Deep Endometriosis and Infertility: What Is the Impact of Surgery?
Abstract
1. Introduction
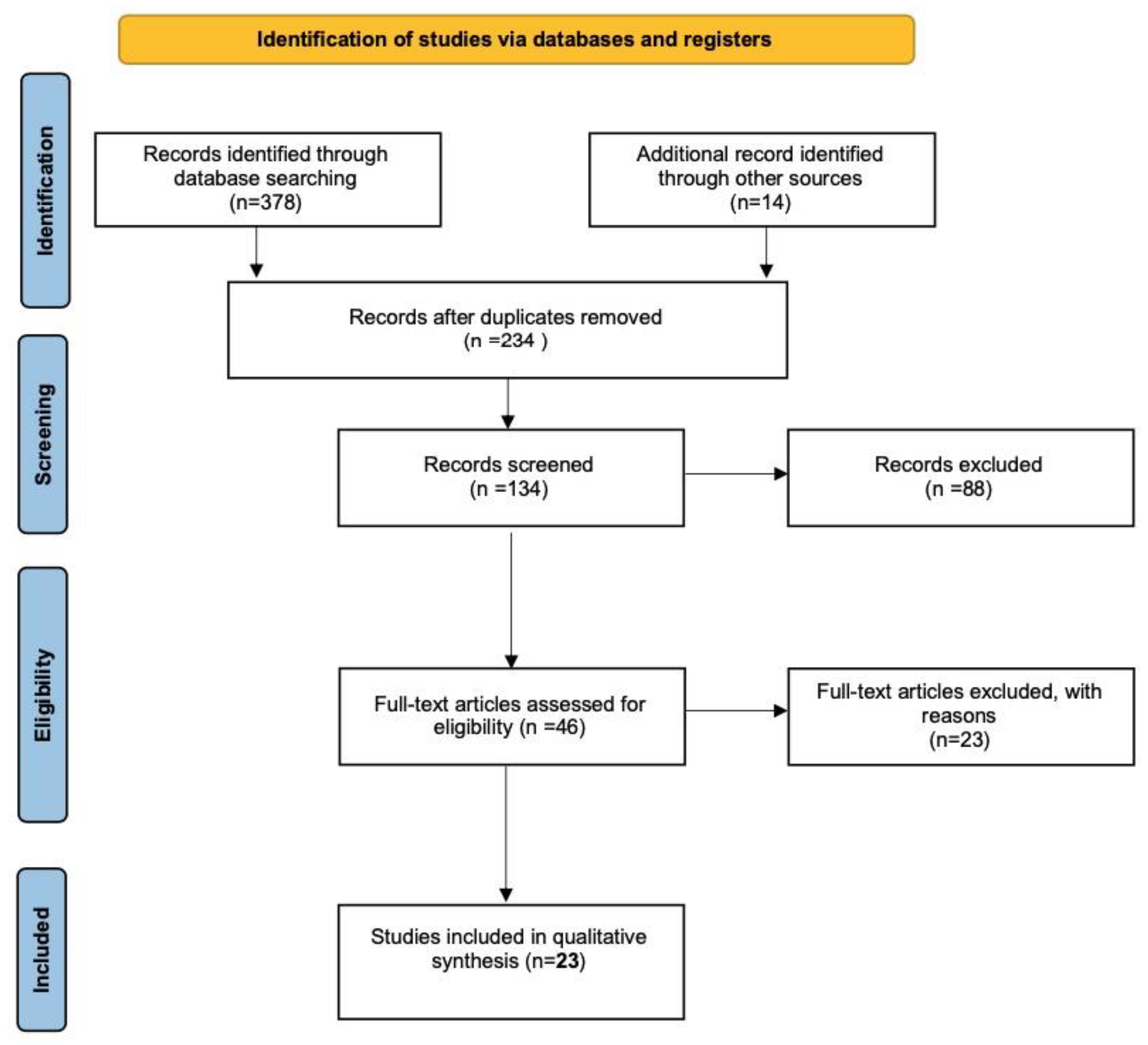
2. Materials and Methods
3. Results
4. Discussion
4.1. Fertility in Women Who Underwent Colorectal Surgery for Deep Endometriosis
4.1.1. Fertility in Women Who Developed Severe Complications after Colorectal Surgery
4.1.2. Choosing the Best Treatment for Removing Bowel Deep Endometriosis to Improve Pregnancy Rate
4.2. Fertility in Women Who Underwent Bladder Surgery for Deep Endometriosis
4.3. Pro and Cons of First-Line Surgery
4.4. Surgery before or after ART
4.5. The Role of Surgery in the Infertility Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angioni, S. New insights on endometriosis. Minerva Ginecol. 2017, 69, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Pontis, A. Umbilical endometriosis primary site without pelvic endometriosis and previous surgery: A case report. G. Ital. Ostet. Ginecol. 2014, 36, 336–338. [Google Scholar] [CrossRef]
- Alio, L.; Angioni, S.; Arena, S.; Bartiromo, L.; Bergamini, V.; Berlanda, N.; Bonanni, V.; Bonin, C.; Buggio, L.; Candiani, M.; et al. Endometriosis: Seeking optimal management in women approaching menopause. Climacteric 2019, 22, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Deiana, D.; Gessa, S.; Anardu, M.; Daniilidis, A.; Nappi, L.; D’Alterio, M.N.; Pontis, A.; Angioni, S. Genetics of endometriosis: A comprehensive review. Gynecol. Endocrinol. 2019, 35, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Angioni, S.; D’Alterio, M.N.; Coiana, A.; Anni, F.; Gessa, S.; Deiana, D. Genetic Characterization of Endometriosis Patients: Review of the Literature and a Prospective Cohort Study on a Mediterranean Population. Int. J. Mol. Sci. 2020, 21, 1765. [Google Scholar] [CrossRef] [PubMed]
- Viganó, D.; Zara, F.; Pinto, S.; Loddo, E.; Casula, L.; Soru, M.B.; D’Ancona, G.; D’Alterio, M.N.; Giuliani, C.; Angioni, S.; et al. How is small bowel permeability in endometriosis patients? a case control pilot study. Gynecol. Endocrinol. 2020, 36, 1010–1014. [Google Scholar] [CrossRef]
- Murgia, F.; Angioni, S.; D’Alterio, M.N.; Pirarba, S.; Noto, A.; Santoru, M.L.; Tronci, L.; Fanos, V.; Atzori, L.; Congiu, F. Metabolic Profile of Patients with Severe Endometriosis: A Prospective Experimental Study. Reprod. Sci. 2021, 28, 728–735. [Google Scholar] [CrossRef]
- Angioni, S.; Saponara, S.; Succu, A.G.; Sigilli, M.; Scicchitano, F.; D’Alterio, M.N. Metabolomic Characteristics in Endometriosis Patients. In Endometriosis Pathogenesis, Clinical Impact and Management; ISGE Series; Springer: Cham, Switzerland, 2021; pp. 9–17. [Google Scholar] [CrossRef]
- D’Alterio, M.N.; Giuliani, C.; Scicchitano, F.; Laganà, A.S.; Oltolina, N.M.; Sorrentino, F.; Nappi, L.; Orrù, G.; Angioni, S. Possible role of microbiome in the pathogenesis of endometriosis. Minerva Obstet. Gynecol. 2021, 73, 193–214. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Tahlak, M.; Keckstein, J.; Wattiez, A.; Martin, D.C. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 14–26. [Google Scholar] [CrossRef]
- Triolo, O.; Laganà, A.S.; Sturlese, E. Chronic Pelvic Pain in Endometriosis: An Overview. J. Clin. Med. Res. 2013, 5, 153–163. [Google Scholar] [CrossRef]
- Melis, I.; Agus, M.; Pluchino, N.; Sardo, A.D.S.; Litta, P.; Melis, G.B.; Angioni, S. Alexithymia in Women with Deep Endometriosis? A Pilot Study. J. Endometr. Pelvic Pain Disord. 2014, 6, 26–33. [Google Scholar] [CrossRef]
- Melis, I.; Litta, P.; Nappi, L.; Agus, M.; Melis, G.B.; Angioni, S. Sexual Function in Women with Deep Endometriosis: Correlation with Quality of Life, Intensity of Pain, Depression, Anxiety, and Body Image. Int. J. Sex. Health 2015, 27, 175–185. [Google Scholar] [CrossRef]
- D’Alterio, M.N.; Saponara, S.; Agus, M.; Laganà, A.S.; Noventa, M.; Loi, E.S.; Feki, A.; Angioni, S. Medical and surgical interventions to improve the quality of life for endometriosis patients: A systematic review. Gynecol. Surg. 2021, 18, 13. [Google Scholar] [CrossRef]
- Angioni, S.; Cela, V.; Sedda, F.; Loi, E.S.; Cofelice, V.; Pontis, A.; Melis, G.B. Focusing on surgery results in infertile patients with deep endometriosis. Gynecol. Endocrinol. 2015, 31, 595–598. [Google Scholar] [CrossRef]
- Stochino-Loi, E.; Millochau, J.-C.; Angioni, S.; Touleimat, S.; Abo, C.; Chanavaz-Lacheray, I.; Hennetier, C.; Roman, H. Relationship between Patient Age and Disease Features in a Prospective Cohort of 1560 Women Affected by Endometriosis. J. Minim. Invasive Gynecol. 2020, 27, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, M.N.; D’Ancona, G.; Raslan, M.; Tinelli, R.; Daniilidis, A.; Angioni, S. Management Challenges of Deep Infiltrating Endometriosis. Int. J. Fertil. Steril. 2021, 15, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Daraï, E.; Marpeau, O.; Thomassin, I.; Dubernard, G.; Barranger, E.; Bazot, M. Fertility after laparoscopic colorectal resection for endometriosis: Preliminary results. Fertil. Steril. 2005, 84, 945–950. [Google Scholar] [CrossRef]
- Angioni, S.; Cofelice, V.; Sedda, F.; Loi, E.; Multinu, F.; Pontis, A.; Melis, G. Progestins for Symptomatic Endometriosis: Results of Clinical Studies. Curr. Drug Ther. 2015, 10, 91–104. [Google Scholar] [CrossRef]
- Vignali, M.; Belloni, G.M.; Pietropaolo, G.; Di Prun, A.B.; Barbera, V.; Angioni, S.; Pino, I. Effect of Dienogest therapy on the size of the endometrioma. Gynecol. Endocrinol. 2020, 36, 723–727. [Google Scholar] [CrossRef]
- Melis, G.B.; Neri, M.; Corda, V.; Malune, M.E.; Piras, B.; Pirarba, S.; Guerriero, S.; Orrù, M.; D’Alterio, M.N.; Angioni, S.; et al. Overview of elagolix for the treatment of endometriosis. Expert Opin. Drug Metab. Toxicol. 2016, 12, 581–588. [Google Scholar] [CrossRef]
- Dababou, S.; Garzon, S.; Laganà, A.S.; Ferrero, S.; Evangelisti, G.; Noventa, M.; D’Alterio, M.N.; Palomba, S.; Uccella, S.; Franchi, M.; et al. Linzagolix: A new GnRH-antagonist under investigation for the treatment of endometriosis and uterine myomas. Expert Opin. Investig. Drugs 2021, 30, 903–911. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, M.N.; Saponara, S.; D’Ancona, G.; Russo, M.; Laganà, A.S.; Sorrentino, F.; Nappi, L.; Angioni, S. Role of surgical treatment in endometriosis. Minerva Obstet. Gynecol. 2021, 73, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Angioni, S.; Nappi, L.; Sorrentino, F.; Peiretti, M.; Daniilidis, A.; Pontis, A.; Tinelli, R.; D’Alterio, M.N. Laparoscopic treatment of deep endometriosis with a diode laser: Our experience. Arch. Gynecol. Obstet. 2021, 304, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Crosignani, P.; Abbiati, A.; Somigliana, E.; Vigano, P.; Fedele, L. The effect of surgery for symptomatic endometriosis: The other side of the story. Hum. Reprod. Updat. 2009, 15, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.; Murk, W.; Arici, A. Endometriosis and Infertility: Epidemiology and Evidence-Based Treatments. Ann. N. Y. Acad. Sci. 2008, 1127, 92–100. [Google Scholar] [CrossRef]
- Eisenberg, V.H.; Weil, C.; Chodick, G.; Shalev, V. Epidemiology of endometriosis: A large population-based database study from a healthcare provider with 2 million members. BJOG: Int. J. Obstet. Gynaecol. 2018, 125, 55–62. [Google Scholar] [CrossRef]
- de Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Macer, M.L.; Taylor, H.S. Endometriosis and Infertility: A Review of the Pathogenesis and Treatment of Endometriosis-Associated Infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef]
- Brosens, I. Endometriosis and the outcome of in vitro fertilization. Fertil. Steril. 2004, 81, 1198–1200. [Google Scholar] [CrossRef]
- Gupta, S.; Goldberg, J.M.; Aziz, N.; Goldberg, E.; Krajcir, N.; Agarwal, A. Pathogenic mechanisms in endometriosis-associated infertility. Fertil. Steril. 2008, 90, 247–257. [Google Scholar] [CrossRef]
- Somigliana, E.; Berlanda, N.; Benaglia, L.; Viganò, P.; Vercellini, P.; Fedele, L. Surgical excision of endometriomas and ovarian reserve: A systematic review on serum antimüllerian hormone level modifications. Fertil. Steril. 2012, 98, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Meana, M.; Hummelshoj, L.; Somigliana, E.; Viganò, P.; Fedele, L. Priorities for Endometriosis Research: A Proposed Focus on Deep Dyspareunia. Reprod. Sci. 2011, 18, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil. Steril. 2012, 98, 591–598. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano, P.; Benaglia, L.; Busnelli, A.; Vercellini, P.; Fedele, L. Adhesion Prevention in Endometriosis: A Neglected Critical Challenge. J. Minim. Invasive Gynecol. 2012, 19, 415–421. [Google Scholar] [CrossRef]
- Somigliana, E.; Infantino, M.; Candiani, M.; Vignali, M.; Chiodini, A.; Busacca, M.; Vignali, M. Association rate between deep peritoneal endometriosis and other forms of the disease: Pathogenetic implications. Hum. Reprod. 2004, 19, 168–171. [Google Scholar] [CrossRef]
- Larsen, S.; Lundorf, E.; Forman, A.; Dueholm, M. Adenomyosis and junctional zone changes in patients with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 206–211. [Google Scholar] [CrossRef]
- Tomassetti, C.; Meuleman, C.; Timmerman, D.; D’Hooghe, T. Adenomyosis and Subfertility: Evidence of Association and Causation. Semin. Reprod. Med. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Vercellini, P.; Consonni, D.; Barbara, G.; Buggio, L.; Frattaruolo, M.P.; Somigliana, E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: A systematic review and meta-analysis. Reprod. Biomed. Online 2014, 28, 704–713. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Somigliana, E.; Viganò, P.; Abbiati, A.; Barbara, G.; Crosignani, P.G. Surgery for endometriosis-associated infertility: A pragmatic approach. Hum. Reprod. 2009, 24, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Roman, H. Colorectal endometriosis and pregnancy wish why doing primary surgery. Front. Biosci. 2015, 7, 83–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; van der Poel, S.; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology, 2009. Hum. Reprod. 2009, 24, 2683–2687. [Google Scholar] [CrossRef]
- Bendifallah, S.; Roman, H.; D’Argent, E.M.; Touleimat, S.; Cohen, J.; Darai, E.; Ballester, M. Colorectal endometriosis-associated infertility: Should surgery precede ART? Fertil. Steril. 2017, 108, 525–531.e4. [Google Scholar] [CrossRef]
- Rubod, C.; Fouquet, A.; Bartolo, S.; Lepage, J.; Capelle, A.; Lefebvre, C.; Kamus, E.; Dewailly, D.; Collinet, P. Factors associated with pregnancy after in vitro fertilization in infertile patients with posterior deep pelvic endometriosis: A retrospective study. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 235–239. [Google Scholar] [CrossRef]
- Kovoor, E.; Nassif, J.; Miranda-Mendoza, I.; Wattiez, A. Endometriosis of Bladder: Outcomes after Laparoscopic Surgery. J. Minim. Invasive Gynecol. 2010, 17, 600–604. [Google Scholar] [CrossRef]
- Kavallaris, A.; Chalvatzas, N.; Hornemann, A.; Banz, C.; Diedrich, K.; Agic, A. 94 months follow-up after laparoscopic assisted vaginal resection of septum rectovaginale and rectosigmoid in women with deep infiltrating endometriosis. Arch. Gynecol. Obstet. 2011, 283, 1059–1064. [Google Scholar] [CrossRef]
- Daraï, E.; Lesieur, B.; Dubernard, G.; Rouzier, R.; Bazot, M.; Ballester, M. Fertility after colorectal resection for endometriosis: Results of a prospective study comparing laparoscopy with open surgery. Fertil. Steril. 2011, 95, 1903–1908. [Google Scholar] [CrossRef]
- Douay-Hauser, N.; Yazbeck, C.; Walker, F.; Luton, D.; Madelenat, P.; Koskas, M. Infertile Women with Deep and Intraperitoneal Endometriosis: Comparison of Fertility Outcome According to the Extent of Surgery. J. Minim. Invasive Gynecol. 2011, 18, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, C.; Tomassetti, C.; D’Hoore, A.; Buyens, A.; Van Cleynenbreugel, B.; Fieuws, S.; Penninckx, F.; Vergote, I.; D’Hooghe, T. Clinical outcome after CO2 laser laparoscopic radical excision of endometriosis with colorectal wall invasion combined with laparoscopic segmental bowel resection and reanastomosis. Hum. Reprod. 2011, 26, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Rozsnyai, F.; Roman, H.; Resch, B.; Dugardin, F.; Berrocal, J.; Descargues, G.; Schmied, R.; Boukerrou, M.; Marpeau, L.; Cirendo Study Group. Outcomes of Surgical Management of Deep Infiltrating Endometriosis of the Ureter and Urinary Bladder. JSLS J. Soc. Laparoendosc. Surg. 2011, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Jelenc, F.; Ribič-Pucelj, M.; Juvan, R.; Kobal, B.; Šinkovec, J.; Šalamun, V. Laparoscopic Rectal Resection of Deep Infiltrating Endometriosis. J. Laparoendosc. Adv. Surg. Tech. 2012, 22, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, C.; Tomassetti, C.; Wolthuis, A.; Van Cleynenbreugel, B.; Laenen, A.; Penninckx, F.; Vergote, I.; D’Hoore, A.; D’Hooghe, T. Clinical Outcome After Radical Excision of Moderate—Severe Endometriosis With or Without Bowel Resection and Reanastomosis: A Prospective Cohort Study. Ann. Surg. 2014, 259, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Tarjanne, S.; Heikinheimo, O.; Mentula, M.; Härkki, P. Complications and long-term follow-up on colorectal resections in the treatment of deep infiltrating endometriosis extending to bowel wall. Acta Obstet. Gynecol. Scand. 2014, 94, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Malzoni, M.; Di Giovanni, A.; Exacoustos, C.; Lannino, G.; Capece, R.; Perone, C.; Rasile, M.; Iuzzolino, D. Feasibility and Safety of Laparoscopic-Assisted Bowel Segmental Resection for Deep Infiltrating Endometriosis: A Retrospective Cohort Study With Description of Technique. J. Minim. Invasive Gynecol. 2016, 23, 512–525. [Google Scholar] [CrossRef]
- Soriano, D.; Bouaziz, J.; Elizur, S.; Zolti, M.; Orvieto, R.; Seidman, D.; Goldenberg, M.; Eisenberg, V.H. Reproductive Outcome Is Favorable After Laparoscopic Resection of Bladder Endometriosis. J. Minim. Invasive Gynecol. 2016, 23, 781–786. [Google Scholar] [CrossRef]
- Saavalainen, L.; Heikinheimo, O.; Tiitinen, A.; Härkki, P. Deep infiltrating endometriosis affecting the urinary tract—surgical treatment and fertility outcomes in 2004–2013. Gynecol. Surg. 2016, 13, 435–444. [Google Scholar] [CrossRef]
- Centini, G.; Afors, K.; Murtada, R.; Argay, I.M.; Lazzeri, L.; Akladios, C.Y.; Zupi, E.; Petraglia, F.; Wattiez, A. Impact of Laparoscopic Surgical Management of Deep Endometriosis on Pregnancy Rate. J. Minim. Invasive Gynecol. 2016, 23, 113–119. [Google Scholar] [CrossRef]
- Ballester, M.; Roman, H.; Mathieu, E.; Touleimat, S.; Belghiti, J.; Daraï, E. Prior colorectal surgery for endometriosis-associated infertility improves ICSI-IVF outcomes: Results from two expert centres. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Millochau, J.-C.; Stochino-Loi, E.; Darwish, B.; Abo, C.; Coget, J.; Chati, R.; Tuech, J.-J.; Roman, H. Multiple Nodule Removal by Disc Excision and Segmental Resection in Multifocal Colorectal Endometriosis. J. Minim. Invasive Gynecol. 2018, 25, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Stochino-Loi, E.; Darwish, B.; Mircea, O.; Touleimat, S.; Millochau, J.-C.; Abo, C.; Angioni, S.; Roman, H. Does preoperative antimüllerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil. Steril. 2017, 107, 707–713.e3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Timoh, K.N.; Ballester, M.; Bendifallah, S.; Fauconnier, A.; Darai, E. Fertility outcomes after laparoscopic partial bladder resection for deep endometriosis: Retrospective analysis from two expert centres and review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 12–17. [Google Scholar] [CrossRef]
- Hudelist, G.; Aas-Eng, M.K.; Birsan, T.; Berger, F.; Sevelda, U.; Kirchner, L.; Salama, M.; Dauser, B. Pain and fertility outcomes of nerve-sparing, full-thickness disk or segmental bowel resection for deep infiltrating endometriosis-A prospective cohort study. Acta Obstet. Gynecol. Scand. 2018, 97, 1438–1446. [Google Scholar] [CrossRef]
- Arfi, A.; Bendifallah, S.; D’Argent, E.M.; Poupon, C.; Ballester, M.; Cohen, J.; Darai, E. Nomogram predicting the likelihood of live-birth rate after surgery for deep infiltrating endometriosis without bowel involvement in women who wish to conceive: A retrospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 235, 81–87. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, S.; Zheng, Y.; Yi, X.; Qiu, J.; Zhang, X.; Hua, K. Reproductive and postsurgical outcomes of infertile women with deep infiltrating endometriosis. BMC Women’s Health 2022, 22, 83. [Google Scholar] [CrossRef]
- Roman, H.; Huet, E.; Bridoux, V.; Khalil, H.; Hennetier, C.; Bubenheim, M.; Braund, S.; Tuech, J.-J. Long-term Outcomes Following Surgical Management of Rectal Endometriosis: Seven-year Follow-up of Patients Enrolled in a Randomized Trial. J. Minim. Invasive Gynecol. 2022, 29, 767–775. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Donnez, O.; Roman, H. Choosing the right surgical technique for deep endometriosis: Shaving, disc excision, or bowel resection? Fertil. Steril. 2017, 108, 931–942. [Google Scholar] [CrossRef]
- Johnson, N.P.; Hummelshoj, L.; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Roman, H. Endometriosis surgery and preservation of fertility, what surgeons should know. J. Visc. Surg. 2018, 155 (Suppl. S1), S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Thomin, A.; Mathieu D’Argent, E.; Laas, E.; Canlorbe, G.; Zilberman, S.; Belghiti, J.; Thomassin-Naggara, I.; Bazot, M.; Ballester, M.; et al. Fertility before and after Surgery for Deep Infiltrating Endometriosis with and without Bowel Involvement: A Literature Review. Minerva Ginecol. 2014, 66, 575–587. [Google Scholar]
- Iversen, M.L.; Seyer-Hansen, M.; Forman, A. Does surgery for deep infiltrating bowel endometriosis improve fertility? A systematic review. Acta Obstet. Gynecol. Scand. 2017, 96, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Daraï, E.; Cohen, J.; Ballester, M. Colorectal endometriosis and fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E. First-line in vitro fertilization or surgery for infertile women with bowel endometriosis? Fertil. Steril. 2021, 115, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Barbara, G.; Buggio, L.; Frattaruolo, M.P.; Somigliana, E.; Fedele, L. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: Literature review. Reprod. Biomed. Online 2012, 24, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, C.; Roman, H.; Alzahrani, Y.; D’Argent, E.M.; Bendifallah, S.; Marty, N.; Perez, M.; Rubod, C.; Collinet, P.; Daraï, E.; et al. Fertility outcomes in women experiencing severe complications after surgery for colorectal endometriosis. Hum. Reprod. 2018, 33, 411–415. [Google Scholar] [CrossRef]
- Roman, H.; Chanavaz-Lacheray, I.; Ballester, M.; Bendifallah, S.; Touleimat, S.; Tuech, J.-J.; Farella, M.; Merlot, B. High postoperative fertility rate following surgical management of colorectal endometriosis. Hum. Reprod. 2018, 33, 1669–1676. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Frattaruolo, M.P.; Borghi, A.; Somigliana, E. Bowel surgery as a fertility-enhancing procedure in patients with colorectal endometriosis: Methodological, pathogenic and ethical issues. Hum. Reprod. 2018, 33, 1205–1211. [Google Scholar] [CrossRef]
- Muzii, L.; DI Tucci, C.; Galati, G.; Mattei, G.; Chinè, A.; Cascialli, G.; Palaia, I.; Benedetti Panici, P. Endometriosis-associated infertility: Surgery or IVF? Minerva Obstet. Gynecol. 2021, 73, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, C.; Boujenah, J.; Poncelet, C.; Chabbert-Buffet, N.; D’Argent, E.M.; Carbillon, L.; Grynberg, M.; Darai, E.; Bendifallah, S. Use of the EFI score in endometriosis-associated infertility: A cost-effectiveness study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Noventa, M.; Gizzo, S.; Saccardi, C.; Borgato, S.; Vitagliano, A.; Quaranta, M.; Litta, P.; Gangemi, M.; Ambrosini, G.; D’Antona, D.; et al. Salpingectomy before assisted reproductive technologies: A systematic literature review. J. Ovarian Res. 2016, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Casals, G.; Carrera, M.; Domínguez, J.A.; Abrão, M.S.; Carmona, F. Impact of Surgery for Deep Infiltrative Endometriosis before In Vitro Fertilization: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2021, 28, 1303–1312.e5. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.; D’Argent, E.M.; Morcel, K.; Belaisch-Allart, J.; Nisolle, M.; Daraï, E. Cumulative pregnancy rate after ICSI-IVF in patients with colorectal endometriosis: Results of a multicentre study. Hum. Reprod. 2012, 27, 1043–1049. [Google Scholar] [CrossRef]
- Iliodromiti, S.; Kelsey, T.; Wu, O.; Anderson, R.A.; Nelson, S. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception: A systematic review and meta-analysis of the literature. Hum. Reprod. Updat. 2014, 20, 560–570. [Google Scholar] [CrossRef]
- Salmassi, A.; Mettler, L.; Hedderich, J.; Jonat, W.; Deenadayal, A.; Von Otte, S.; Eckmann-Scholz, C.; Schmutzler, A.G. Cut-Off Levels of Anti-Mullerian Hormone for The Prediction of Ovarian Response, In Vitro Fertilization Outcome and Ovarian Hyperstimulation Syndrome. Int. J. Fertil. Steril. 2015, 9, 157–167. [Google Scholar] [CrossRef]
- Streuli, I.; de Mouzon, J.; Paccolat, C.; Chapron, C.; Petignat, P.; Irion, O.P.; de Ziegler, D. AMH concentration is not related to effective time to pregnancy in women who conceive naturally. Reprod. Biomed. Online 2014, 28, 216–224. [Google Scholar] [CrossRef]
- Raos, M.; Roman, H.; Seyer-Hansen, M.; Kesmodel, U.S.; Knudsen, U.B. EFFORT study: Comparing impact of operation and assisted reproductive technologies on fertility for women with deep infiltrating endometriosis—Study protocol for a multicentre randomised trial. BMJ Open 2022, 12, e052877. [Google Scholar] [CrossRef]
| Article | Study Design | Patients Studied for PR n | Endometriosis Localization (Main Site) | Documented Infertility before Surgery % | WTC % | TPR % (SP + MAR) | TPR in WTC % | Llive Birth % |
|---|---|---|---|---|---|---|---|---|
| Kovoor et al., 2010 [49] | Retrospective observational | 10 | Bladder | 100% | 100% | 60% | 60% | NR |
| Kavallaris et al., 2011 [50] | Retrospective observational | 30 | Rectovaginal and bowel | NR | 56.6% | 36.6% | 64.7% | 81.8% |
| Daraı et al., 2011 [51] | RCT | 52 | Colorectal | 44.3% | 53.8% | 53.8% | 39.3% | NR |
| Douay-Hauser et al., 2011 [52] | Retrospective controlled | 75 | No specific involvement | 100% | 100% | 18.7% | 18.7% | NR |
| Meuleman et al., 2011 [53] | Retrospective observational | 28 | Colorectal | NR | 100% | 46.4% | 46.4% | NR |
| Rosznyai et al., 2011 [54] | Retrospective observational | 9 | Bladder and ureteral | NR | 100% | 66.6% | 66.6% | NR |
| Jelenc et al., 2012 [55] | Retrospective observational | 14 | Colorectal | NR | 100% | 71.4% | 71.4% | NR |
| Meuleman et al., 2014 [56] | Prospective controlled | 148 | Colorectal | NR | NR | 50.7% | NR | 81.3% |
| Tarjanne et al., 2014 [57] | Retrospective observational | 164 | Colorectal | NR | 53.7% | 25% | 46.5% | 100% |
| Malzoni et al., 2016 [58] | Retrospective observational | 72 | Colorectal | NR | 100% | 69.4% | 69.4% | 68% |
| Soriano et al., 2016 [59] | Retrospective observational | 42 | Bladder | 64.3% | 100% | 80.9% | 85.7% | 100% |
| Saavalainen et al., 2016 [60] | Retrospective observational | 28 | Bladder | NR | NR | 64.2% | NR | NR |
| Centini et al., 2016 [61] | Retrospective observational | 115 | No specific involvement | 100% | NR | 54.8% | NR | 77.7% |
| Ballester et al., 2017 [62] | Prospective observational | 60 | Colorectal | 90% | 100% | 60% | 60% | 58.3% |
| Millochau et al., 2017 [63] | Retrospective observational | 9 | Colorectal | NR | NR | 66.6% | NR | 83% |
| Bendifallah et al., 2017 [47] | Retrospective controlled | 55 | Colorectal | 100% | 100% | 49.1% | 49.1% | 100% |
| Stochino Loi et al., 2017 [64] | Retrospective controlled | 180 | No specific involvement | 75% | 75% | 74.4% | NR | 72.4% |
| Timoh et al., 2018 [65] | Retrospective observational | 34 | Bladder with and without posterior compartment involvement | 50% | 73.5% | 50% | 68% | 76.5% |
| Hudelist et al., 2018 [66] | Prospective observational | 61 | Bowel | 54.5% | 100% | 63.9% | 63.9% | 76.9% |
| Rubod et al., 2019 [48] | Retrospective observational | 152 | No specific involvement | 100% | 100% | 49.3% | 49.3% | NR |
| Arfi et al., 2019 [67] | Retrospective observational | 118 | No specific involvement | 55.9% | 100% | 39% | 39% | 78.2% |
| Zhang et al., 2022 [68] | Retrospective observational | 55 | No specific involvement | 100% | 100% | 61.8% | 61.8% | 82.3% |
| Roman et al., 2022 [69] | RCT | 37 | Colorectal | NR | 100% | 83.8% | 83.8% | NR |
| Total | 1548 | 79.7% | 86.6% | 52.6% 95% CI (49.7–63) | 53% 95% CI (40.2–77.4) | 78.3% 95% CI (60.8–80.2) |
| Article | Infertile Women WTC n | TPR % | SP % | MAR % |
|---|---|---|---|---|
| Kovoor et al., 2010 [49] | 10 | 60% | 50% | 10% |
| Daraı et al., 2011 [51] | 15 | 33.3% | 26.7% | 6.6% |
| Douay-Hauser et al., 2011 [52] | 75 | 18.7% | 18.7% | 0% |
| Meuleman et al., 2011 [53] | 28 | 46.4% | 28.6% | 17.8% |
| Jelenc et al., 2012 [55] | 14 | 71.4% | 57.1% | 14.3% |
| Malzoni et al., 2016 [58] | 72 | 69.4% | 61.1% | 8.3% |
| Ballester et al., 2017 [62] | 54 | 66.6% | 0% | 66.6% |
| Soriano et al., 2016 [59] | 27 | 70.4% | 18.5% | 51.9% |
| Bendifallah et al., 2017 [47] | 55 | 49.1% | 0% | 49.1% |
| Timoh et al., 2018 [65] | 17 | 52.9% | 35.3% | 17.6% |
| Rubod et al., 2019 [48] | 152 | 49.3% | 0% | 49.3% |
| Hudelist et al., 2018 [66] | 61 | 63.9% | 42.6% | 21.3% |
| Zhang et al., 2022 [68] | 55 | 61.8% | 43.6% | 18.2% |
| Total | 635 | 53% 95% CI (46.3–63.4) | 22.7% 95% CI (15.3–57.2) | 30.3% 95% CI (7.6–58) |
| Article Focusing on Bowel Involvement | Number of Patients n | Women Studied for PR n | TPR % | SP % | MAR % | Type of Treatment | Clavien–Dindo Complications Grade % and Recurrences % |
|---|---|---|---|---|---|---|---|
| Kavallaris et al., 2011 [50] | 55 | 30 | 36.6% | 23.3% | 13.3% | Combined LPS/vaginal technique en bloc excision of the posterior vaginal wall, rectovaginal septum, and a part of the rectosigmoid | Grade II: 1.8% Grade III: 5.4% 25.5% bladder atony requiring self- catheterization for a mean of 3 months 3.6% anastomotic leakage 3.6% bowel recurrence reoperation Recurrences NR |
| Darai et al., 2011 [51] | 52 | 52 | 53.8% | 11.5% | 42.3% | LPS or LPT colorectal resection LPS: 17.3% LPT 28.8% | Grade I: 3.8% Grade II: 42.3% Grade III: 23% Recurrences NR |
| Meuleman et al., 2011 [53] | 45 | 28 | 46.4% | 28.6% | 17.8% | CO2 laser lps radical deep endometriosis excision followed by bowel resection and reanastomosis | Grade III: 2.2% 2.2% bladder atony required self- catheterization for 10 weeks 2.2% LPS salpingectomy for hydrosalpinx 1 year after the surgery Recurrences 4.4% |
| Jelenc et al., 2012 [55] | 56 | 14 | 71.4% | 57.1% | 14.3% | LPS segmental bowel resection and reanastomosis | Grade III: 10.7% 5.4% anastomotic leakage 3.6% rectovaginal fistula 1.8% LPT for bleeding in pelvic region 3.6% anastomotic stricture Recurrences NR |
| Meuleman et al., 2014 [56] | 203 | 148 | 50.7% | 20.9% | 29.7% | Radical excision of moderate–severe endometriosis with or without LPS bowel resection and reanastomosis | Grade I–II: 4.4% Grade III: 2% 1.3% anastomotic leakage 1.3% rectovaginal fistula 1% bladder atony 0.98% bladder leakage Recurrerences: 3.9% |
| Tarjanne et al., 2014 [57] | 164 | 164 | 25% | NR | NR | LPS or LPT colorectal resection | Grade III: 8.5% 2.4% anastomotic leakage 1.8% ureteral fistulae 1.2% rectovaginal fistula 0.6% bladder atony for 3 months Recurrences: 6.7% |
| Malzoni et al., 2016 [58] | 248 | 72 | 69.4% | 61.1% | 8.3% | LPS bowel segmental resection and deep endometriosis excision | Grade II: 2.8% Grade III: 4.8% Grade IV: 0.4% 1.6% anastomotic leakage 2.4% rectovaginal fistula 0.8% severe peritonitis Recurrences NR |
| Ballester et al., 2017 [62] | 60 | 60 | 60% | 0% | 60% | LPS surgery: rectal shaving, full thickness disc excision or segmental colorectal resection | NR |
| Millochau et al., 2017 [63] | 21 | 9 | 66.6% | 22.2% | 44.4% | LPS rectal disc excision or short segmental resection | Grade II: 19% Grade III: 28.6% 19% rectovaginal fistula Recurrences NR |
| Bendifallah et al., 2017 [47] | 55 | 55 | 49.1% | 0% | 49.1% | LPS surgery: rectal shaving, full thickness disc excision or segmental colorectal resection | NR |
| Hudelist et al., 2018 [66] | 134 | 61 | 63.9% | 42.6% | 21.3% | LPS disc and limited nerve- and vessel-sparing segmental resection | Grade I: 7.5% Grade II: 2.2% Grade III: 6% 6.7% temporary bladder atony 1.5% anastomotic leakage 0.7% rectovaginal fistula Recurrences NR |
| Roman et al., 2022 [69] | 55 | 37 | 83.8% | NR | NR | LPS surgery: rectal shaving, full thickness disc excision or segmental colorectal resection | Grade III: 20% 3.6% rectovaginal fistula 1.8% bladder fistula 12.7% bladder atony required self- catheterization for 30 days Recurrences 3.6% |
| Total | 1148 | 730 | 50.3% 95% CI (47.2–65.6) | 24.9% 95% CI (8.1–34.5) | 30.8% 95% CI (19–41) | Grade I: 1.2% Grade II: 4.45% Grade III: 7.45% Grade IV: 0.09% 2% anastomotic leakage 5.2% bladder atony required self- catheterization 2.4% rectovaginal fistula Recurrences 4.8% |
| Studies | Patients n | DR and/or Shaving % | TPR in Conservative Surgery % | SP in Conservative Surgery % | MAR in Conservative Surgery % | SR % | TPR in SR % | SPR in SR % | MAR in SR % | TPR in Patients WTC % |
|---|---|---|---|---|---|---|---|---|---|---|
| Kavallaris et al., 2011 [50] | 30 | 0 | 0 | 0 | 0 | 100% | 36.6% | 23.3% | 13.3 % | 64.7% |
| Daraı et al., 2011 [51] | 52 | 0 | 0 | 0 | 0 | 100% | 53.8% | 11.5% | 42.3% | 39.3% |
| Meuleman et al., 2011 [53] | 28 | 0 | 0 | 0 | 0 | 100% | 46.4% | 28.6% | 17.8% | 46.4% |
| Jelenc et al., 2012 [55] | 14 | 0 | 0 | 0 | 0 | 100% | 71.4% | 57.1% | 14.3% | 71.4% |
| Tarjanne et al., 2014 [57] | 164 | 0 | 0 | 0 | 0 | 100% | 25% | NR | NR | 46.5% |
| Malzoni et al., 2016 [58] | 72 | 0 | 0 | 0 | 0 | 100% | 69.4% | 61.1% | 8.3% | 69.4% |
| Ballester et al., 2017 [62] | 9 | 22.2% | 100% | 0% | 100% | 77.7% | 42.8% | 0% | 42.8% | 55.5% |
| Hudelist et al., 2018 [66] | 61 | 18% | 63.6% | 54.5% | 9% | 82% | 64% | 40% | 24% | 63.9% |
| Total | 430 | 3% | 69.2% | 46.2% | 23% | 97% | 45% | 36.7% | 21.4% | 56.8% |
| Article Focusing on Bladder Involvement | Number of Patients n | Women Studied for PR n | TPR % | SP % | MAR % | Type of Treatment | Clavien–Dindo Complications Grade % and Recurrences % |
|---|---|---|---|---|---|---|---|
| Kovoor et al., 2010 [49] | 21 | 10 | 60% | 50% | 10% | LPS partial cystectomy or partial thickness excision of the detrusor muscle | Grade III: 19% 9.5% vescicovaginal fistula Recurrences NR |
| Rozsnyai et al., 2011 [54] | 30 | 9 | 66.6% | 55.5% | 11.1% | LPS partial cystectomy or partial thickness excision of the detrusor muscle and/or ureteral surgery | Grade II: 6.6% Grade III: 13.3% 6.6% bladder atony required self- catheterization for >6 months 3.3% vescico vaginal fistula Recurrences NR |
| Soriano et al., 2016 [59] | 69 | 42 | 80.9% | 38.1.% | 42.8% | LPS partial cystectomy or LPS partial thickness excision of the detrusor muscle | Grade III: 2.9% Recurrences 2.9% |
| Saavalainen et al., 2016 [60] | 53 | 28 | 64.2% | 25% | 39.3% | LPS and/or partial cystectomy or LPS partial thickness excision of the detrusor muscle | Grade I: 3.8% Grade II: 39.6% Grade III: 9.4% Recurrences NR |
| Timoh et al., 2018 [65] | 34 | 34 | 50% | 35.3% | 14.7% | LPS bladder resection associated with or without posterior DE resection | Grade I: 8.8% Grade II: 5.8% 5.8% bladder atony required self-catheterization Recurrences NR |
| Total | 207 | 123 | 65.9% 95% CI (54.5–74.2) | 36.6% 95% CI (30.2–51) | 29.3% 95% CI (9.5–37.7) | Grade I: 2.4% Grade II: 12% Grade III: 7.2% |
| Article Without Specific Involvement | Number of Patients n | Women Studied for PR n | TPR % | SP % | MAR % | Type of Treatment | Clavien–Dindo Complications Grade % and Recurrences % |
|---|---|---|---|---|---|---|---|
| Douay-hauser et al., 2011 [52] | 75 | 75 | 18.7% | 18.7% | 0% | LPS/LPT excision or coagulation of all visible endometriosis lesions. Segmental LPT resection in case of bowel involvement | Grade III: 8% 2.6% ureteral injuries 1.38% bowel perforation 1.38% abdominal wall abscess 1.38% ovarian abscess 1.38% anastomotic fistula Recurrences NR |
| Centini et al., 2016 [61] | 115 | 115 | 54.8% | 26.1% | 28.7% | Laparoscopic treatment of anterior, posterior, and lateral compartment | Grade III: 7.8% 1.7% vescicovaginal fistula 0.9% urinoma 0.9% rectovaginal fistula Recurrences 16.5% |
| Stochino loi et al., 2017 [64] | 180 | 180 | 74.4% | 41.1% | 33.3% | Laparoscopic treatment of anterior, posterior, and lateral compartment. | NR |
| Rubod et al., 2019 [48] | 152 | 152 | 49.3% | 0% | 49.3% | Excision of deep posterior endometriosis | NR |
| Arfi et al., 2019 [67] | 118 | 118 | 39% | 20.4% | 18.6% | LPS excision without bowel involvement | NR |
| Zhang et al., 2022 [68] | 55 | 55 | 61.8% | 43.6% | 18.2% | LPS excision with and without bowel involvement (shaving or segmental resection) | NR |
| Total | 695 | 695 | 52.7% IC95% (34.2–65.1) | 23.9% IC95% (12.1–37) | 28.8% IC95% (11.4–38) | Grade III: 7.9% 1% rectovaginal fistula |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniilidis, A.; Angioni, S.; Di Michele, S.; Dinas, K.; Gkrozou, F.; D’Alterio, M.N. Deep Endometriosis and Infertility: What Is the Impact of Surgery? J. Clin. Med. 2022, 11, 6727. https://doi.org/10.3390/jcm11226727
Daniilidis A, Angioni S, Di Michele S, Dinas K, Gkrozou F, D’Alterio MN. Deep Endometriosis and Infertility: What Is the Impact of Surgery? Journal of Clinical Medicine. 2022; 11(22):6727. https://doi.org/10.3390/jcm11226727
Chicago/Turabian StyleDaniilidis, Angelos, Stefano Angioni, Stefano Di Michele, Konstantinos Dinas, Fani Gkrozou, and Maurizio Nicola D’Alterio. 2022. "Deep Endometriosis and Infertility: What Is the Impact of Surgery?" Journal of Clinical Medicine 11, no. 22: 6727. https://doi.org/10.3390/jcm11226727
APA StyleDaniilidis, A., Angioni, S., Di Michele, S., Dinas, K., Gkrozou, F., & D’Alterio, M. N. (2022). Deep Endometriosis and Infertility: What Is the Impact of Surgery? Journal of Clinical Medicine, 11(22), 6727. https://doi.org/10.3390/jcm11226727