A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiochemistry of 68Ga-Nitroimidazole
2.3. 68Ga-Nitroimidazole PET/CT Imaging Procedure
2.4. 18F-FDG PET/CT Imaging Procedure
2.5. Image Analysis
2.6. Qualitative Analysis
2.7. Semi-Quantitative Analysis
2.8. Immunohistochemical Assessment
2.9. Statistical Analysis
3. Results
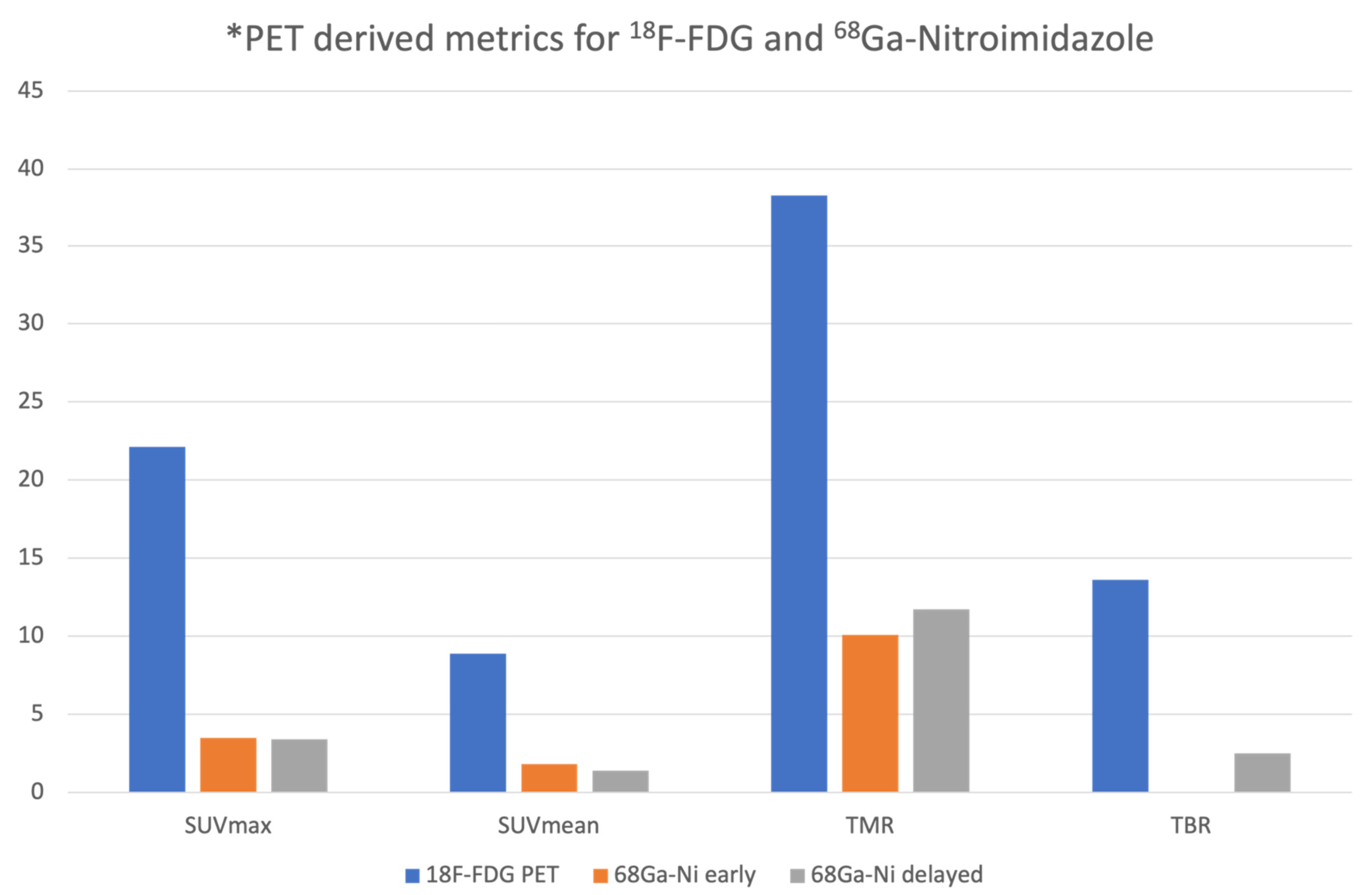
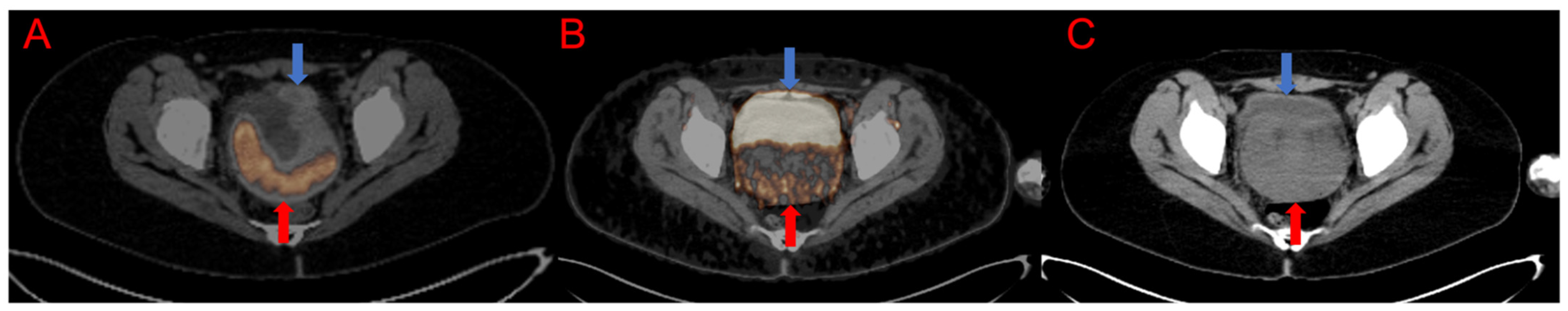
3.1. Qualitative and Semiquantitative Analysis of the PET/CT Images
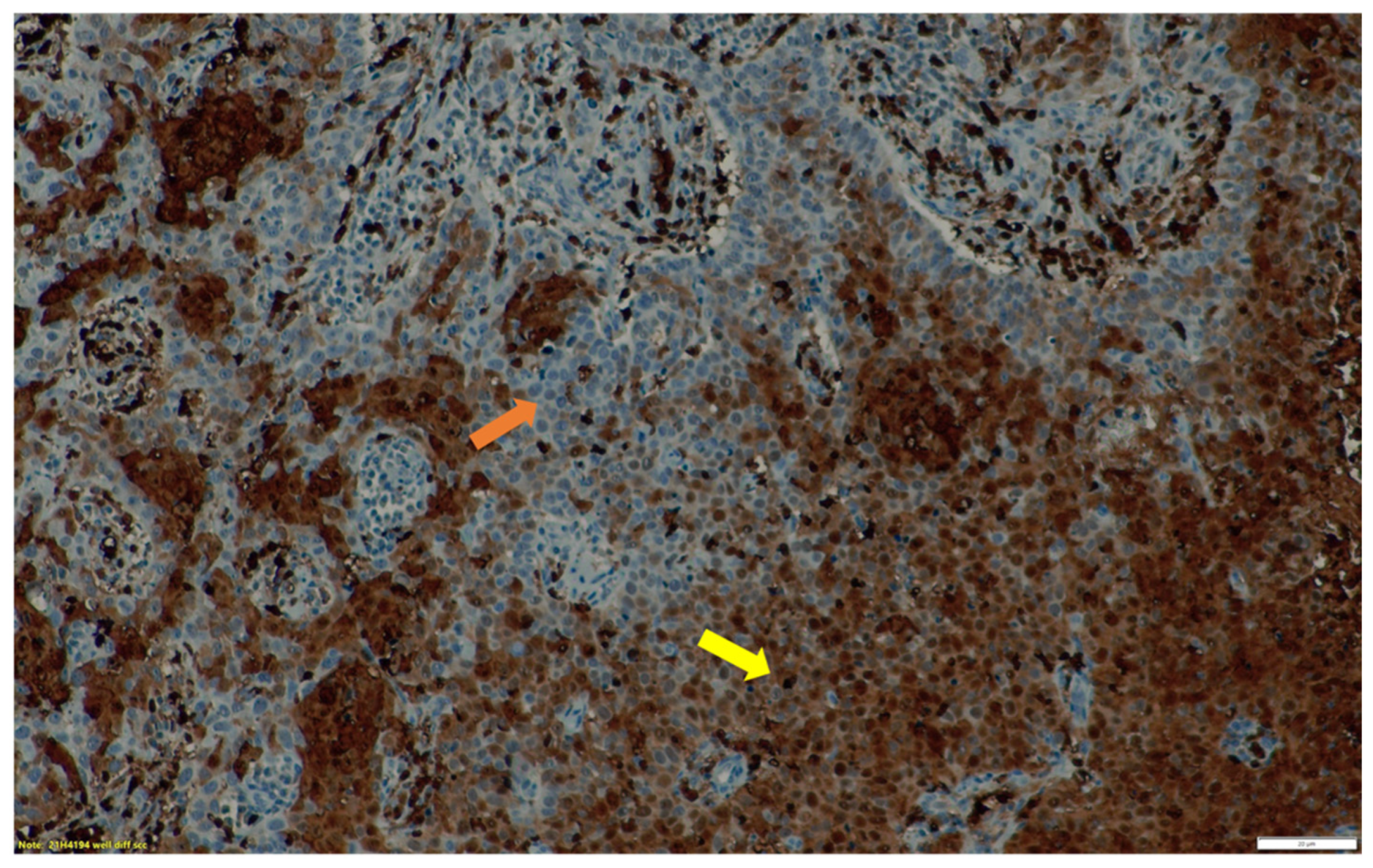
3.2. Immunohistochemistry Findings
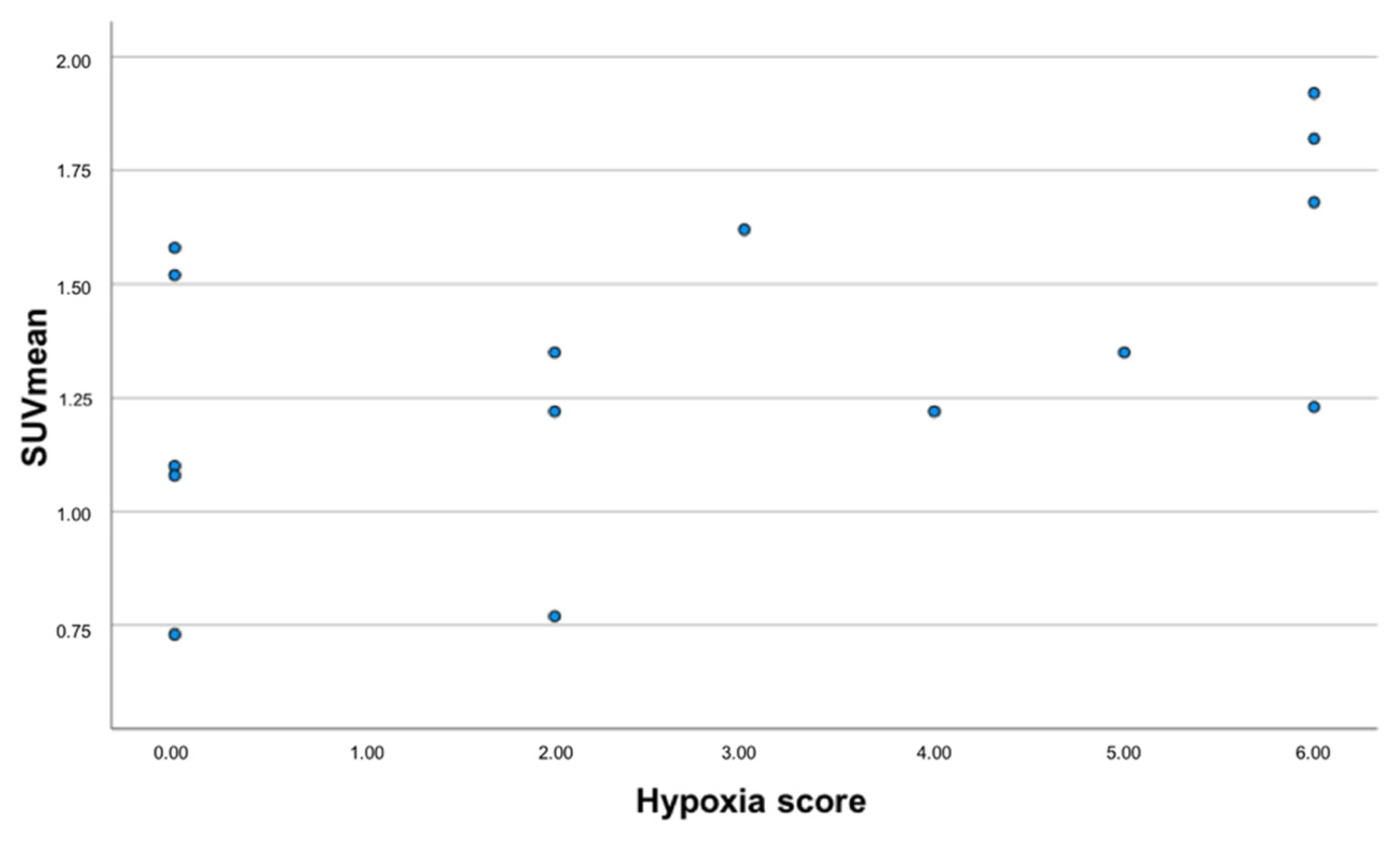
3.3. Correlation between 68Ga-Nitroimidazole PET/CT, 18F-FDG PET/CT Imaging and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Denny, L.; Anorlu, R. Cervical cancer in Africa. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1434–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denny, L. Cervical cancer treatment in Africa. Curr. Opin. Oncol. 2011, 23, 469–474. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Thews, O.; Hoeckel, M. Treatment resistance of solid tumors: Role of hypoxia and anemia. Med. Oncol. 2001, 18, 243–259. [Google Scholar] [CrossRef]
- Hockel, M.; Vorndran, B.; Schlenger, K.; Baussmann, E.; Knapstein, P.G. Tumor oxygenation: A new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol. Oncol. 1993, 51, 141–149. [Google Scholar] [CrossRef]
- Hockel, M.; Vaupel, P. Oxygenation of cervix cancers: Impact of clinical and pathological parameters. Adv. Exp. Med. Biol. 2003, 510, 31–35. [Google Scholar]
- Hockel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.C.; Humm, J.; Larson, S.; Amols, H.; Fuks, Z.; Leibel, S.; Koutcher, J.A. Towards multidimensional radiotherapy (MD-CRT): Biological imaging and biological conformality. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 551–560. [Google Scholar] [CrossRef]
- Supiot, S.; Lisbona, A.; Paris, F.; Azria, D.; Fenoglietto, P. [“Dose-painting”: Myth or reality?]. Cancer Radiother. 2010, 14, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life 2008, 60, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.F.; Zou, H.; Sun, X.; Shen, B. 18F-Fluoromisonidazole in tumor hypoxia imaging. Oncotarget 2017, 8, 94969–94979. [Google Scholar] [CrossRef] [Green Version]
- Busk, M.; Horsman, M.R.; Jakobsen, S.; Keiding, S.; van der Kogel, A.J.; Bussink, J.; Overgaard, J. Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: A comparative study involving microPET, autoradiography, PO2-polarography, and fluorescence microscopy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Wallace, S.; Cherif, A.; Li, C.; Gretzer, M.B.; Kim, E.E.; Podoloff, D.A. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology 1995, 194, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; Wiel, A.; Lieverse, R.I.Y.; Marcus, D.; Ibrahim, A.; Primakov, S.; Wu, G.; Theys, J.; Yaromina, A.; Dubois, L.J.; et al. Hypoxia PET Imaging with [18F]-HX4-A Promising Next-Generation Tracer. Cancers 2020, 12, 1322. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38, 1155–1160. [Google Scholar]
- Roesch, F. Maturation of a key resource—The germanium-68/gallium-68 generator: Development and new insights. Curr. Radiopharm. 2012, 5, 202–211. [Google Scholar] [CrossRef]
- Hoigebazar, L.; Jeong, J.M.; Hong, M.K.; Kim, Y.J.; Lee, J.Y.; Shetty, D.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C. Synthesis of 68Ga-labeled DOTA-nitroimidazole derivatives and their feasibilities as hypoxia imaging PET tracers. Bioorg. Med. Chem. 2011, 19, 2176–2181. [Google Scholar] [CrossRef]
- Hoigebazar, L.; Jeong, J.M.; Choi, S.Y.; Choi, J.Y.; Shetty, D.; Lee, Y.S.; Lee, D.S.; Chung, J.K.; Lee, M.C.; Chung, Y.K. Synthesis and characterization of nitroimidazole derivatives for 68Ga-labeling and testing in tumor xenografted mice. J. Med. Chem. 2010, 53, 6378–6385. [Google Scholar] [CrossRef]
- Seelam, S.R.; Lee, J.Y.; Lee, Y.S.; Hong, M.K.; Kim, Y.J.; Banka, V.K.; Lee, D.S.; Chung, J.K.; Jeong, J.M. Development of 68Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Bioorg. Med. Chem. 2015, 23, 7743–7750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, S.; Dematteis, S.; Giglio, J.; Cerecetto, H.; Rey, A. Synthesis, in vitro and in vivo characterization of two novel 68Ga-labelled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl. Med. Biol. 2013, 40, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, R.; Mallia, M.B.; Sarma, H.D. 68Ga-labeled PET tracers for targeting tumor hypoxia: Role of bifunctional chelators on pharmacokinetics. Nucl. Med. Biol. 2021, 96–97, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ramogida, C.F.; Pan, J.; Ferreira, C.L.; Patrick, B.O.; Rebullar, K.; Yapp, D.T.; Lin, K.S.; Adam, M.J.; Orvig, C. Nitroimidazole-Containing H2dedpa and H2CHXdedpa Derivatives as Potential PET Imaging Agents of Hypoxia with 68Ga. Inorg. Chem. 2015, 54, 4953–4965. [Google Scholar] [CrossRef]
- Boellaard, R.; O’Doherty, M.J.; Weber, W.A.; Mottaghy, F.M.; Lonsdale, M.N.; Stroobants, S.G.; Oyen, W.J.; Kotzerke, J.; Hoekstra, O.S.; Pruim, J.; et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Souvatzoglou, M.; Grosu, A.L.; Roper, B.; Krause, B.J.; Beck, R.; Reischl, G.; Picchio, M.; Machulla, H.J.; Wester, H.J.; Piert, M. Tumour hypoxia imaging with [18F]FAZA PET in head and neck cancer patients: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1566–1575. [Google Scholar] [CrossRef]
- Fyles, A.; Milosevic, M.; Hedley, D.; Pintilie, M.; Levin, W.; Manchul, L.; Hill, R.P. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J. Clin. Oncol. 2002, 20, 680–687. [Google Scholar] [CrossRef]
- Dehdashti, F.; Grigsby, P.W.; Lewis, J.S.; Laforest, R.; Siegel, B.A.; Welch, M.J. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone). J. Nucl. Med. 2008, 49, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Pinker, K.; Andrzejewski, P.; Baltzer, P.; Polanec, S.H.; Sturdza, A.; Georg, D.; Helbich, T.H.; Karanikas, G.; Grimm, C.; Polterauer, S.; et al. Multiparametric [18F]Fluorodeoxyglucose/[18F]Fluoromisonidazole Positron Emission Tomography/Magnetic Resonance Imaging of Locally Advanced Cervical Cancer for the Non-Invasive Detection of Tumor Heterogeneity: A Pilot Study. PLoS ONE 2016, 11, e0155333. [Google Scholar] [CrossRef]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellas, K.; Bache, M.; Pigorsch, S.U.; Taubert, H.; Kappler, M.; Holzapfel, D.; Zorn, E.; Holzhausen, H.J.; Haensgen, G. Prognostic impact of HIF-1alpha expression in patients with definitive radiotherapy for cervical cancer. Strahlenther. Onkol. 2008, 184, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.W.; Malyapa, R.S.; Higashikubo, R.; Schwarz, J.K.; Welch, M.J.; Huettner, P.C.; Dehdashti, F. Comparison of molecular markers of hypoxia and imaging with (60)Cu-ATSM in cancer of the uterine cervix. Mol. Imaging Biol. 2007, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; Committee, E.G. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv72–iv83. [Google Scholar] [CrossRef]
- Clavo, A.C.; Brown, R.S.; Wahl, R.L. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J. Nucl. Med. 1995, 36, 1625–1632. [Google Scholar]
- Minn, H.; Clavo, A.C.; Wahl, R.L. Influence of hypoxia on tracer accumulation in squamous-cell carcinoma: In vitro evaluation for PET imaging. Nucl. Med. Biol. 1996, 23, 941–946. [Google Scholar] [CrossRef]
- Dierckx, R.A.; Van de Wiele, C. FDG uptake, a surrogate of tumour hypoxia? Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1544–1549. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, J.G.; Wilson, D.C.; Conrad, E.U.; Peterson, L.M.; Bruckner, J.D.; Rasey, J.S.; Chin, L.K.; Hofstrand, P.D.; Grierson, J.R.; Eary, J.F.; et al. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: Correlation of hypoxia, metabolism and VEGF expression. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 695–704. [Google Scholar] [CrossRef]
- Zimny, M.; Gagel, B.; DiMartino, E.; Hamacher, K.; Coenen, H.H.; Westhofen, M.; Eble, M.; Buell, U.; Reinartz, P. FDG--a marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1426–1431. [Google Scholar] [CrossRef]
- Kietzmann, T.; Mennerich, D.; Dimova, E.Y. Hypoxia-Inducible Factors (HIFs) and Phosphorylation: Impact on Stability, Localization, and Transactivity. Front. Cell Dev. Biol. 2016, 4, 11. [Google Scholar] [CrossRef]
- Uchida, T.; Rossignol, F.; Matthay, M.A.; Mounier, R.; Couette, S.; Clottes, E.; Clerici, C. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: Implication of natural antisense HIF-1alpha. J. Biol. Chem. 2004, 279, 14871–14878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Frequency | Percent |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 44.65 ± 11.43 | |
| Range | 26–72 | |
| Histological subtype | ||
| Mucinous endocervical carcinoma | 1 | 5.0 |
| Papillary squamourethelial carcinoma | 1 | 5.0 |
| Squamous cell carcinoma | 16 | 80.0 |
| Papillary surface serous carcinoma | 1 | 5.0 |
| Adenocarcinoma | 1 | 5.0 |
| FIGO stage | ||
| IIB | 3 | 15.0 |
| IIIA | 1 | 5.0 |
| IIIB | 9 | 45.0 |
| IIIC | 2 | 10.0 |
| IIIC1 | 1 | 5.0 |
| IIIC2 | 1 | 5.0 |
| IVA | 2 | 10.0 |
| IVB | 1 | 5.0 |
| Patient No. | Histological Subtype | Intensity of Staining of HIF-1α | Percentage Distribution of HIF-1α Expression | Sum of Intensity and Distribution (Out of 6) | Qualitative 68Ga-Nitroimidazole PET Findings |
|---|---|---|---|---|---|
| 1 | Squamous cell carcinoma | 3 | 25% | 4 | 2 |
| 2 | Squamous cell carcinoma | 3 | 75 % | 6 | 2 |
| 3 | Squamous cell carcinoma | 3 | 75% | 6 | 2 |
| 4 | Papillary surface serous carcinoma | 2 | 75% | 5 | 0 |
| 5 | Squamous cell carcinoma | 3 | 75% | 6 | 0 |
| 6 | Adenocarcinoma | 3 | 75% | 6 | 2 |
| 7 | Squamous cell carcinoma | 0 | - | 0 | 2 |
| 8 | Squamous cell carcinoma | 2 | 25% | 3 | 2 |
| 9 | Squamous cell carcinoma | 0 | - | 0 | 2 |
| 10 | Papillary squamo-urothelial carcinoma | 1 | 25% | 2 | 1 |
| 11 | Squamous cell carcinoma | 0 | - | 0 | 1 |
| 12 | Squamous cell carcinoma | 1 | 25% | 2 | 2 |
| 13 | Squamous cell carcinoma | 0 | - | 0 | 2 |
| 14 | Squamous cell carcinoma | 0 | - | 0 | 0 |
| 15 | Squamous cell carcinoma | 1 | 25% | 2 | 2 |
| Hypoxia on Staining | ||||||
|---|---|---|---|---|---|---|
| Negative | Weak | Moderate | Strong | |||
| Median (Range) | Median (Range) | Median (Range) | Median (Range) | K | p Value | |
| 18F-FDG SUVmax | 17.32 (11.82–17.81) | 21.21 (11.64–22.42) | 13.70 (13.41–13.99) | 17.50 (16.09–30.77) | 2.927 | 0.403 |
| 18F-FDG TMR | 30.00 (21.00–43.00) | 38.00 (22.00–46.00) | 27.00 (27.00–27.00) | 32.00 (18.00–50.00) | 0.670 | 0.880 |
| 68Ga-Ni SUVmax(1) | 3.73 (1.00–4.00) | 3.38 (3.00–4.00) | 2.08 (2.00–2.00) | 4.10 (2.00–5.00) | 2.795 | 0.424 |
| 68Ga-Ni SUVmax(2) | 3.71 (1.60–4.19) | 3.06 (3.04–3.15) | 1.77 (1.62–1.91) | 3.57 (2.78–4.32) | 4.342 | 0.227 |
| 68Ga-Ni TMR | 8.00 (4.00–10.00) | 9.50 (8.00–11.00) | 10.00 (6.00–14.00) | 15.00 (7.00–24.00) | 2.669 | 0.446 |
| 68Ga-Ni TMR 2 | 11.00 (6.00–16.00) | 15.00 (7.00–17.00) | 7.50 (7.00–8.00) | 12.00 (7.00–26.00) | 1.852 | 0.604 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokoala, K.M.G.; Lawal, I.O.; Maserumule, L.C.; Hlongwa, K.N.; Ndlovu, H.; Reed, J.; Bida, M.; Maes, A.; van de Wiele, C.; Mahapane, J.; et al. A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry. J. Clin. Med. 2022, 11, 962. https://doi.org/10.3390/jcm11040962
Mokoala KMG, Lawal IO, Maserumule LC, Hlongwa KN, Ndlovu H, Reed J, Bida M, Maes A, van de Wiele C, Mahapane J, et al. A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry. Journal of Clinical Medicine. 2022; 11(4):962. https://doi.org/10.3390/jcm11040962
Chicago/Turabian StyleMokoala, Kgomotso M. G., Ismaheel O. Lawal, Letjie C. Maserumule, Khanyisile N. Hlongwa, Honest Ndlovu, Janet Reed, Meshack Bida, Alex Maes, Christophe van de Wiele, Johncy Mahapane, and et al. 2022. "A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry" Journal of Clinical Medicine 11, no. 4: 962. https://doi.org/10.3390/jcm11040962
APA StyleMokoala, K. M. G., Lawal, I. O., Maserumule, L. C., Hlongwa, K. N., Ndlovu, H., Reed, J., Bida, M., Maes, A., van de Wiele, C., Mahapane, J., Davis, C., Jeong, J. M., Popoola, G., Vorster, M., & Sathekge, M. M. (2022). A Prospective Investigation of Tumor Hypoxia Imaging with 68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry. Journal of Clinical Medicine, 11(4), 962. https://doi.org/10.3390/jcm11040962









