KD Diagnosis Does Not Increase Cardiovascular Risk in Children According to Dynamic Intima–Media Roughness Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
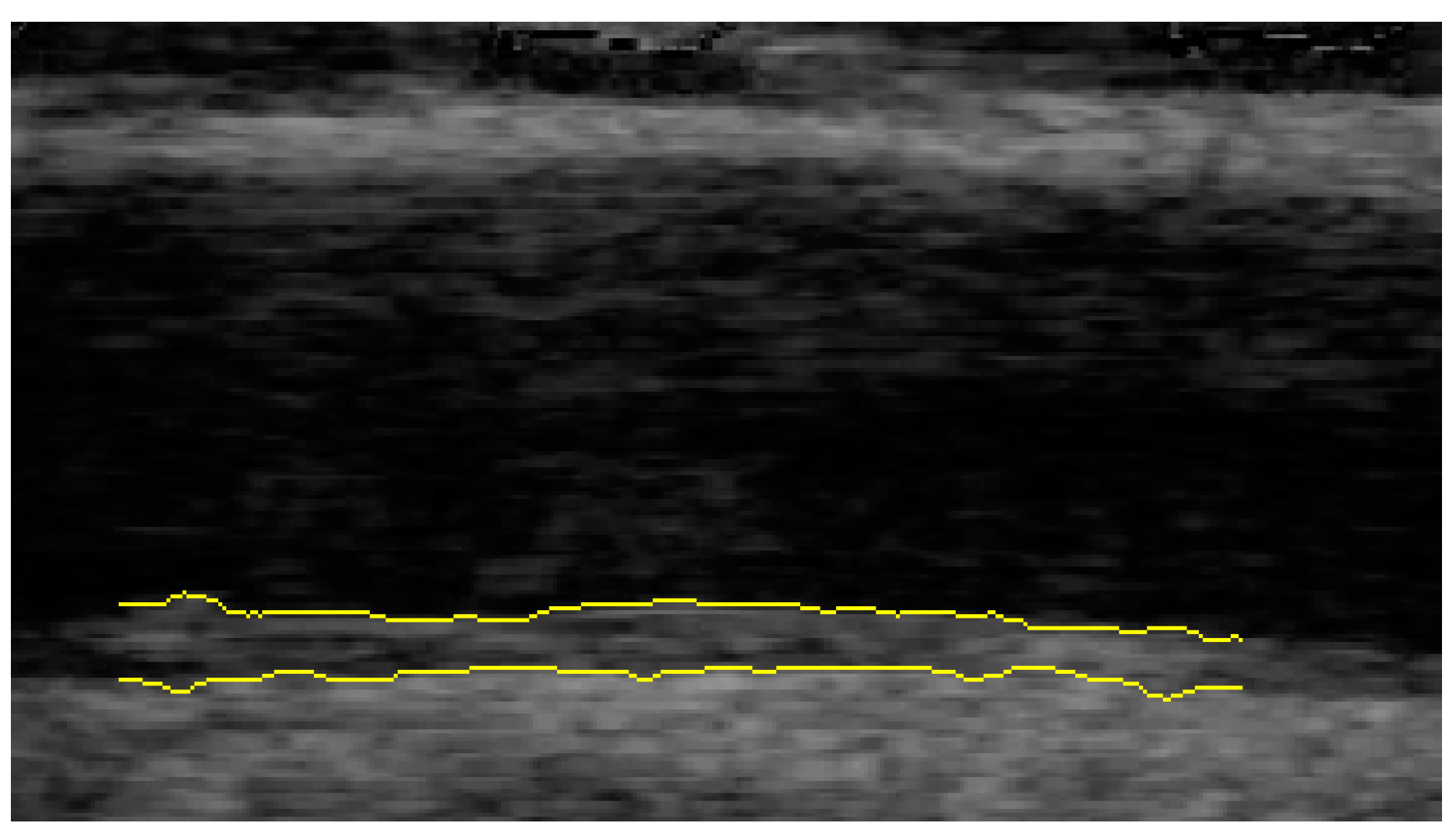
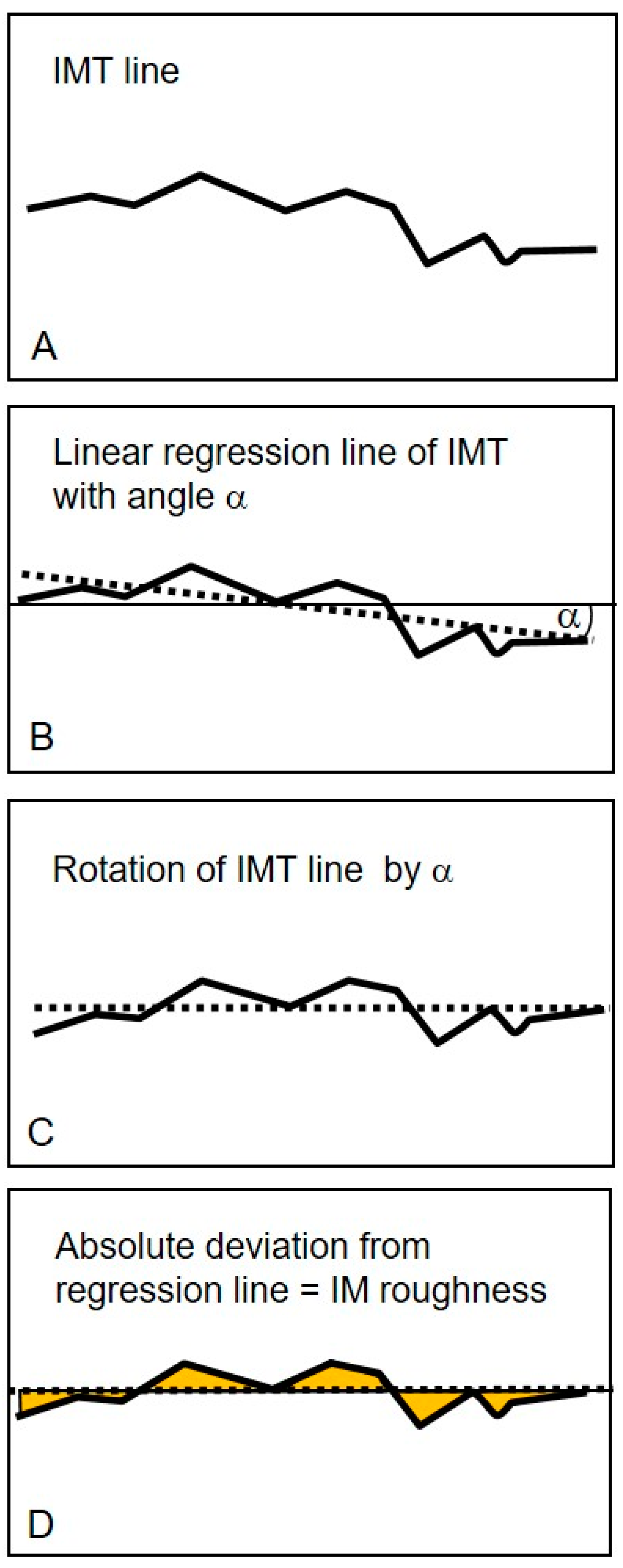
2.2. Ultrasound Examination
2.3. Statistical Analysis
3. Results
3.1. Comparison of Baseline Characteristics between Patients and Controls
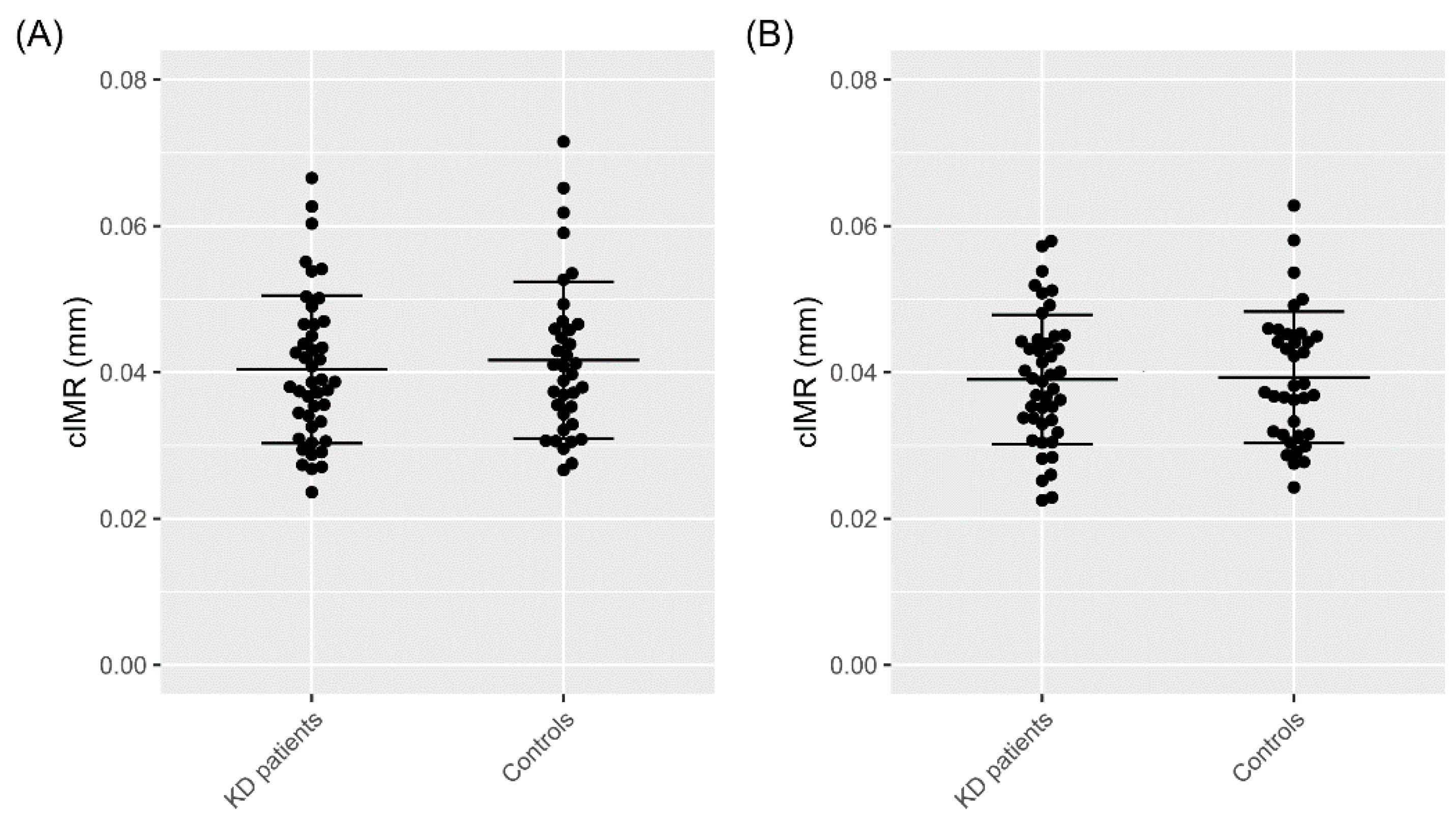
3.2. Carotid Intima–Media Roughness
3.3. Influence of Coronary Artery Status and Acute Phase Parameters on cIMR
4. Discussion
4.1. Dynamic Carotid Intima–Media Roughness, a Potential Vascular Parameter
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jakob, A. Kawasaki-Syndrom. Mon. Kinderheilkd 2016, 164, 241–256. [Google Scholar] [CrossRef]
- Ae, R.; Makino, N.; Kosami, K.; Kuwabara, M.; Matsubara, Y.; Nakamura, Y. Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017–2018. J. Pediatr. 2020, 225, 23–29.e22. [Google Scholar] [CrossRef] [PubMed]
- Jakob, A.; Whelan, J.; Kordecki, M.; Berner, R.; Stiller, B.; Arnold, R.; von Kries, R.; Neumann, E.; Roubinis, N.; Robert, M. Kawasaki Disease in Germany. Pediatric Infect. Dis. J. 2016, 35, 129–134. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Amano, S.; Hazama, F.; Kubagawa, H.; Tasaka, K.; Haebara, H.; Hamashima, Y. General pathology of Kawasaki disease: On the morphological alterations corresponding to the clinical manifestations. Pathol. Int. 1980, 30, 681–694. [Google Scholar] [CrossRef]
- Silva, A.A.; Maeno, Y.; Hashmi, A.; Smallhorn, J.F.; Silverman, E.D.; McCrindle, B.W. Cardiovascular risk factors after Kawasaki disease: A case-control study. J. Pediatr. 2001, 138, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.F.; Laranjo, S.; Parames, F.; Freitas, I.; Mota-Carmo, M. Long-term evaluation of endothelial function in Kawasaki disease patients. Cardiol. Young 2013, 23, 517–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dietz, S.M.; Tacke, C.E.; Hutten, B.A.; Kuijpers, T.W. Peripheral Endothelial (Dys)Function, Arterial Stiffness and Carotid Intima-Media Thickness in Patients after Kawasaki Disease: A Systematic Review and Meta-Analyses. PLoS ONE 2015, 10, e0130913. [Google Scholar] [CrossRef]
- Shah, V.; Christov, G.; Mukasa, T.; Brogan, K.S.; Wade, A.; Eleftheriou, D.; Levin, M.; Tulloh, R.M.; Almeida, B.; Dillon, M.J.; et al. Cardiovascular status after Kawasaki disease in the UK. Heart 2015, 101, 1646–1655. [Google Scholar] [CrossRef]
- Dalla Pozza, R.; Bechtold, S.; Urschel, S.; Kozlik-Feldmann, R.; Netz, H. Subclinical atherosclerosis, but normal autonomic function after Kawasaki disease. J. Pediatr. 2007, 151, 239–243. [Google Scholar] [CrossRef]
- Schmidt-Trucksass, A.; Sandrock, M.; Cheng, D.C.; Muller, H.M.; Baumstark, M.W.; Rauramaa, R.; Berg, A.; Huonker, M. Quantitative measurement of carotid intima–media roughness--effect of age and manifest coronary artery disease. Atherosclerosis 2003, 166, 57–65. [Google Scholar] [CrossRef]
- Homma, S.; Hirose, N.; Ishida, H.; Ishii, T.; Araki, G. Carotid plaque and intima–media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke 2001, 32, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Dallaire, F.; Dahdah, N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J. Am. Soc. Echocardiogr. 2011, 24, 60–74. [Google Scholar] [CrossRef]
- Teynor, A.; Caviezel, S.; Dratva, J.; Kunzli, N.; Schmidt-Trucksass, A. An automated, interactive analysis system for ultrasound sequences of the common carotid artery. Ultrasound Med. Biol. 2012, 38, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Trucksäss, A.; Cheng, D.C.; Sandrock, M.; Schulte-Mönting, J.; Rauramaa, R.; Huonker, M.; Burkhardt, H. Computerized analysing system using the active contour in ultrasound measurement of carotid artery intima–media thickness. Clin. Physiol. 2001, 21, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.C.; Schmidt-Trucksäss, A.; Cheng, K.S.; Burkhardt, H. Using snakes to detect the intimal and adventitial layers of the common carotid artery wall in sonographic images. Comput. Methods Programs Biomed. 2002, 67, 27–37. [Google Scholar] [CrossRef]
- Dalla Pozza, R.; Pirzer, R.; Beyerlein, A.; Weberruss, H.; Oberhoffer, R.; Schmidt-Trucksass, A.; Netz, H.; Haas, N. Beyond intima–media-thickness: Analysis of the carotid intima–media-roughness in a paediatric population. Atherosclerosis 2016, 251, 164–169. [Google Scholar] [CrossRef]
- Rueb, K.; Mynard, J.; Liu, R.; Wake, M.; Vuillermin, P.; Ponsonby, A.L.; Zannino, D.; Burgner, D.P. Changes in carotid artery intima–media thickness during the cardiac cycle—A comparative study in early childhood, mid-childhood, and adulthood. Vasa 2017, 46, 275–281. [Google Scholar] [CrossRef]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: Executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010, 122, 2748–2764. [Google Scholar]
- Chambless, L.E.; Heiss, G.; Folsom, A.R.; Rosamond, W.; Szklo, M.; Sharrett, A.R.; Clegg, L.X. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am. J. Epidemiol. 1997, 146, 483–494. [Google Scholar] [CrossRef]
- Gardin, J.M.; Bartz, T.M.; Polak, J.F.; O’Leary, D.H.; Wong, N.D. What do carotid intima–media thickness and plaque add to the prediction of stroke and cardiovascular disease risk in older adults? The cardiovascular health study. J. Am. Soc. Echocardiogr. 2014, 27, 998–1005.e1002. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Nicolaides, A.N.; Laurora, G.; Cesarone, M.R.; De Sanctis, M.; Incandela, L.; Barsotti, A. Progression of subclinical atherosclerosis in 6 years. Ultrasound evaluation of the average, combined femoral and carotid bifurcation intima-media thickness. Vasa 1995, 24, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Nicolaides, A.N.; Laurora, G.; Cesarone, M.R.; De Sanctis, M.; Incandela, L.; Barsotti, A. Ultrasound morphology classification of the arterial wall and cardiovascular events in a 6-year follow-up study. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.F.; O’Leary, D.H.; Kronmal, R.A.; Wolfson, S.K.; Bond, M.G.; Tracy, R.P.; Gardin, J.M.; Kittner, S.J.; Price, T.R.; Savage, P.J.; et al. Sonographic evaluation of carotid artery atherosclerosis in the elderly: Relationship of disease severity to stroke and transient ischemic attack. Radiology 1993, 188, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Hoen, H.; Byington, R.; Howard, G.; Riley, W.A.; Furberg, C.D. Spatial distribution of carotid intimal-medial thickness as measured by B-mode ultrasonography. Stroke 1994, 25, 1812–1819. [Google Scholar] [CrossRef]
- Wu, Y.; Xi, M.; Zhang, L.; Lu, X.; Cheng, X.; Lv, Q. Carotid Intima-Media Roughness and Elasticity in Hypertensive Patients With Normal Carotid Intima-Media Thickness. J. Ultrasound Med. 2019, 38, 1545–1552. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, X.; Zhang, L.; Cheng, X.; Yuan, L.; Xie, M.; Lv, Q. Correlation between carotid intima-media roughness and cardiovascular risk factors. Exp. Ther. Med. 2019, 18, 49–56. [Google Scholar] [CrossRef]
- Krebs, A.; Schmidt-Trucksass, A.; Wagner, J.; Krebs, K.; Doerfer, J.; Schwab, K.O. Adult-like but regressive increase of intima–media thickness and roughness in a child with type 1 diabetes. Pediatr. Diabetes 2005, 6, 161–164. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Peleteiro, B.; Álvarez-Bueno, C.; Rodriguez-Artalejo, F.; Martínez-Vizcaíno, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: A systematic review and meta-analysis. BMJ Open 2017, 7, e015949. [Google Scholar] [CrossRef]
- Dionne, A.; Ibrahim, R.; Gebhard, C.; Bakloul, M.; Selly, J.B.; Leye, M.; Déry, J.; Lapierre, C.; Girard, P.; Fournier, A.; et al. Coronary Wall Structural Changes in Patients With Kawasaki Disease: New Insights From Optical Coherence Tomography (OCT). J. Am. Heart Assoc. 2015, 4, e001939. [Google Scholar] [CrossRef]
| n | KD Patients (n = 44) | n | Controls (n = 36) | p-Value * | |
|---|---|---|---|---|---|
| Female, n (%) | 44 | 15 (34.1) | 36 | 14 (38.9) | 0.657 |
| Age (years) | 44 | 13.4 (7.5) | 36 | 12.1 (5.3) | 0.372 |
| Height (cm) | 44 | 149.3 (24.2) | 36 | 148.4 (22.5) | 0.864 |
| Weight (kg) | 44 | 43.5 (21.3) | 36 | 42.2 (18.6) | 0.763 |
| BMI (kg/m2) | 44 | 18.2 (3.7) | 36 | 18.1 (3.1) | 0.834 |
| Blood pressure | |||||
| SBP (mmHg) | 44 | 117.3 (13.6) | 36 | 113.4 (8.2) | 0.117 |
| DBP (mmHg) | 44 | 70.9 (10.0) | 36 | 68.2 (8.9) | 0.207 |
| MAP (mmHg) | 44 | 92.1 (10.5) | 36 | 89 (7.7) | 0.128 |
| HR (1/min) | 44 | 88.5 (12.4) | 36 | 82.9 (12.6) | 0.053 |
| Laboratory data | |||||
| Total cholesterol (mg/dL) | 38 | 163.6 (31.5) | 24 | 171.2 (30.7) | 0.356 |
| Triglycerides (mg/dL) | 38 | 112.5 (65.3) | 24 | 88.5 (35.4) | 0.067 |
| LDL (mg/dL) | 26 | 104.7 (25.5) | 24 | 104 (26.2) | 0.925 |
| VLDL (mg/dL) | 20 | 16.6 (6.4) | 24 | 13.9 (6.5) | 0.176 |
| HDL (mg/dL) | 26 | 50.1 (13.0) | 24 | 53.9 (8.7) | 0.224 |
| CAA Status | |||||
| Group A | 27 (69.2) | ||||
| Group B | 6 (15.4) | ||||
| Group C | 6 (15.4) |
| cIMR End-Diastolic (mm) | p-Value | cIMR End-Systolic (mm) | p-Value | |
|---|---|---|---|---|
| Kawasaki disease (Yes vs. No) | −0.128 | 0.584 | −0.044 | 0.851 |
| n | ||
|---|---|---|
| Aneurysms (cases, %) | 39 | 12 (30.8%) |
| Therapy refractory (>1 times IVIG) (cases, %) | 37 | 14 (37.8%) |
| Duration of fever (days) | 33 | 9.70 (5.3) |
| Age at disease (months) | 41 | 4.4 (3.2) |
| BMI (kg/m2) | 30 | 15.3 (1.4) |
| CRP (mg/L) | 35 | 115.4 (92.0) |
| Thrombocytes (g/L) | 32 | 534.9 (175.6) |
| Hb (g/dL) | 34 | 10.2 (1.9) |
| Leukocytes (g/L) | 35 | 19.1 (10.9) |
| cIMR End-Diastolic (mm) | p-Value | cIMR End-Systolic (mm) | p-Value | |
|---|---|---|---|---|
| Group A (vs. healthy) | −0.140 | 0.599 | −0.143 | 0.583 |
| Group B (vs. healthy) | −0.403 | 0.391 | 0.293 | 0.524 |
| Group C (vs. healthy) | 0.006 | 0.990 | 0.280 | 0.554 |
| Group B + C (vs. A) | −0.056 | 0.888 | 0.543 | 0.150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, M.; Ullmann, T.; Pastor-Villaescusa, B.; Dalla-Pozza, R.; Bohlig, S.; Schmidt-Trucksäss, A.; Pattathu, J.; Haas, N.A.; Jakob, A. KD Diagnosis Does Not Increase Cardiovascular Risk in Children According to Dynamic Intima–Media Roughness Measurements. J. Clin. Med. 2022, 11, 1177. https://doi.org/10.3390/jcm11051177
König M, Ullmann T, Pastor-Villaescusa B, Dalla-Pozza R, Bohlig S, Schmidt-Trucksäss A, Pattathu J, Haas NA, Jakob A. KD Diagnosis Does Not Increase Cardiovascular Risk in Children According to Dynamic Intima–Media Roughness Measurements. Journal of Clinical Medicine. 2022; 11(5):1177. https://doi.org/10.3390/jcm11051177
Chicago/Turabian StyleKönig, Miriam, Theresa Ullmann, Belén Pastor-Villaescusa, Robert Dalla-Pozza, Sarah Bohlig, Arno Schmidt-Trucksäss, Joseph Pattathu, Nikolaus A. Haas, and André Jakob. 2022. "KD Diagnosis Does Not Increase Cardiovascular Risk in Children According to Dynamic Intima–Media Roughness Measurements" Journal of Clinical Medicine 11, no. 5: 1177. https://doi.org/10.3390/jcm11051177
APA StyleKönig, M., Ullmann, T., Pastor-Villaescusa, B., Dalla-Pozza, R., Bohlig, S., Schmidt-Trucksäss, A., Pattathu, J., Haas, N. A., & Jakob, A. (2022). KD Diagnosis Does Not Increase Cardiovascular Risk in Children According to Dynamic Intima–Media Roughness Measurements. Journal of Clinical Medicine, 11(5), 1177. https://doi.org/10.3390/jcm11051177








