Abstract
Neonatal sepsis remains a leading cause of morbidity and mortality worldwide. It is widely considered that exchange transfusion (ET) as an adjunctive treatment for neonatal sepsis has the ability to reduce mortality. This review summarizes the current knowledge regarding the efficacy of ET for neonatal sepsis. In neonatal sepsis, immune responses such as proinflammatory and anti-inflammatory cytokines play an important role in pathogenesis and can lead to septic shock, multiple organ failure, and death. Between the 1970s and 1990s several authors reported that ET was effective in the treatment of neonatal sepsis with sclerema. ET removes bacterial toxins and inflammatory cytokines from the blood by replacing it with fresh and immunologically abundant blood, thereby leading to improvement in tissue perfusion and oxygenation. Moreover, ET with fresh whole blood increases neutrophil count and immunoglobulin levels as well as enhancing neutrophil function. However, there is a lack of clear evidence for the clinical efficacy of ET. In addition, adverse events associated with ET have been reported. Although most complications are transient, ET can lead to life-threatening complications. Therefore, ET can be considered a last resort treatment to rescue neonates with severe sepsis with sclerema and disseminated intravascular coagulation.
1. Introduction
Sepsis is one of the most common infectious conditions that occurs during the neonatal period. Although advancements in prenatal screening and intrapartum antibiotic prophylaxis over the past two decades have led to a significant reduction in risk and severity, sepsis remains a major cause of morbidity and mortality in neonates. Globally, an estimated three million neonates develop sepsis every year, and 11–19% of neonatal mortality is related to sepsis [1]. The incidence of neonatal sepsis is inversely associated with gestational age and birth weight. While up to 20% of very low-birthweight infants develop sepsis, the rate in most immature infants may be up to 60% [2], with a mortality rate of up to 71% in neonates weighing <1000 g at the onset of sepsis [3]. Despite the development of new antibiotics and treatment with specific antibiotics, neonates with either severe sepsis along with sclerema (sclerema neonatorum, a unique panniculitis characterized by rapidly progressive hardening of the skin and subcutaneous adipose tissue) or disseminated intravascular coagulation (DIC, a secondary syndrome characterized by excessive systemic activation of coagulation resulting in both hemorrhage and thrombosis) present high mortality rates [4,5]. Moreover, the incidence of neonatal sepsis has increased with the increasing survival of highly-susceptible preterm and/or low-birthweight infants.
A greater understanding of the pathophysiology of sepsis has led to multiple potential therapeutic targets for interventions to improve outcomes. Although routine treatment strategies have remained almost unchanged, to demonstrate enhancement in the host defense mechanisms several types of adjunctive therapies including granulocyte transfusion [6], granulocyte-colony stimulating factors (G-CSF) [7], and intravenous immunoglobulins (IVIG) [8] have been utilized and studied in cases of neonatal septicemia with varying degrees of success. However, these therapies have been ineffective in improving treatment outcomes [9,10]. In such a scenario, clinicians often attempt drastic but unproven and more invasive treatment methods to save rapidly deteriorating neonates with severe sepsis. Exchange transfusion (ET) is one such treatment method; it can rapidly remove circulating bacterial endotoxins and inflammatory cytokines. In the 1970s, case reports noted the effective use of ET in severe neonatal infections along with sclerema [11,12,13]. Thereafter, especially in the 1980s and 1990s, ET was proposed as an adjunct therapy to improve the survival in neonates with severe sepsis [14,15,16,17,18]. However, only a few studies have reported the impact of this treatment (such as reduction in the mortality rate of neonatal sepsis), and reporting is particularly sparse in developing countries [17,18]. Moreover, no definitive recommendations have been provided on the use of ET in neonates with septicemia.
In this narrative review, we summarize the current knowledge regarding neonatal sepsis and ET as a treatment method for sepsis, demonstrate the most significant new findings concerning the efficacy and adverse effects of ET by using diverse data, and introduce alternative therapeutic candidate to ET for the optimal management of neonatal sepsis in order to guide clinicians confronting this life-threatening condition.
2. Literature Research Methods
A narrative approach was chosen for this literature review, aiming to provide background information on the postulated pathophysiological mechanisms underlying neonatal sepsis and the history and theoretical efficacy of ET as a treatment method. Moreover, we aimed to provide the main topics with a focus on the challenges posed by therapeutical considerations regarding ET.
The literature research was conducted using the well-established PubMed, Cochran Library, Google Scholar, and ResearchGate databases. In addition, we conducted a search using two major internet search engines Google and Yahoo! Animal models, case series, controlled and uncontrolled studies, and meta-analyses were included, while case reports were excluded; only english-language papers were considered. We did not set a specific time window for the research; however, concerning the adverse effects of ET, we focused on studies conducted in the 2000s. This research used various combinations of the following keywords: “neonate”, “infection”, “sepsis”, “septic shock”, “sclerema”, “disseminated intravascular coagulation”, “pathophysiology”, “adjunctive therapies”, “exchange transfusion”, “adverse effect”, “plasma exchange”, and/or “extracorporeal blood purification.” In the first step, papers were screened by abstract and title. Thereafter, the full text of the selected papers was examined. Papers were excluded if their content was not relevant to the topics of neonatal sepsis, ET, or plasma exchange (PE). Additional publications were identified from the references cited in the initial papers.
3. Pathophysiology of Neonatal Sepsis
Neonatal sepsis differs from sepsis in adults, and is defined as a systemic inflammatory response syndrome due to suspected or proven infectious etiology in neonates with implications for epidemiology and pathophysiology [19,20]. The clinical spectrum of sepsis begins when a systemic or localized infection progresses to sepsis. Further clinical deterioration leads to septic shock through the persistence of hypoperfusion or hypotension despite adequate fluid resuscitation and use of vasopressor agents, ultimately leading to multiorgan dysfunction syndrome and possibly death.
A pathogen in the bloodstream is recognized by the innate immune system and consumed by macrophages and other phagocytes, leading to lysis of the organism [21]. This leads to the release of an endotoxin known as lipopolysaccharide, a component of a gram-negative organism, and an exotoxin known as peptidoglycan, a component of a gram-positive organism. Activation of macrophages stimulates the release of arachidonic acid metabolites such as prostaglandins and proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL)-1β, IL-6, and IL-8. This in turn leads to the release of anti-inflammatory cytokines, including IL-4, IL-10, and transforming growth factor-β, further activating the complement and coagulation cascades [20]. The complement cascade can activate coagulation and proinflammatory cytokine production and provoke tissue injury. In this way, systemic sepsis becomes a multisystem disorder with varying manifestations, such as hypotension and shock in the cardiovascular system; hemopoietic manifestations may include neutropenia, anemia, and DIC (Figure 1) [19].
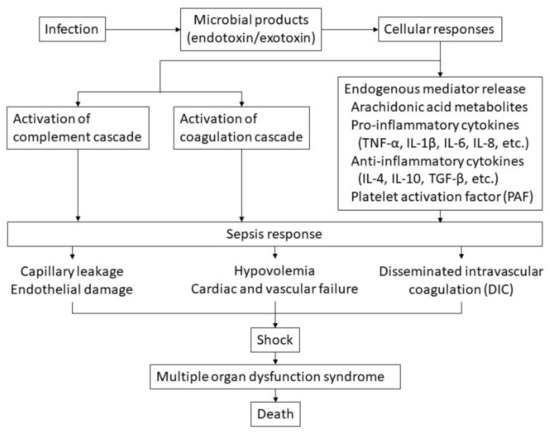
Figure 1.
Hypothetical pathophysiology of sepsis. The cascade of sepsis and its influencing factors are shown.
Particularly in low-birthweight infants, defense against bacterial infections in neonates may be weakened by such factors as neutrophil deficiency, impairment of chemotaxis of neutrophils and monocytes, complement deficiency, and decreased bactericidal activity of neutrophils [14]. Moreover, the imbalance between proinflammatory and anti-inflammatory cytokines appears to be associated with the severity of sepsis in neonates. Thus, the incomplete development of the host defense system of neonates is largely responsible for the high mortality rate of neonatal sepsis.
4. Exchange Transfusion as a Treatment Method for Neonatal Sepsis
ET is a form of blood transfusion in which the patient’s total blood volume is replaced within a few hours. It was introduced in the late 1940s to decrease the mortality of hemolytic disease in newborns and to prevent kernicterus due to hyperbilirubinemia in surviving patients. It is used to remove antibody-coated erythrocytes and products of hemolysis such as bilirubin in various immune or non-immune hemolytic diseases. Subsequently, ET has become one of the most commonly performed neonatal procedures for severe hyperbilirubinemia. Over the decades and coupled with advances in prenatal interventions such as anti-Rh-globulin for Rh-negative mothers and postnatal care such as effective phototherapy, indications for ET have significantly decreased due to the decreased incidence of hemolytic disease [22].
Considering the persistently high case fatality rate associated with neonatal sepsis and septic shock in spite of appropriate antimicrobial and optimal supportive therapy, various adjunctive therapies such as granulocyte transfusion, G-CSF, and IVIG, have been evaluated for the treatment of sepsis and septic shock [7,8]. However, none of these strategies has been demonstrated to reduce the mortality rate in cases of sepsis and septic shock [9,10].
In the 1970s, published case reports described the effective use of ET in severe neonatal sepsis with sclerema [11,13]. In subsequent years, particularly in the 1980s and the 1990s, many clinicians used this procedure as a rescue therapy in neonates with severe sepsis [14,15,16,17,18].
The possible rationales for performing ET using fresh, whole, adult blood include the following:
- (i)
- Elimination of bacteria, bacterial toxins, and circulating inflammatory cytokines; Togari et al. investigated ten neonates who received ET for treatment of septic shock and reported that in six of eight patients with positive endotoxins the endotoxins were completely eliminated by ET. All six patients recovered from shock and survived; however, the two patients who remained endotoxin-positive after ET died [23]. Sugiura et al. reported that in an extremely low-birthweight infant with septic shock due to necrotizing enterocolitis, the elevated serum IL-8 and calprotectin levels decreased after ET and the blood pressure increased and stabilized [24]. Moreover, Chishiki et al. reported that ET saved the life of a neonate with septic shock due to severe Group B streptococcus meningitis, and that the procedure reduced the levels of both proinflammatory and anti-inflammatory cytokines [25].
- (ii)
- Improvement of pulmonary perfusion, ventilation, and tissue oxygenation; Gottuso et al. reported significantly improved pulmonary perfusion and ventilation after ET in low-birth-weight infants with severe respiratory distress [26]. The mechanism for this (as suggested by Delivoria-Papadopoulos et al.) is the improved oxygenation of the tissues following ET with fresh whole blood as a result of either the increase in 2,3 diphosphoglycerate or adult hemoglobin or the removal of vasoactive substances such as prostaglandin F2α [27].
- (iii)
- Correction of the plasma coagulation system; it is thought that ET supplements various coagulation factors, increases the platelet count, and affects DIC to improve coagulation ability [28]. Ugwu et al. reported the case of a neonate with DIC due to severe infection whose coagulation profile was corrected by replacement of consumed coagulation factors using ET and whose clinical condition improved [29].
- (iv)
- Enhancement of immunological defense mechanisms; Sadana et al. demonstrated that serum immunoglobulin (Ig)G, IgA, and IgM levels rose significantly 12–24 h after ET in neonatal sepsis with sclerema [17]. Hall et al. reported significantly increased peripheral neutrophil counts after ET among survivors in neonates with early onset Group B streptococcal septicemia [30]. Furthermore, Mathur et al. reported that leukocyte/neutrophil count increased and granulocyte function improved significantly immediately after ET in septicemic neonates [31]. Suppression of lipid peroxidation [32] and increased blood volume and oxygen supply in the brain [33] have been reported as well.
5. Adverse Effects of Exchange Transfusion
ET is not a risk-free procedure, and adverse events associated with ET have been reported even in healthcare settings with advanced clinical care [34,35]. As ET may lead to changes in the internal environment of neonates, adverse events associated with ET may be attributable to fluctuations in blood volume and pressure, changes in acid–base status, alterations in platelet count due to the use of packed red cells that lack platelets and coagulation factors, electrolyte disturbances, and the introduction of infectious agents. The frequency of ET-related adverse events varies in different studies (15–74%) [36,37]. The reasons behind the wide variation in the frequencies of different adverse events include differences in the definition of adverse events, enrollment criteria, and inconsistent follow-up periods after ET. Although the frequency of ET has declined remarkably, the incidence of ET-related morbidity and mortality has not followed suit [38]. Table 1 shows the complications of ET and their incidence reported in studies conducted in the 2000s, although these are all studies on ET for neonatal hyperbilirubinemia [34,35,39,40,41,42,43,44,45,46,47,48]. The most common complications related to ET procedures are thrombocytopenia and hypocalcemia [38,49]. It was found that most adverse events resolve without special treatment [36,40]. However, ET can lead to more severe complications, such as life-threatening bleeding, sepsis, cardiac dysfunction, necrotizing enterocolitis, and even death, apart from transient hypocalcemia, hyperkalemia, bradycardia, and thrombocytopenia. The mortality rate attributable to the ET procedure has been reported to be 0–8% in several previous studies [34,38,39,43,48,50].

Table 1.
Adverse events associated with exchange transfusion in neonates and their incidence rates, as reported in studies conducted in the 2000s.
Few studies have reported adverse events associated with ET in neonatal sepsis. Pungi et al. reported that serious adverse events were not attributable to the ET procedure in 50 neonates with septic shock [28]. Although approximately half of all patients required platelet transfusion after ET, none of them had bleeding associated with reduction in platelet counts. Moreover, the authors reported that there was no hypocalcemia during or after ET. Aradhya et al. reported that among 41 patients who received ET for sepsis, twelve (29%) developed mild hypothermia, two (5%) had transient bradycardia, two (5%) had hyperkalemia, and two (5%) had hypernatremia, all of whom recovered spontaneously [51]. Verma et al. reported that among seven neonates who required ET for severe sepsis, 14% developed hyperkalemia and 71% developed moderate thrombocytopenia, and all patients recovered spontaneously [52]; however, only one patient developed necrotizing enterocolitis within 12 h of ET. Adverse events of ET in patients with poor clinical conditions such as sepsis may differ in severity from those of ET in patients with hyperbilirubinemia. In their study regarding adverse events related to ET for hyperbilirubinemia Patra et al. observed a high incidence of adverse events, as the enrolled neonates had a clinical condition with a more severe profile [49]. Jackson reported that among all patients who underwent ET for hyperbilirubinemia, the rate of severe complications observed in ill infants (12%) was significantly greater than that observed in healthy infants (1.2%) [38]. Moreover, the author reported that the mortality rate was 2% in the entire group and 8% in the subset classified as ill [38].
6. Efficacy of Exchange Transfusion in Managing Neonatal Sepsis
In 1974, Prod’hom et al. proposed ET as an adjunctive therapy for severely septic neonates [11]. Subsequently, various physicians have attempted ET as a treatment modality for severe septicemia and demonstrated its effectiveness in the treatment of neonatal sepsis and septic shock. Xanthou et al. reported that two critically ill neonates with severe infection associated with sclerema were successfully treated with repeated ET [13]. Töllner et al. examined 22 neonates who received ET for sepsis [12]. Both studies demonstrated impressive clinical improvement and decreased mortality following ET. Vein et al. performed ET on ten neonates with refractory sepsis with progressive sclerema [15]. Seven of the ten patients showed immediate improvement and ultimately survived. Dalvi et al. studied 53 neonates with severe or unresponsive sepsis (51 neonates had sclerema) treated with ET and reported an overall survival rate of 77.4% [16]. However, despite the potential benefits of ET evidence-based clinical efficacy is lacking in these studies, as most were anecdotal reports or used small population sizes without comparative controls. To date, only a small number of randomized controlled trials or case-control studies on the efficacy of ET have been published. Table 2 shows prospective and retrospective studies evaluating the efficacy of ET for neonatal sepsis [17,18,28,31,51,52,53,54,55,56,57]. Among the seven prospective studies, two demonstrated that ET significantly reduced mortality. One of the four retrospective studies reported a significant reduction in mortality in the ET group compared to the non-ET group. Moreover, four of the total eleven studies reported significantly increased levels of serum IG and one study showed significantly increased levels of leukocytes and neutrophils. Although most studies have shown clinical benefits, other authors have reported different results. The majority of studies have inadequate sample sizes and follow-up durations, varying inclusion and exclusion criteria, and different or vague septic shock definitions and criteria for ET. Moreover, they do not have mortality rate as their primary objective. Recently, Mathias et al. conducted a meta-analysis in order to evaluate the effect of ET on mortality in neonatal sepsis [58]. The authors demonstrated a mortality benefit (relative risk: 0.72, p = 0.01) and a significant increase in immunological parameters (IG and complement levels) (p = 0.02) and neutrophil counts (p = 0.03) compared with controls in septic neonates who underwent ET. This study suggested that ET can be an adjunctive method for the treatment of neonatal sepsis, although the certainty of the evidence is low.

Table 2.
Previous studies evaluating the efficacy of ET for neonatal sepsis.
Regarding blood products used for ET procedure in the present review (Table 2), eight of the total eleven studies and two of the three studies demonstrating significant reduction in mortality in the ET group used fresh whole blood. While ET using reconstituted blood (packed red blood cells and fresh frozen plasma) is expected to achieve elimination of bacterial toxins and inflammatory cytokines, ET using fresh whole blood is expected to improve neutrophil function. However, until recently the emergent availability of fresh whole blood has been limited in most centers. A Japanese survey examined the availability of blood for ET in neonates and reported that the blood products used in 367 ET cases performed during a three-year period (from 2013 to 2016) all used reconstituted blood [59].
In this literature review, the first related article found was from the mid-1970s. The number of articles published in the 1980s increased, that in the 1990s decreased, and only a few articles were published in the 2000s (Figure 2). Moreover, in 2016 Cochrane published a review protocol to determine the efficacy of ET in reducing the mortality rate in neonates with septicemia and to ascertain the adverse effects associated with ET; however, the protocol was withdrawn in 2021 because it was out of date [60]. In the latest editions of a textbook on neonatal medicine [61] and in a representative textbook of neonatal infections [62], ET was not suggested as a treatment method for neonatal sepsis.
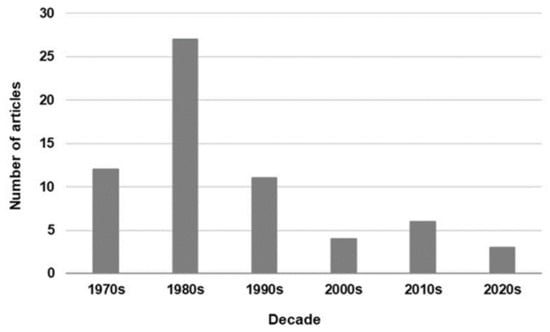
Figure 2.
Trend in the number of journal articles on exchange transfusion for neonatal sepsis published per decade.
7. Plasma Exchange as an Alternative to Exchange Transfusion
Cytokines play an important role in the pathophysiology of sepsis, even in neonates [20]. Therefore, cytokine elimination therapy may improve the condition of neonates with sepsis. Although ET has been reported to have a cytokine elimination effect [24,25], the direct removal of inflammatory cytokines from whole blood using therapeutic PE is a potential alternative [63]. PE is a well-established therapeutic procedure commonly used in adults; it is based on the removal of plasma from whole blood followed by replacement of that volume with fresh frozen plasma. Evidence suggests that PE can reduce mortality in adult patients with sepsis and septic shock [64]. Several studies have reported that PE in neonates results in improvement of multiple organ failure caused by septicemia [65]. Recently, as the new technology of extracorporeal PE hemoabsorption devices for cytokine reduction (such as Toraymyxin (Toray, Tokyo, Japan) [66] and CytoSorb (CytoSorbents Europe, GmbH, Berlin, Germany) [67,68]) has been introduced, contributing to the achievement of rapid hemodynamic stabilization in neonatal and pediatric patients with refractory septic shock. However, using PE in neonates is considered a challenge due to the relatively large extracorporeal blood volume in relation to the apheresis machines, which are developed for adults. Especially in low-birthweight infants, insufficient blood drainage due to limited vascular access may cause inadequate extracorporeal blood volume to maintain the established flow of the PE system, interruption of the pump, and clotting complications. Moreover, factors such as insertion of large-bore catheters for vascular access and hemodynamic changes due to altered blood volume may make it difficult to use PE as an adjunctive therapy for neonatal sepsis.
8. Limitations and Strengths of This Review
This narrative review can be justified on the basis of the diverse data regarding neonatal sepsis and its adjunctive treatment focusing on ET, as well as the needs for this area of interest to be mapped out for the benefit of clinicians engaged in relevant practice.
However, this review has certain limitations. First, the main limitation is the narrative design, which is a source of bias inherent to such studies. The database searches and hand searches referred to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines [69]; however, they could not be rigorously followed because this was not a systematic review and meta-analysis. Moreover, study selection was determined by only one researcher. These issues may have resulted in selection bias. However, the author believes that the search was comprehensive with respect to the current state of neonatal sepsis and its therapeutic considerations. Moreover, in order to reduce the impact of publication bias, this review research included grey literature and books in addition to peer-reviewed journals, as these could provide a source of practical experience and important information on neonatal sepsis and ET.
Second, the majority of studies evaluating the efficacy of ET for neonatal sepsis were not recent. The quality of neonatal intensive care and the blood products, devices, and methods used for ET have changed during the last quarter century; evaluations of efficacy and safety made in previous studies may not be acceptable today.
This review did not focus on drawing conclusions as to whether ET is effective for sepsis; instead, the focus has been to comprehensively map the various research results and adverse effects reported to date in order to provide clinicians with information on the respective advantages and disadvantages of this treatment. Moreover, regarding the adverse effects of ET the focus has been on those complications reported in the 2000s, particularly those associated with ET for sepsis. These can be considered strengths of this work.
9. Conclusions
Previous studies have shown that ET for neonatal sepsis may have biological plausibility and a probability of benefit. However, the current evidence does neither supports nor refutes the use of ET in the management of severe neonatal sepsis. However, the evidence in question has low certainty, inadequate power, and a moderate-to-high risk of bias. Therefore, adequately powered and well-designed randomized controlled trials are essential for assessing the use of ET for neonatal sepsis. For refractory neonatal sepsis with sclerema and/or DIC, which is likely to be life-threatening, ET can be considered a worthwhile treatment as a last resort. However, ET, which requires the use of fresh whole blood to improve neutrophil function cannot be expected to advance in the future, as the emergent availability of fresh whole blood has until recently been limited in most centers. If a more efficient and safer blood purification method can be developed, ET will not be necessary for neonatal sepsis.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Kermorvant-Duchemin, E.; Laborie, S.; Rabilloud, M.; Lapillonne, A.; Claris, O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 2008, 9, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Darmstadt, G.L. Sclerema neonatorum: A review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J. Perinatol. 2008, 28, 453–460. [Google Scholar] [CrossRef] [PubMed]
- VanVooren, D.M.; Bradshaw, W.T.; Blake, S.M. Disseminated Intravascular Coagulation in the Neonate. Neonatal Netw. 2018, 37, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Brocklehurst, P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropenia. Cochrane Database Syst. Rev. 2011, 2011, CD003956. [Google Scholar] [CrossRef]
- Bernstein, H.M.; Pollock, B.H.; Calhoun, D.A.; Christensen, R.D. Administration of recombinant granulocyte colony-stimulating factor to neonates with septicemia: A meta-analysis. J. Pediatr. 2001, 138, 917–920. [Google Scholar] [CrossRef]
- INIS Collaborative Group; Brocklehurst, P.; Farrell, B.; King, A.; Juszczak, E.; Darlow, B.; Haque, K.; Salt, A.; Stenson, B.; Tarnow-Mordi, W.; et al. Treatment of neonatal sepsis with intravenous immune globulin. N. Engl. J. Med. 2011, 365, 1201–1211. [Google Scholar]
- Ohlsson, A.; Lacy, J.B. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst. Rev. 2020, 1, CD001239. [Google Scholar] [CrossRef]
- Carr, R.; Modi, N.; Doré, C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst. Rev. 2003, 2003, CD003066. [Google Scholar] [CrossRef]
- Prod’hom, L.S.; Choffat, J.M.; Frenck, N.; Mazoumi, M.; Relier, J.P.; Torrado, A. Care of the seriously ill neonate with hyaline membrane disease and with sepsis (sclerema neonatorum). Pediatrics 1974, 53, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Töllner, U.; Pohlandt, F.; Heinze, F.; Henrichs, I. Treatment of septicaemia in the newborn infant: Choice of initial antimicrobial drugs and the role of exchange transfusion. Acta Paediatr. 1977, 66, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Xanthou, M.; Xypolyta, A.; Anagnostakis, D.; Economou-Mavrou, C.; Matsaniotis, N. Exchange transfusion in severe neonatal infection with sclerema. Arch. Dis. Child. 1975, 50, 901–902. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoder, M.C.; Polin, R.A. Immunotherapy of neonatal septicemia. Pediatr. Clin. N. Am. 1986, 33, 481–501. [Google Scholar] [CrossRef]
- Vain, N.E.; Mazlumian, J.R.; Swarner, O.W.; Cha, C.C. Role of exchange transfusion in the treatment of severe septicemia. Pediatrics 1980, 66, 693–697. [Google Scholar] [CrossRef]
- Dlavi, R.; Rao, S.; Rangnekar, J.; Fernandez, A. Exchange transfusions in neonatal sepsis. Indian Pediatr. 1991, 28, 39–43. [Google Scholar]
- Sadana, S.; Mathur, N.B.; Thakur, A. Exchange transfusion in septic neonates with sclerema: Effect on immunoglobulin and complement levels. Indian Pediatr. 1997, 34, 20–25. [Google Scholar]
- Narayanan, I.; Mitter, A.; Gujral, V.V. A comparative study on the value of exchange and blood transfusion in the management of severe neonatal septicaemia with sclerema. Indian J. Pediatr. 1982, 49, 519–523. [Google Scholar] [CrossRef]
- Wynn, J.L.; Wong, H.R. Pathophysiology and treatment of septic shock in neonates. Clin. Perinatol. 2010, 37, 439–479. [Google Scholar] [CrossRef]
- Machado, J.R.; Soave, D.F.; da Silva, M.V.; de Menezes, L.B.; Etchebehere, R.M.; Monteiro, M.L.; Antônia dos Reis, M.; Correa, R.R.M.; Celes, M.R. Neonatal sepsis and inflammatory mediators. Mediat. Inflamm. 2014, 2014, 269681. [Google Scholar] [CrossRef]
- Pius, S.; Bello, M. Neonatal septicaemia in poor resource settings. Pediatric Infect. Dis. 2017, 2, 34. [Google Scholar]
- Bhutani, V.K.; Meng, N.F.; Knauer, Y.; Danielsen, B.H.; Wong, R.J.; Stevenson, D.K.; Gould, J.B. Extreme hyperbilirubinemia and rescue exchange transfusion in California from 2007 to 2012. J. Perinatol. 2016, 36, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Togari, H.; Mikawa, M.; Iwanaga, T.; Matsumoto, N.; Kawase, A.; Hagisawa, M.; Ogino, T.; Goto, R.; Watanabe, I.; Kito, H.; et al. Endotoxin clearance by exchange blood transfusion in septic shock neonate. Acta Paediatr. 1983, 72, 87–91. [Google Scholar] [CrossRef]
- Sugiura, T.; Kouwaki, M.; Goto, K.; Endo, T.; Ito, K.; Koyama, N.; Togari, H. Effects of exchange transfusion on cytokine profiles in necrotizing enterocolitis. Pediatr. Int. 2012, 54, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Chishiki, M.; Go, H.; Endo, K.; Ueda, N.K.; Takehara, H.; Namai, Y. Cytokine profiles before and after exchange transfusions in severe late-onset neonatal group B streptococcus meningitis: A case report. Tohoku J. Exp. Med. 2021, 253, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Gottuso, M.A.; Williams, M.L.; Oski, F.A. The role of exchange transfusions in the management of low-birth-weight infants with and without severe respiratory distress syndrome. II. Further observations and studies of mechanisms of action. J. Pediatr. 1976, 89, 279–285. [Google Scholar] [CrossRef]
- Delivoria-Papadopoulos, M.; Roncevic, N.P.; Oski, F.A. Postnatal changes in oxygen transport of term, premature, and sick infants: The role of red cell 2,3-diphosphoglycerate and adult hemoglobin. Pediatr. Res. 1971, 5, 235–245. [Google Scholar] [CrossRef]
- Pugni, L.; Ronchi, A.; Bizzarri, B.; Consonni, D.; Pietrasanta, C.; Ghirardi, B.; Fumagalli, M.; Ghirardello, S.; Mosca, F. Exchange transfusion in the treatment of neonatal septic shock: A ten-year experience in a neonatal intensive care unit. Int. J. Mol. Sci. 2016, 17, 695. [Google Scholar] [CrossRef]
- Ugwu, R.O.; Eneh, A.U.; Nwauche, C.A. Disseminated intravascular coagulopathy in a neonate: Management challenges. Niger. J. Med. 2007, 16, 177–180. [Google Scholar] [CrossRef]
- Hall, R.T.; Shigeoka, A.O.; Hill, H.R. Serum opsonic activity and peripheral neutrophil counts before and after exchange transfusion in infants with early onset group B streptococcal septicemia. Pediatric Infect. Dis. 1983, 2, 356–358. [Google Scholar] [CrossRef]
- Mathur, N.B.; Subramanian, B.K.; Sharma, V.K.; Puri, R.K. Exchange transfusion in neutropenic septicemic neonates: Effect on granulocyte functions. Acta Paediatr. 1993, 82, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Kokk, T.; Talvik, R.; Zilmer, M.; Kütt, E.; Talvik, T. Markers of oxidative stress before and after exchange transfusion for neonatal sepsis. Acta Paediatr. 1996, 85, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yamashita, Y.; Nishimi, T.; Inoue, T.; Matsuishi, T.; Kato, H. Changes of cerebral hemodynamics and oxygenation in unstable septic newborns during exchange transfusion. Kurume Med. J. 1998, 45, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, A.; Okan, F.F.; Ünsür, E.K.; Nuhoğlu, A. Adverse events associated with exchange transfusion and etiology of severe hyperbilirubinemia in near-term and term newborns. Turk. J. Med. Sci. 2011, 41, 93–100. [Google Scholar]
- Chitlangia, M.; Shah, G.S.; Poudel, P.; Mishra, O.P. Adverse events of exchange transfusion in neonatal hyperbilirubinemia. J. Nepal Paediatr. Soc. 2014, 34, 7–13. [Google Scholar] [CrossRef]
- Bujandric, N.; Grujic, J. Exchange transfusion for severe neonatal hyperbilirubinemia: 17 years’ experience from Vojvodina, Serbia. Indian J. Hematol. Blood Transfus. 2016, 32, 208–214. [Google Scholar] [CrossRef]
- Sá, C.A.M.; Santos, M.C.P.; Carvalho, M.D.; Moreira, M.C.P. Adverse events related to exchange transfusion in newborn infants with hemolytic disease: Ten years of experience. Rev. Paul. Pediatr. 2009, 27, 168–172. [Google Scholar] [CrossRef]
- Jackson, J.C. Adverse events associated with exchange transfusion in healthy and ill newborns. Pediatrics 1997, 99, E7. [Google Scholar] [CrossRef]
- Chacham, S.; Kumar, J.; Dutta, S.; Kumar, P. Adverse events following blood exchange transfusion for neonatal hyperbilirubinemia: A prospective study. J. Clin. Neonatol. 2019, 8, 79–84. [Google Scholar]
- Malla, T.; Singh, S.; Poudyal, P.; Sathian, B.; Bk, G.; Malla, K.K. A prospective study on exchange transfusion in neonatal unconjugated hyperbilirubinemia--in a tertiary care hospital, Nepal. Kathmandu Univ. Med. J. 2015, 13, 102–108. [Google Scholar] [CrossRef][Green Version]
- Okulu, E.; Erdeve, O.; Tuncer, Ö.; Ertuğrul, S.; Özdemir, H.; Çiftdemir, N.A.; Zenciroğlu, A.; Atasay, B. Exchange transfusion for neonatal hyperbilirubinemia: A multicenter, prospective study of Turkish Neonatal Society. Turk. Arch. Pediatr. 2021, 56, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Badiee, Z. Exchange transfusion in neonatal hyperbilirubinaemia: Experience in Isfahan, Iran. Singap. Med. J. 2007, 48, 421–423. [Google Scholar]
- Yu, C.; Li, H.; Zhang, Q.; He, H.; Chen, X.; Hua, Z. Report about term infants with severe hyperbilirubinemia undergoing exchange transfusion in Southwestern China during an 11-year period, from 2001 to 2011. PLoS ONE 2017, 12, e0179550. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarpour, B.; Ebrahimi, H.; Karkan, M.F.; Farahmand, N.; Karambin, M.M. Neonatal exchange transfusion for hyperbilirubinemia in Guilan (the north province of Iran): A 3-year experience. Turk. J. Pediatr. 2012, 54, 626–631. [Google Scholar]
- Hakan, N.; Zenciroğlu, A.; Aydin, M.; Okumus, N.; Dursun, A.; Dilli, D. Exchange transfusion for neonatal hyperbilirubinemia: An 8-year single center experience at a tertiary neonatal intensive care unit in Turkey. J. Matern. Fetal Neonatal Med. 2015, 28, 1537–1541. [Google Scholar] [CrossRef]
- Al-Lawama, M.; Al-Rimawi, E.; Al-Shibi, R.; Badran, E. Adoption of the American Academy of Pediatrics’ neonatal hyperbilirubinemia guidelines and its effect on blood exchange transfusion rate in a tertiary care center in Amman, Jordan. J. Blood Med. 2018, 9, 61–66. [Google Scholar] [CrossRef]
- Dey, S.K.; Jahan, S.; Jahan, I.; Islam, M.S.; Shabuj, M.K.; Shahidullah, M. Exchange transfusion for hyperbilirubinemia among term and near term in NICU of a tertiary care hospital of Bangladesh: Findings from a prospective study. Euroasian J. Hepatogastroenterol. 2021, 11, 21–26. [Google Scholar] [CrossRef]
- Duan, L.; Gan, S.; Hu, H. A single-center experience on exchange transfusion therapy in 123 full-term cases of severe neonatal hyperbilirubinemia in Wuhan. J. Matern. Fetal Neonatal Med. 2021, 34, 466–472. [Google Scholar] [CrossRef]
- Patra, K.; Storfer-Isser, A.; Siner, B.; Moore, J.; Hack, M. Adverse events associated with neonatal exchange transfusion in the 1990s. J. Pediatr. 2004, 144, 626–631. [Google Scholar] [CrossRef]
- Chitty, H.E.; Ziegler, N.; Savoia, H.; Doyle, L.W.; Fox, L.M. Neonatal exchange transfusions in the 21st century: A single hospital study. J. Paediatr. Child Health 2013, 49, 825–832. [Google Scholar] [CrossRef]
- Aradhya, A.S.; Sundaram, V.; Kumar, P.; Ganapathy, S.M.; Jain, A.; Rawat, A. Double volume exchange transfusion in severe neonatal sepsis. Indian J. Pediatr. 2016, 83, 107–113. [Google Scholar] [CrossRef]
- Verma, A.; Pandita, A.; Gupta, G.; Naranje, K.M.; Singh, A. Role of DVET in severe neonatal sepsis in an era of high antibiotic resistance: A retrospective observational study. J. Matern. Fetal Neonatal Med. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Belohradsky, B.H.; Simon, G.; Spier, J.M.; Ross, R.; Marger, W. The immunoglobulin state of the neonate following surgery: Consequences for immunotherapy of neonatal septicemia. Prog. Pediatr. Surg. 1979, 13, 39–51. [Google Scholar] [PubMed]
- Bossi, E.; Meister, B.; Pfenninger, J. Exchange transfusion for severe neonatal septicemia. Pediatrics 1981, 67, 941. [Google Scholar] [CrossRef] [PubMed]
- Gunes, T.; Koklu, E.; Buyukkayhan, D.; Kurtoglu, S.; Karakukcu, M.; Patiroglu, T. Exchange transfusion or intravenous immunoglobulin therapy as an adjunct to antibiotics for neonatal sepsis in developing countries: A pilot study. Ann. Trop. Paediatr. 2006, 26, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Rajput, U.; Deshmukh, L.S. Exchange transfusion in neonatal sepsis dose not reduces overall mortality. J. Evol. Med. Dent. Sci. 2013, 2, 8297–8301. [Google Scholar] [CrossRef]
- Chillal, R.; Ahmed, F.K.R.; Umakanth, R. Exchange transfusion in neonatal sepsis- an old wine in new bottle. Sch. J. App. Med. Sci. 2021, 9, 799–804. [Google Scholar]
- Mathias, S.; Balachander, B.; Bosco, A.; Britto, C.; Rao, S. The effect of exchange transfusion on mortality in neonatal sepsis: A meta-analysis. Eur. J. Pediatr. 2022, 181, 369–381. [Google Scholar] [CrossRef]
- Koyama, N.; Amizuka, T.; Oku, K.; Kawaguchi, C.; Shirakawa, Y.; Cho, K.; Hosono, S. Survey of exchange transfusions for neonates in Japan and availability of blood. J. Jpn. Soc. Neonat. Health Dev. 2020, 32, 138–143. (In Japanese) [Google Scholar]
- Mishra, S.; Chawla, D.; Agarwal, R. Effect of exchange transfusion on mortality in neonates with septicemia. Cochrane Database Syst. Rev. 2021, 8, CD012477. [Google Scholar] [CrossRef]
- Dhudasia, M.B.; Flannery, D.D.; Puopolo, K.M. Evaluation and management of infection in the newborn. In Avery & MacDonald’s Neonatology: Pathophysiology and Management of the Newborn, 8th ed.; Boardman, J., Groves, A., Ramasethu, J., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 767–778. [Google Scholar]
- Nizet, V.; Klein, J.O. Bacterial sepsis and meningitis. In Remington and Klein’s Infectious Diseases of the Fetus and Newborn Infant, 8th ed.; Wilson, C.B., Nizet, V., Maldonado, Y.A., Remington, J.S., Klein, J.O., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2016; pp. 217–271. [Google Scholar]
- Iwai, H.; Nagaki, M.; Naito, T.; Ishiki, Y.; Murakami, N.; Sugihara, J.; Muto, Y.; Moriwaki, H. Removal of endotoxin and cytokines by plasma exchange in patients with acute hepatic failure. Crit. Care Med. 1998, 26, 873–876. [Google Scholar] [CrossRef]
- Knaup, H.; Stahl, K.; Schmidt, B.M.W.; Idowu, T.O.; Busch, M.; Wiesner, O.; Welte, T.; Haller, H.; Kielstein, J.T.; Hoeper, M.M.; et al. Early therapeutic plasma exchange in septic shock: A prospective open-label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit. Care 2018, 22, 285. [Google Scholar] [CrossRef]
- Sawyer, T.; Billimoria, Z.; Handley, S.; Smith, K.; Yalon, L.; Brogan, T.V.; Di Geronimo, R. Therapeutic plasma exchange in neonatal septic shock: A retrospective cohort study. Am. J. Perinatol. 2020, 37, 962–969. [Google Scholar] [CrossRef]
- Nishizaki, N.; Nakagawa, M.; Hara, S.; Oda, H.; Kantake, M.; Obinata, K.; Uehara, Y.; Hiramatsu, K.; Shimizu, T. Effect of PMX-DHP for sepsis due to ESBL-producing E. coli in an extremely low-birthweight infant. Pediatr. Int. 2016, 58, 411–414. [Google Scholar] [CrossRef]
- Milella, L.; Ficarella, M.T.; Calabrese, G.; Sisto, M.; Grieco, R.L.; Moliterini, P.; Raimondo, P.; Cito, F.; Bellino, V.; Ranieri, A.; et al. Application of hemoadsorption in neonatal and pediatric hyperinflammatory states: A case series. Am. J. Pediatr. 2019, 5, 34–42. [Google Scholar] [CrossRef]
- Bottari, B.; Guzzo, I.; Marano, M.; Stoppa, F.; Ravà, L.; Di Nardo, M.; Cecchetti, C. Hemoperfusion with Cytosorb in pediatric patients with septic shock: A retrospective observational study. Int. J. Artif. Organs 2020, 43, 587–593. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).