Endometriosis and Isthmocele: Common or Rare?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Performed Surgery
2.2. Follow-Up
2.3. Outcome Measures
- The presence of extrauterine endometriosis and uterine scar endometriosis.
- Secondary outcome measures:
- The correlation between uterine scar endometriosis and endometriosis.
- Surgery outcomes, the improvement of symptoms, the achievement of pregnancy, pregnancy outcome, and complications in patients with and without extrauterine or uterine scar endometriosis.
2.4. Data Collection
- Patient characteristics: age, body mass index at the date of treatment;
- Patient’s medical history: number of preoperative pregnancies and deliveries, type of prior cesarean section (elective or emergency), previous surgeries, pre-existing endometriosis;
- Symptoms that indicate isthmocele surgery: abnormal uterine bleeding, dysmenorrhea, secondary infertility, or uterine scar pregnancy;
- Surgery characteristics: surgery time, intraoperative blood loss, complications (classified with Clavien–Dindo), suture technique for hysterotomy closure used during the procedure;
- Outcomes: histologic findings, newly diagnosed endometriosis, postoperative symptoms, pregnancies, and their outcomes.
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics, Medical History, Symptoms
3.2. Surgery Characteristics
3.3. Outcomes
3.3.1. Histological Findings
Endometriosis
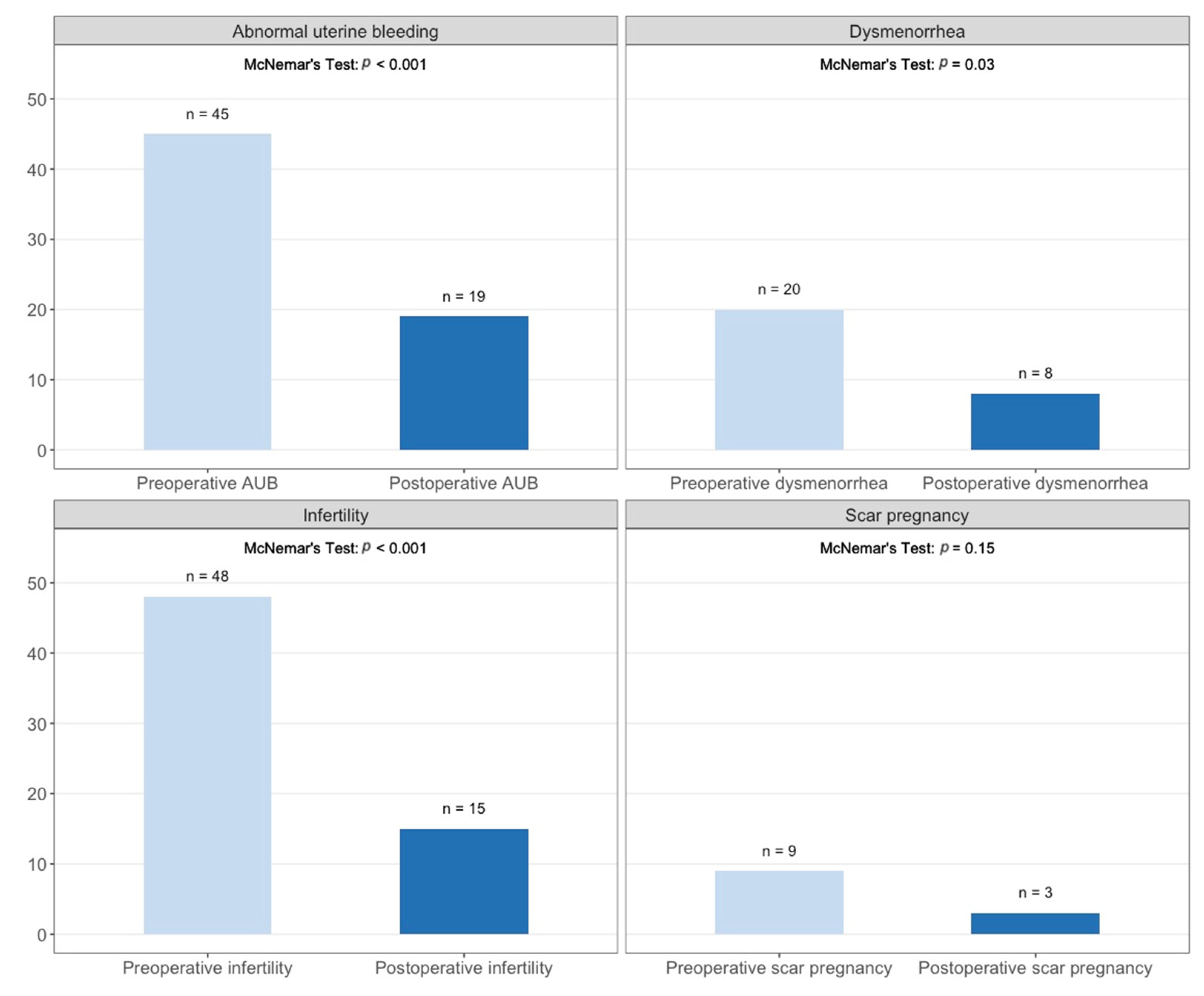
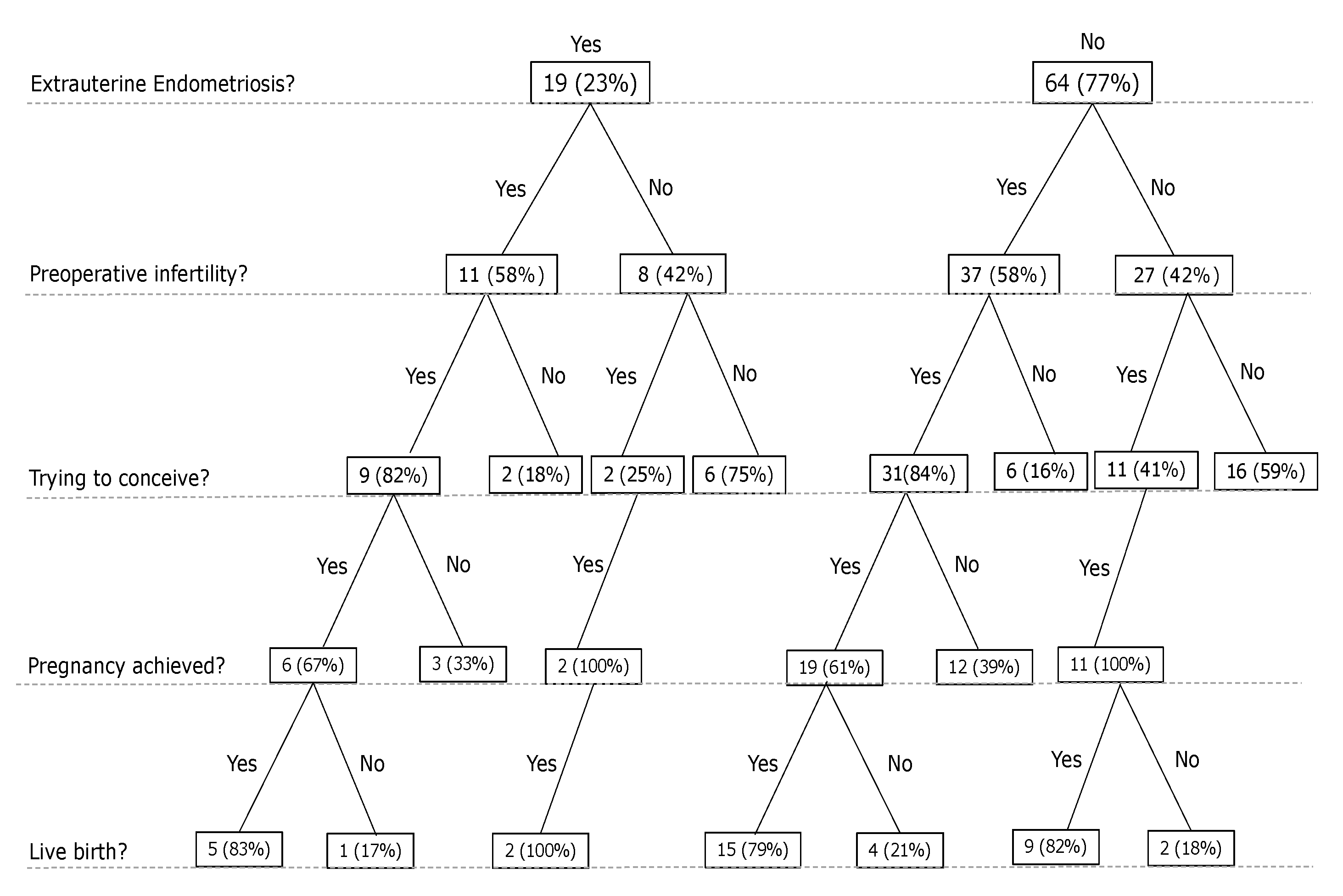
3.3.2. Pregnancy
3.3.3. Infertility
3.3.4. Pregnancy Complications
3.3.5. Abnormal Uterine Bleeding
3.3.6. Dysmenorrhea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bij de Vaate, A.J.; Van der Voet, L.F.; Naji, O.; Witmer, M.; Veersema, S.; Brölmann, H.A.; Bourne, T.; Huirne, J.A. Prevalence, potential risk factors for development and symptoms related to the presence of uterine niches following Cesarean section: Systematic review. Ultrasound Obstet. Gynecol. 2014, 43, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Tulandi, T.; Cohen, A. Emerging Manifestations of Cesarean Scar Defect in Reproductive-aged Women. J. Minim. Invasive Gynecol. 2016, 23, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, G.; Grifone, G.; Raimondo, D.; Seracchioli, R.; Scambia, G.; Masciullo, V. Hysteroscopic Treatment of Symptomatic Cesarean-induced Isthmocele: A Prospective Study. J. Minim. Invasive Gynecol. 2015, 22, 297–301. [Google Scholar] [CrossRef]
- Vikhareva Osser, O.; Jokubkiene, L.; Valentin, L. High prevalence of defects in Cesarean section scars at transvaginal ultrasound examination. Ultrasound Obstet. Gynecol. 2009, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Park, I.Y.; Kim, M.R.; Lee, H.N.; Gen, Y.; Kim, M.J. Risk factors for Korean women to develop an isthmocele after a cesarean section. BMC Pregnancy Childbirth 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Osser, O.V.; Valentin, L. Risk factors for incomplete healing of the uterine incision after caesarean section. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; DeZee, K.J.; Ahnfeldt, E.P.; Wagner, M. Abdominal wall endometriosis: A surgeon’s perspective and review of 445 cases. Am. J. Surg. 2008, 196, 207–212. [Google Scholar] [CrossRef]
- Tanimura, S.; Funamoto, H.; Hosono, T.; Shitano, Y.; Nakashima, M.; Ametani, Y.; Nakano, T. New diagnostic criteria and operative strategy for cesarean scar syndrome: Endoscopic repair for secondary infertility caused by cesarean scar defect. J. Obstet. Gynaecol. Res. 2015, 41, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Donnez, O.; Donnez, J.; Orellana, R.; Dolmans, M.M. Gynecological and obstetrical outcomes after laparoscopic repair of a cesarean scar defect in a series of 38 women. Fertil. Steril. 2017, 107, 289–296.e2. [Google Scholar] [CrossRef] [Green Version]
- Morris, H. Surgical pathology of the lower uterine segment caesarean section scar: Is the scar a source of clinical symptoms? Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 1995, 19, 16–20. [Google Scholar] [CrossRef]
- Shapira, M.; Mashiach, R.; Meller, N.; Watad, H.; Baron, A.; Bouaziz, J.; Cohen, S.B. Clinical Success Rate of Extensive Hysteroscopic Cesarean Scar Defect Excision and Correlation to Histologic Findings. J. Minim. Invasive Gynecol. 2020, 27, 129–134. [Google Scholar] [CrossRef]
- Tsuji, S.; Takahashi, A.; Higuchi, A.; Yamanaka, A.; Amano, T.; Kimura, F.; Seko-Nitta, A.; Murakami, T. Pregnancy outcomes after hysteroscopic surgery in women with cesarean scar syndrome. PLoS ONE 2020, 15, e0243421. [Google Scholar] [CrossRef] [PubMed]
- Nirgianakis, K.; Oehler, R.; Mueller, M. The Rendez-vous technique for treatment of caesarean scar defects: A novel combined endoscopic approach. Surg. Endosc. 2016, 30, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Ludwin, A.; Vilos, G.A.; Török, P.; Tesarik, J.; Vitagliano, A.; Lasmar, R.B.; Chiofalo, B. From hysteroscopy to laparoendoscopic surgery: What is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 301, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Tulandi, T. Cesarean Scar Pregnancy: A Systematic Review. J. Minim. Invasive Gynecol. 2017, 24, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, A.L.; Farland L v Shah, D.K.; Harris, H.R. Best Practice & Research Clinical Obstetrics and Gynaecology Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Andolf, E.; Thorsell, M.; Källén, K. Caesarean section and risk for endometriosis: A prospective cohort study of Swedish registries. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1061–1065. [Google Scholar] [CrossRef]
- Marcoux, S.; Maheux, R.; Bérubé, S. Laparoscopic Surgery in Infertile Women with Minimal or Mild Endometriosis. N. Engl. J. Med. 1997, 337, 217–222. [Google Scholar] [CrossRef]
- Hodgson, R.M.; Lee, H.L.; Wang, R.; Mol, B.W.; Johnson, N. Interventions for endometriosis-related infertility: A systematic review and network meta-analysis. Fertil. Steril. 2020, 113, 374–382.e2. [Google Scholar] [CrossRef]
- Bafort, C.; Beebeejaun, Y.; Tomassetti, C.; Bosteels, J.; Duffy, J.M.N. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Fabres, C.; Arriagada, P.; Fernández, C.; Mackenna, A.; Zegers, F.; Fernández, E. Surgical treatment and follow-up of women with intermenstrual bleeding due to Cesarean section scar defect. J. Minim. Invasive Gynecol. 2005, 12, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Karampelas, S.; Salem Wehbe, G.; de Landsheere, L.; Badr, D.A.; Tebache, L.; Nisolle, M. Laparoscopic Isthmocele Repair: Efficacy and Benefits before and after Subsequent Cesarean Section. J. Clin. Med. 2021, 10, 5785. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.E.; Steller, C.; Cholkeri-Singh, A.S.K. Laparoscopic repair and resection of uterine isthmocele. Fertil. Steril. 2016, 106, e219–e220. [Google Scholar] [CrossRef]
- Tanos, V.; Toney, Z.A. Uterine scar rupture—Prediction, prevention, diagnosis, and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 53, 115–131. [Google Scholar] [CrossRef]
- Bujold, E.; Jastrow, N.; Simoneau, J.; Brunet, S.; Gauthier, R.J. SMFM Papers Prediction of complete uterine rupture by sonographic evaluation of the lower uterine segment. YMOB 2009, 201, 320.e1–320.e6. [Google Scholar]
- Vervoort, A.J.M.W.; Uittenbogaard, L.B.; Hehenkamp, W.J.K.; Brölmann, H.A.M.; Mol, B.W.J.; Huirne, J.A.F. Why do niches develop in Caesarean uterine scars? Hypotheses on the aetiology of niche development. Hum. Reprod. 2015, 30, 2695–2702. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, H.; Fu, X.D. Identifying possible risk factors for cesarean scar pregnancy based on a retrospective study of 291 cases. J. Obstet. Gynaecol. Res. 2020, 46, 272–278. [Google Scholar] [CrossRef]
- Vikhareva Osser, O.; Valentin, L. Clinical importance of appearance of cesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet. Gynecol. 2011, 117, 525–532. [Google Scholar] [CrossRef]
- Zhang, Y. A Comparative Study of Transvaginal Repair and Laparoscopic Repair in the Management of Patients with Previous Cesarean Scar Defect. J. Minim. Invasive Gynecol. 2016, 23, 535–541. [Google Scholar] [CrossRef]
| Characteristics | Means ± SD or n (%) |
|---|---|
| Age (years) | 34.07 ± 4.3 |
| Body mass index (kg/m2) | 24.8 ± 4.5 |
| Number of previous cesarean sections | |
| 1 | 58 (70%) |
| 2 | 19 (23%) |
| >2 | 6 (7%) |
| Pre-existing endometriosis | 7 (8%) |
| Prior isthmocele correction | 5 (6%) |
| Indication for surgery | |
| 45 (54%) 20 (24%) 48 (58%) 9 (11%) |
| Surgery time (min) | 125 ± 33.3 |
| Blood loss (mL) | 42.77 ± 89 |
| Suture technique | |
| 62 (75%) 20 (24%) |
| Histological findings of the uterine scar | |
| 60 (72%) 9 (11%) 5 (6%) 2 (2%) |
| Extrauterine endometriosis | 19 (23%) |
| Intra-/postoperative complications | 4 (5%) |
| Characteristics | Extrauterine Endometriosis n = 19 (23%) | No Endometriosis n = 64 (77%) |
|---|---|---|
| Means ± SD or n (%) | ||
| Age (years) | 34.6 ± 4.2 | 33.9 ± 4.3 |
| Body mass index (kg/m2) | 25.2 ± 3.2 | 24.7 ± 4.88 |
| Previous cesarean section | ||
| 1 | 15 (79%) | 43 (67%) |
| 2 | 3 (16%) | 16 (25%) |
| >2 | 1 (5%) | 5 (8%) |
| Pre-existing endometriosis | 1 (5%) | 6 (9%) |
| Histologic findings in the uterine scar | ||
| Scar tissue/fibrosis | 10 (53%) | 48 (75%) |
| Endometriosis | 6 (32%) ** | 3 (5%) |
| Inflammation | 0 (0%) | 5 (8%) |
| Other | 0 (0%) | 5 (8%) |
| Unknown | 3 (15%) | 4 (6%) |
| Surgery time (min) | 143 ± 38 * | 120 ± 30 |
| Blood loss (mL) | 64 ± 85 | 42.3 ± 87.8 |
| Indication for surgery | ||
| Abnormal uterine bleeding | 10 (53%) | 35 (55%) |
| Dysmenorrhea | 6 (32%) | 14 (22%) |
| Secondary infertility | 11 (58%) | 37 (58%) |
| Cesarean scar pregnancy | 0 (0%) | 9 (14%) |
| Persistence of symptoms/de novo symptoms | ||
| Abnormal uterine bleeding | 6 (32%) | 13 (20%) |
| Dysmenorrhea | 3 (16%) | 5 (8%) |
| Secondary infertility | 3 (16%) | 12 (19%) |
| Cesarean scar pregnancy | 0 (0%) | 3 (5%) |
| Revised American Society of Reproductive Medicine (rASRM) score | ||
| rASRM I | 14 (74%) | |
| rASRM II | 3 (16%) | |
| rASRM III | 2 (10%) | |
| rASRM IV | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulz, M.; Imboden, S.; Nirgianakis, K.; Siegenthaler, F.; Rau, T.T.; Mueller, M.D. Endometriosis and Isthmocele: Common or Rare? J. Clin. Med. 2022, 11, 1158. https://doi.org/10.3390/jcm11051158
Gulz M, Imboden S, Nirgianakis K, Siegenthaler F, Rau TT, Mueller MD. Endometriosis and Isthmocele: Common or Rare? Journal of Clinical Medicine. 2022; 11(5):1158. https://doi.org/10.3390/jcm11051158
Chicago/Turabian StyleGulz, Marietta, Sara Imboden, Konstantinos Nirgianakis, Franziska Siegenthaler, Tilman T. Rau, and Michael D. Mueller. 2022. "Endometriosis and Isthmocele: Common or Rare?" Journal of Clinical Medicine 11, no. 5: 1158. https://doi.org/10.3390/jcm11051158
APA StyleGulz, M., Imboden, S., Nirgianakis, K., Siegenthaler, F., Rau, T. T., & Mueller, M. D. (2022). Endometriosis and Isthmocele: Common or Rare? Journal of Clinical Medicine, 11(5), 1158. https://doi.org/10.3390/jcm11051158






