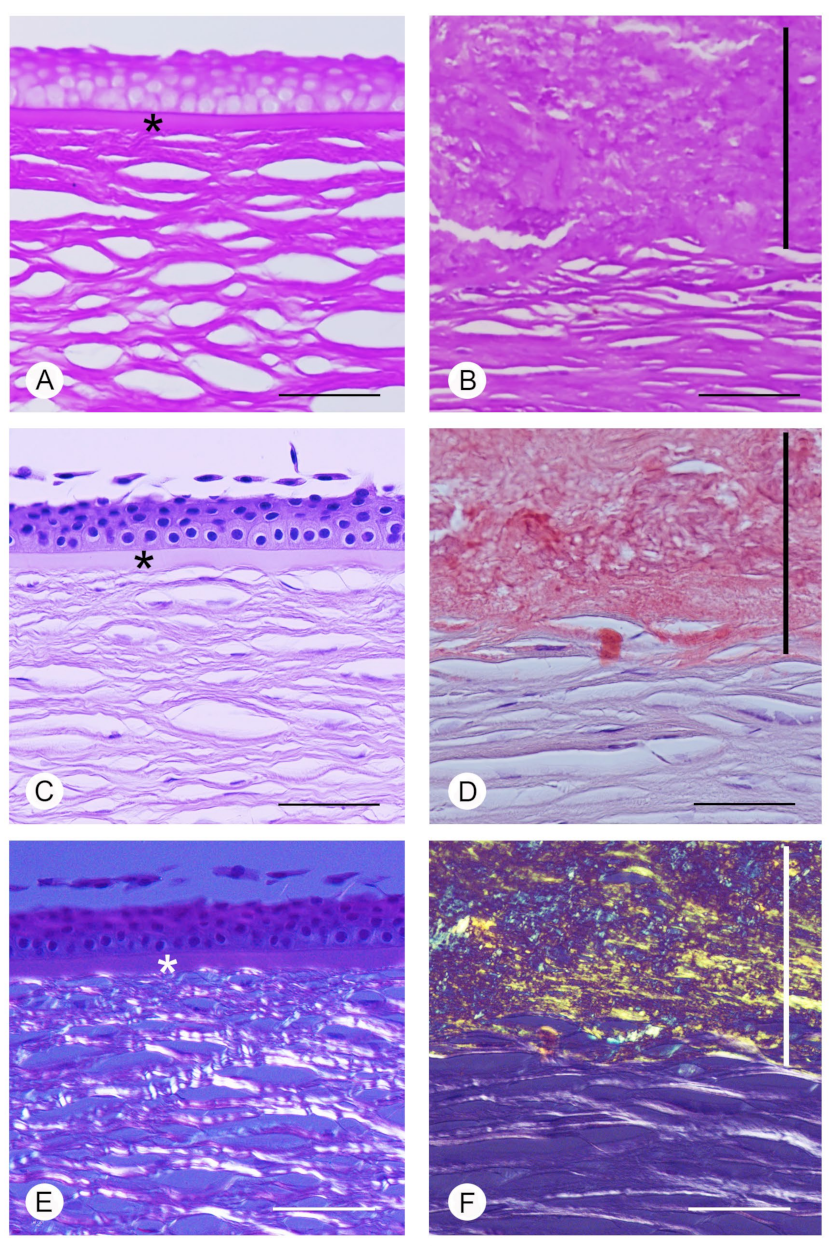
2.4. Light Microscopy
2.5. Raman Spectroscopy Measurements
3. Results
3.1. Genetic Analysis Data
3.2. Light Microscopy Data
3.3. Raman Spectroscopy Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Beuerman, R.W.; Pedroza, L. Ultrastructure of the human cornea. Microsc. Res. Tech. 1996, 33, 320–335. [Google Scholar] [CrossRef]
- Moshirfar, M.; Bennett, P.; Ronquillo, Y. Corneal Dystrophy. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shukla, A.N.; Cruzat, A.; Hamrah, P. Confocal Microscopy of Corneal Dystrophies. Semin. Ophthalmol. 2012, 27, 107–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markoff, A.; Bogdanova, N.; Uhlig, C.E.; Groppe, M.; Horst, J.; Kennerknecht, I. A novel TACSTD2 gene mutation in a Turkish family with a gelatinous drop-like corneal dystrophy. Mol. Vis. 2006, 12, 1473–1476. [Google Scholar] [PubMed]
- Kaza, H.; Barik, M.R.; Reddy, M.M.; Mittal, R.; Das, S. Gelatinous drop-like corneal dystrophy: A review. Br. J. Ophthalmol. 2016, 101, 10–15. [Google Scholar] [CrossRef]
- Weiss, J.S.; Møller, H.U.; Aldave, A.J.; Seitz, B.; Bredrup, C.; Kivelä, T.; Munier, F.L.; Rapuano, C.J.; Nischal, K.K.; Kim, E.K.; et al. IC3D Classification of Corneal Dystrophies—Edition 2. Cornea 2015, 34, 117–159. [Google Scholar] [CrossRef] [Green Version]
- Tsujikawa, M.; Kurahashi, H.; Tanaka, T.; Okada, M.; Yamamoto, S.; Maeda, N.; Watanabe, H.; Inoue, Y.; Kiridoshi, A.; Matsumoto, K.; et al. Homozygosity Mapping of a Gene Responsible for Gelatinous Drop–like Corneal Dystrophy to Chromosome 1p. Am. J. Hum. Genet. 1998, 63, 1073–1077. [Google Scholar] [CrossRef] [Green Version]
- Tsujikawa, M. Road to a Genetic Model of Gelatinous Drop-Like Corneal Dystrophy. Cornea 2018, 37 (Suppl. S1), S91–S93. [Google Scholar] [CrossRef]
- Oliver, V.F.; Vincent, A.L. The Genetics and Pathophysiology of IC3D Category 1 Corneal Dystrophies: A Review. Asia Pac. J. Ophthalmol. 2016, 5, 1–281. [Google Scholar] [CrossRef]
- Dammacco, R.; Merlini, G.; Lisch, W.; Kivelä, T.T.; Giancipoli, E.; Vacca, A.; Dammacco, F. Amyloidosis and Ocular Involvement: An Overview. Semin. Ophthalmol. 2020, 35, 7–26. [Google Scholar] [CrossRef]
- Masmali, A.; Alkanaan, A.; Alkatan, H.M.; Kirat, O.; Almutairi, A.A.; Almubrad, T.; Akhtar, S. Clinical and Ultrastructural Studies of Gelatinous Drop-Like Corneal Dystrophy (GDLD) of a Patient with TACSTD2 Gene Mutation. J. Ophthalmol. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venuti, V.; Crupi, V.; Fazio, B.; Majolino, D.; Acri, G.; Testagrossa, B.; Stancanelli, R.; De Gaetano, F.; Gagliardi, A.; Paolino, D.; et al. Physicochemical Characterization and Antioxidant Activity Evaluation of Idebenone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Biomolecules 2019, 9, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, C.; de la Lastra, J.P.; Plou, F.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A.; Matuszyk, E.; Radwan, B.; Rocchetti, S.; Chlopicki, S.; Baranska, M. Toward Raman Subcellular Imaging of Endothelial Dysfunction. J. Med. Chem. 2021, 64, 4396–4409. [Google Scholar] [CrossRef]
- Chen, T.; Yavuz, A.; Wang, M.C. Dissecting lipid droplet biology with coherent Raman scattering microscopy. J. Cell Sci. 2021, 135, jcs252353. [Google Scholar] [CrossRef]
- Ma, L.; Chen, L.; Chou, K.C.; Lu, X. Campylobacter jejuni Antimicrobial Resistance Profiles and Mechanisms Determined Using a Raman Spectroscopy-Based Metabolomic Approach. Appl. Environ. Microbiol. 2021, 87, e0038821. [Google Scholar] [CrossRef]
- Choo-Smith, L.-P.; Edwards, H.G.M.; Endtz, H.P.; Kros, J.M.; Heule, F.; Barr, H.; Jrobinson, J.S.R.; Bruining, H.A.; Puppels, G.J. Medical applications of Raman spectroscopy: From proof of principle to clinical implementation. Biopolymers 2002, 67, 1–9. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Acri, G.; Venuti, V.; Costa, S.; Testagrossa, B.; Pellegrino, S.; Crupi, V.; Majolino, D. Raman Spectroscopy as Noninvasive Method of Diagnosis of Pediatric Onset Inflammatory Bowel Disease. Appl. Sci. 2020, 10, 6974. [Google Scholar] [CrossRef]
- Acri, G.; Testagrossa, B.; Giudice, E.; Arfuso, F.; Piccione, G.; Giannetto, C. Application of Raman Spectroscopy for the Evaluation of Metabolomic Dynamic Analysis in Athletic Horses. J. Equine Vet. Sci. 2021, 96, 103319. [Google Scholar] [CrossRef]
- Tanwar, S.; Paidi, S.K.; Prasad, R.; Pandey, R.; Barman, I. Advancing Raman spectroscopy from research to clinic: Translational potential and challenges. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119957. [Google Scholar] [CrossRef]
- Filik, J.; Stone, N. Analysis of human tear fluid by Raman spectroscopy. Anal. Chim. Acta 2008, 616, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Goddard, J.M.; Barr, H.; Stone, N. Surface enhanced Raman scattering of herpes simplex virus in tear film. Photodiagnosis Photodyn. Ther. 2008, 5, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Kruger, E.F.; Minko, G.; Liu, C.H.; Rosen, R.B.; Alfano, R.R. Detection of glutamate in the eye by Raman spectroscopy. J. Biomed. Opt. 2003, 8, 167–172. [Google Scholar] [CrossRef]
- Pelletier, C.C.; Lambert, J.L.; Borchert, M. Determination of Glucose in Human Aqueous Humor Using Raman Spectroscopy and Designed-Solution Calibration. Appl. Spectrosc. 2005, 59, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Marro, M.; Taubes, A.; Abernathy, A.; Balint, S.; Moreno, B.; Sanchez-Dalmau, B.; Martínez-Lapiscina, E.H.; Amat-Roldan, I.; Petrov, D.; Villoslada, P. Dynamic molecular monitoring of retina inflammation by in vivo Raman spectroscopy coupled with multivariate analysis. J. Biophotonics 2014, 7, 724–734. [Google Scholar] [CrossRef]
- Hu, J.; Wu, F.; Huang, Z.; Ma, S.; Zhang, J.; Yang, J.; Han, X.; Xu, G. Raman Spectroscopy Analysis of the Biochemical Characteristics of Experimental Keratomycosis. Curr. Eye Res. 2016, 41, 1408–1413. [Google Scholar] [CrossRef]
- Cinotti, E.; Perrot, J.L.; Labeille, B.; Espinasse, M.; Ouerdane, Y.; Boukenter, A.; Thuret, G.; Gain, P.; Campolmi, N.; Douchet, C.; et al. Optical diagnosis of a metabolic disease: Cystinosis. J. Biomed. Opt. 2013, 18, 046013. [Google Scholar] [CrossRef]
- Kaji, Y.; Akiyama, T.; Segawa, H.; Oshika, T.; Kano, H. Raman Microscopy: A Noninvasive Method to Visualize the Localizations of Biomolecules in the Cornea. Cornea 2017, 36 (Suppl. S1), S67–S71. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman Spectroscopy for Monitoring Protein Structure in Muscle Food Systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 512–523. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Acri, G.; Giudice, E.; Arfuso, F.; Testagrossa, B.; Piccione, G. Quantifying Serum Total Lipids and Tryptophan Concentrations by Raman Spectroscopy during Standardized Obstacle Course in Horses. J. Equine Vet. Sci. 2022, 108, 103820. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Montorio, D.; Morra, V.B.; Criscuolo, C.; Lanzillo, R.; Salvatore, E.; Camerlingo, C.; Lisitskiy, M.; Delfino, I.; Portaccio, M.; et al. Surface-enhanced Raman spectroscopy of tears: Toward a diagnostic tool for neurodegenerative disease identification. J. Biomed. Opt. 2020, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zavatski, S.; Khinevich, N.; Girel, K.; Redko, S.; Kovalchuk, N.; Komissarov, I.; Lukashevich, V.; Semak, I.; Mamatkulov, K.; Vorobyeva, M.; et al. Surface Enhanced Raman Spectroscopy of Lactoferrin Adsorbed on Silvered Porous Silicon Covered with Graphene. Biosensors 2019, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camerlingo, C.; Lisitskiy, M.; Lepore, M.; Portaccio, M.; Montorio, D.; Del Prete, S.; Cennamo, G. Characterization of Human Tear Fluid by Means of Surface-Enhanced Raman Spectroscopy. Sensors 2019, 19, 1177. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, S.; Nishida, K.; Dota, A.; Inatomi, T.; Koizumi, N.; Elliott, A.; Lewis, D.; Quantock, A.; Fullwood, N. Epithelial Barrier Function and Ultrastructure of Gelatinous Drop-like Corneal Dystrophy. Cornea 2000, 19, 551–555. [Google Scholar] [CrossRef]
- Takaoka, M.; Nakamura, T.; Ban, Y.; Kinoshita, S. Phenotypic Investigation of Cell Junction–Related Proteins in Gelatinous Drop-Like Corneal Dystrophy. Investig. Opthalmol. Vis. Sci. 2007, 48, 1095–1101. [Google Scholar] [CrossRef]
- Acri, G.; Romano, C.; Costa, S.; Pellegrino, S.; Testagrossa, B. Raman Spectroscopy Technique: A Non-Invasive Tool in Celiac Disease Diagnosis. Diagnostics 2021, 11, 1277. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: A review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Kurouski, D.; Van Duyne, R.P.; Lednev, I.K. Exploring the structure and formation mechanism of amyloid fibrils by Raman spectroscopy: A review. Analyst 2015, 140, 4967–4980. [Google Scholar] [CrossRef]
- McKay, T.B.; Priyadarsini, S.; Rowsey, T.; Karamichos, D. Arginine Supplementation Promotes Extracellular Matrix and Metabolic Changes in Keratoconus. Cells 2021, 10, 2076. [Google Scholar] [CrossRef] [PubMed]
- Mencucci, R.; Marini, M.; Gheri, G.; Vichi, D.; Sarchielli, E.; Bonaccini, L.; Ambrosini, S.; Thyrion, G.D.Z.; Paladini, I.; Vannelli, G.B.; et al. Lectin binding in normal, keratoconus and cross-linked human corneas. Acta Histochem. 2011, 113, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, B.; Foley, S.; Cardey, B.; Enescu, M. An experimental and theoretical study of the amino acid side chain Raman bands in proteins. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Punihaole, D.; Workman, R.J.; Hong, Z.; Madura, J.D.; Asher, S.A. Polyglutamine Fibrils: New Insights into Antiparallel β-Sheet Conformational Preference and Side Chain Structure. J. Phys. Chem. B 2016, 120, 3012–3026. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.; Singh, V.K.; Baral, B.; Pathak, D.K.; Jayabalan, J.; Kumar, R.; Tapryal, S.; Jha, H.C. Indication of Neurodegenerative Cascade Initiation by Amyloid-like Aggregate-Forming EBV Proteins and Peptide in Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, S.R.; Aspden, R.M. Raman Microscopy and Bone. Methods Mol. Biol. 2019, 1914, 651–659. [Google Scholar] [CrossRef]
- Faoláin, E.O.; Hunter, M.B.; Byrne, J.M.; Kelehan, P.; Lambkin, H.A.; Byrne, H.J.; Lyng, F.M. Raman Spectroscopic Evaluation of Efficacy of Current Paraffin Wax Section Dewaxing Agents. J. Histochem. Cytochem. 2005, 53, 121–129. [Google Scholar] [CrossRef] [Green Version]
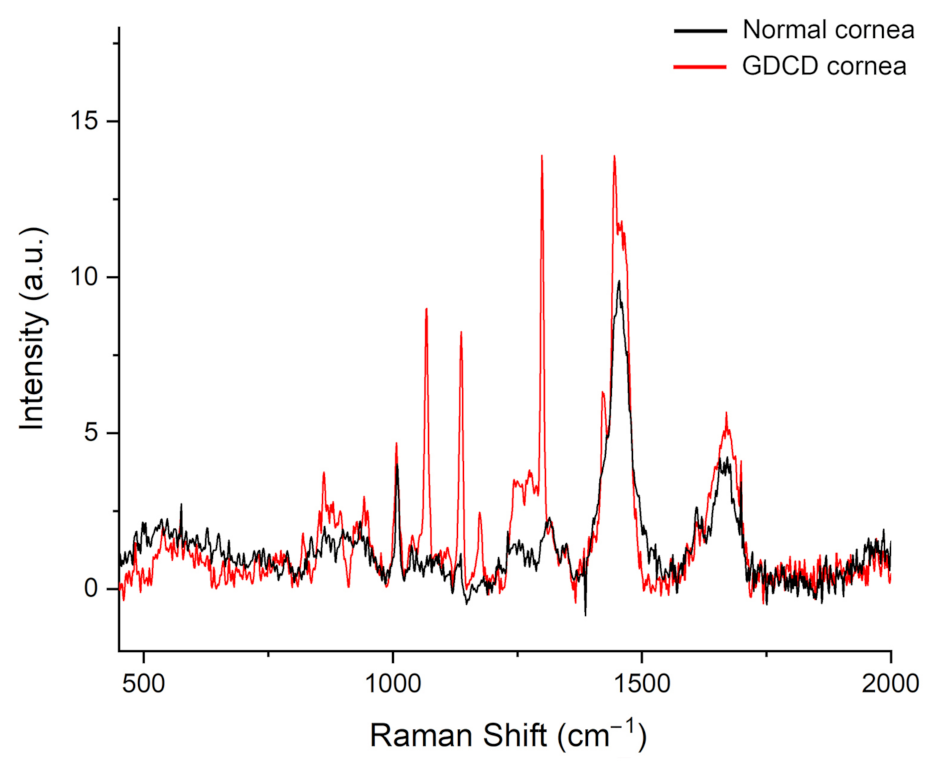
| Frequency (cm−1) | Tentative Assignment | Normal Cornea | GDCD Cornea | References |
|---|---|---|---|---|
| 855 | Tyrosine | visible | visible | [32] |
| 886 | Tryptophan | visible | visible | [25,33] |
| 940 | Proline, valine | visible | visible | [28] |
| 1005 | Phenylalanine | visible | visible | [28,32] |
| 1068 | Unassigned | ----- | visible | ----- |
| 1138 | Unassigned | ----- | visible | ----- |
| 1173 | Tyrosine | visible | visible | [34] |
| 1200–1300 | Amide III band | visible | visible | [34] |
| 1421 | Unassigned | ------ | visible | ----- |
| 1440 | Vibrational stretching CH2 | visible | visible | [35] |
| 1650 | Amide I band | visible | visible | [35,36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acri, G.; Micali, A.; D’Angelo, R.; Puzzolo, D.; Aragona, P.; Testagrossa, B.; Aragona, E.; Wylegala, E.; Nowinska, A. Raman Spectroscopic Study of Amyloid Deposits in Gelatinous Drop-like Corneal Dystrophy. J. Clin. Med. 2022, 11, 1403. https://doi.org/10.3390/jcm11051403
Acri G, Micali A, D’Angelo R, Puzzolo D, Aragona P, Testagrossa B, Aragona E, Wylegala E, Nowinska A. Raman Spectroscopic Study of Amyloid Deposits in Gelatinous Drop-like Corneal Dystrophy. Journal of Clinical Medicine. 2022; 11(5):1403. https://doi.org/10.3390/jcm11051403
Chicago/Turabian StyleAcri, Giuseppe, Antonio Micali, Rosalia D’Angelo, Domenico Puzzolo, Pasquale Aragona, Barbara Testagrossa, Emanuela Aragona, Edward Wylegala, and Anna Nowinska. 2022. "Raman Spectroscopic Study of Amyloid Deposits in Gelatinous Drop-like Corneal Dystrophy" Journal of Clinical Medicine 11, no. 5: 1403. https://doi.org/10.3390/jcm11051403
APA StyleAcri, G., Micali, A., D’Angelo, R., Puzzolo, D., Aragona, P., Testagrossa, B., Aragona, E., Wylegala, E., & Nowinska, A. (2022). Raman Spectroscopic Study of Amyloid Deposits in Gelatinous Drop-like Corneal Dystrophy. Journal of Clinical Medicine, 11(5), 1403. https://doi.org/10.3390/jcm11051403







