An Innovative Ultrasound Technique for Early Detection of Kidney Dysfunction: Superb Microvascular Imaging as a Reference Standard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
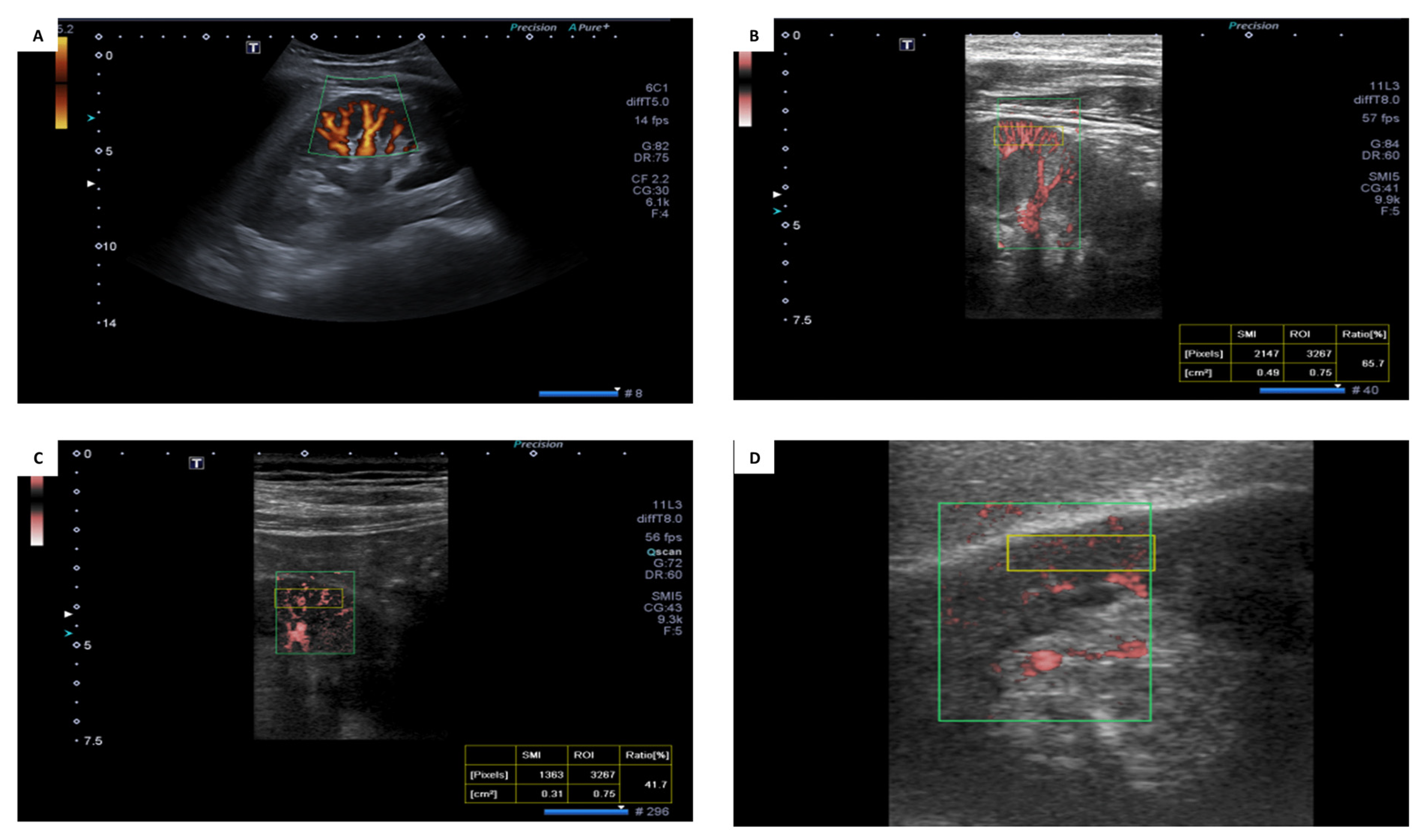
2.2. Superb Microvascular Ultrasound Imaging
2.3. Subjects and Parameters
- –
- Inclusion Criteria:
- Patients with CKD at stages 2–5 (pre-dialytic).
- Patients with known CKD, abnormal serum creatinine, and proteinuria levels even when GFR apparently normal.
- At age ≥18 y.
- –
- Exclusion Criteria:
- Acute kidney injury.
- Pregnant women.
- Patients with severe liver diseases.
- Inter-current illness such as fever or sepsis.
- Allergic rhinitis.
- Hydro-nephrosis.
2.4. Statistical Analysis
3. Results
3.1. Description of Main Data of the Participants
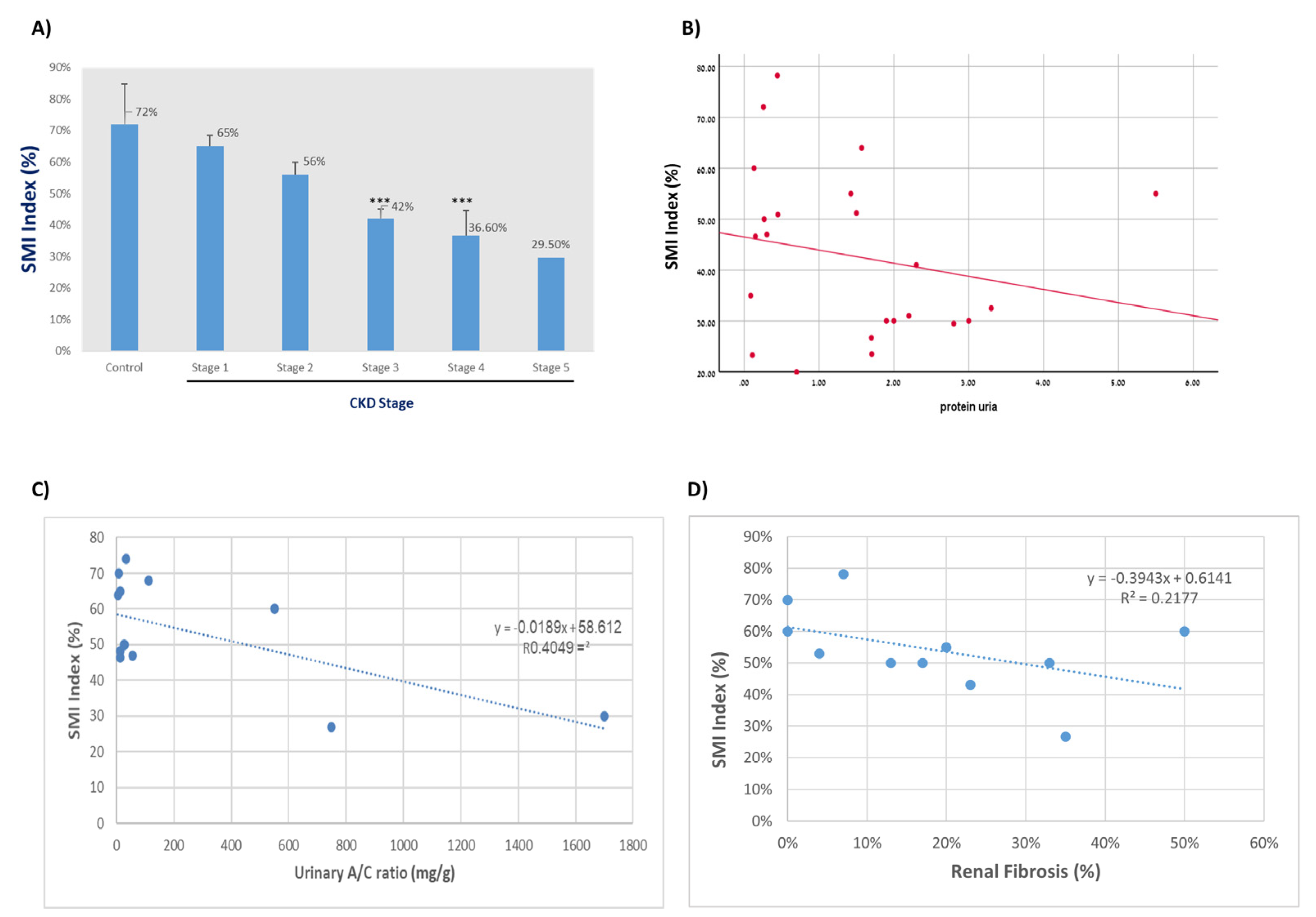
3.2. Kidney Function Parameters and SMI Evaluations in All Subjects
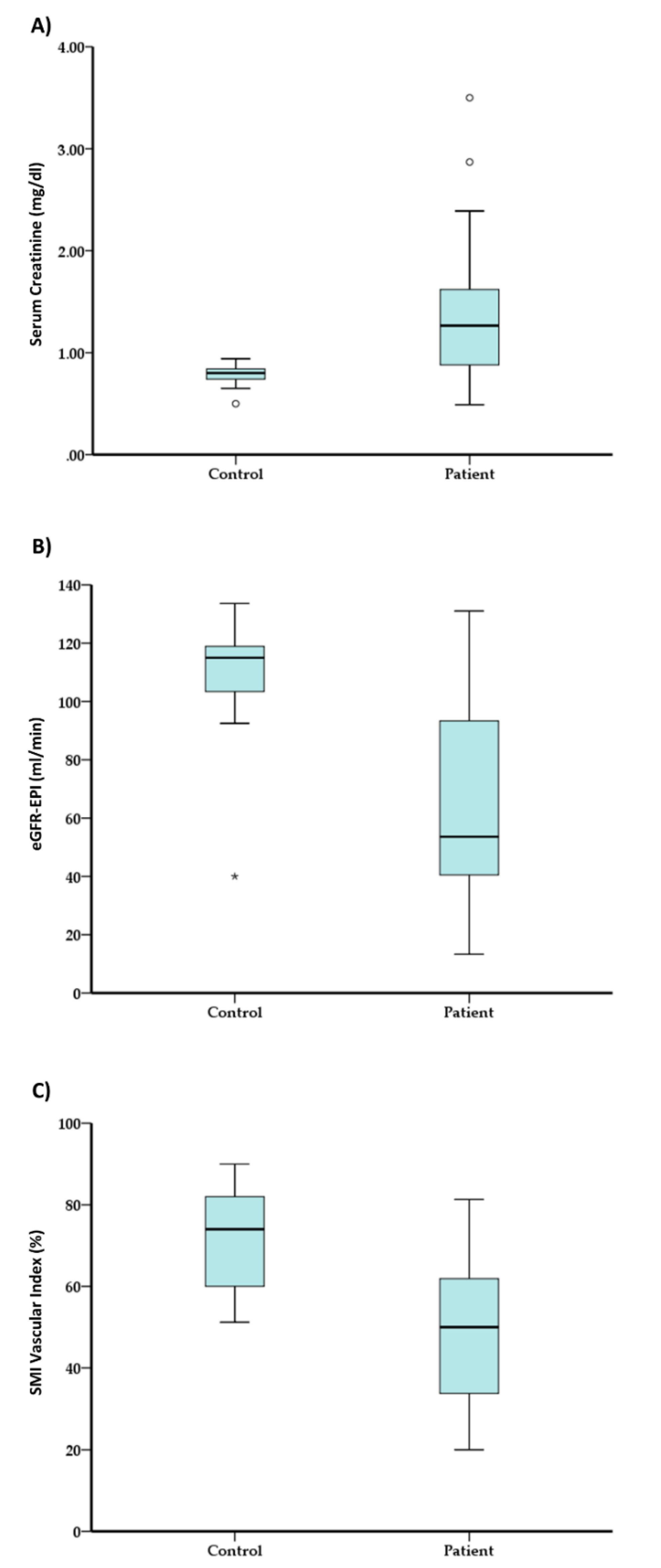
3.3. Comparison between Control and CKD Patients in Main Tested Parameters
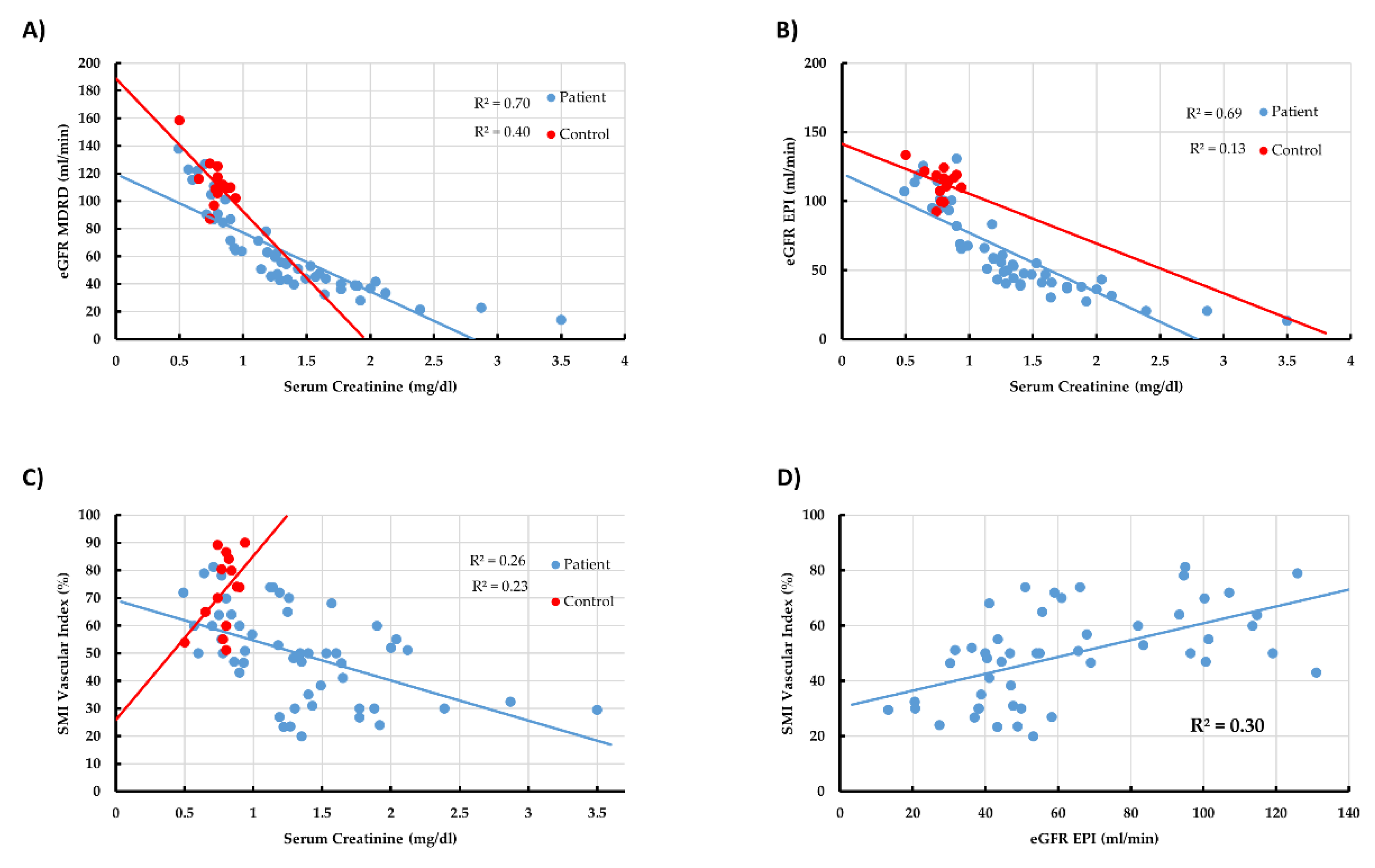
3.4. Linear Correlation between All Main Tested Parameters in All Participants
3.5. Linear Correlation between All Main Tested Parameters in CKD Patients
4. Discussion
Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Kdoqi. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am. J. Kidney Dis. 2007, 49, S12–S154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicari, R.; Gargani, L.; Wiecek, A.; Covic, A.; Goldsmith, D.; Suleymanlar, G.; Parati, G.; Ortiz, A.; Massy, Z.; Martinez-Castelao, A.; et al. The use of echocardiography in observational clinical trials: The EURECA-m registry. Nephrol. Dial. Transpl. 2013, 28, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Hassan, K.; Loberant, N.; Abbas, N.; Fadi, H.; Shadia, H.; Khazim, K. Shear wave elastography imaging for assessing the chronic pathologic changes in advanced diabetic kidney disease. Ther. Clin. Risk Manag. 2016, 12, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Goya, C.; Kilinc, F.; Hamidi, C.; Yavuz, A.; Yildirim, Y.; Cetincakmak, M.G.; Hattapoglu, S. Acoustic radiation force impulse imaging for evaluation of renal parenchyma elasticity in diabetic nephropathy. AJR Am. J. Roentgenol. 2015, 204, 324–329. [Google Scholar] [CrossRef]
- Sarvazyan, A.P.; Rudenko, O.V.; Swanson, S.D.; Fowlkes, J.B.; Emelianov, S.Y. Shear wave elasticity imaging: A new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 1998, 24, 1419–1435. [Google Scholar] [CrossRef]
- Samir, A.E.; Allegretti, A.S.; Zhu, Q.L.; Dhyani, M.; Anvari, A.; Sullivan, D.A.; Trottier, C.A.; Dougherty, S.; Williams, W.W.; Babitt, J.L.; et al. Shear wave elastography in chronic kidney disease: A pilot experience in native kidneys. BMC Nephrol. 2015, 16, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaffanello, M.; Piacentini, G.; Bruno, C.; Brugnara, M.; Fanos, V. Renal elasticity quantification by acoustic radiation force impulse applied to the evaluation of kidney diseases: A review. J. Investig. Med. 2015, 63, 605–612. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, Y.; Xu, Y. Value of virtual touch tissue quantification in stages of diabetic kidney disease. J. Ultrasound Med. 2014, 33, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef]
- Ohno, Y.; Fujimoto, T.; Shibata, Y. A New Era in Diagnostic Ultrasound, Superb Microvascular Imaging: Preliminary Results in Pediatric Hepato-Gastrointestinal Disorders. Eur. J. Pediatr. Surg. 2017, 27, 20–25. [Google Scholar] [PubMed]
- Cantisani, V.; David, E.; Ferrari, D.; Fanelli, F.; Di Marzo, L.; Catalano, C.; Benedetto, F.; Spinelli, D.; Katsargyris, A.; Blandino, A.; et al. Color Doppler Ultrasound with Superb Microvascular Imaging Compared to Contrast-enhanced Ultrasound and Computed Tomography Angiography to Identify and Classify Endoleaks in Patients Undergoing EVAR. Ann. Vasc. Surg. 2017, 40, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Lee, J.Y.; Han, J.K. Superb microvascular imaging technology for ultrasound examinations: Initial experiences for hepatic tumors. Eur. J. Radiol. 2016, 85, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Yongfeng, Z.; Ping, Z.; Wengang, L.; Yang, S.; Shuangming, T. Application of a Novel Microvascular Imaging Technique in Breast Lesion Evaluation. Ultrasound Med. Biol. 2016, 42, 2097–2105. [Google Scholar] [CrossRef]
- Park, A.Y.; Seo, B.K.; Cha, S.H.; Yeom, S.K.; Lee, S.W.; Chung, H.H. An Innovative Ultrasound Technique for Evaluation of Tumor Vascularity in Breast Cancers: Superb Micro-Vascular Imaging. J. Breast Cancer 2016, 19, 210–213. [Google Scholar] [CrossRef]
- Ma, Y.; Li, G.; Li, J.; Ren, W.D. The Diagnostic Value of Superb Microvascular Imaging (SMI) in Detecting Blood Flow Signals of Breast Lesions: A Preliminary Study Comparing SMI to Color Doppler Flow Imaging. Medicine 2015, 94, e1502. [Google Scholar] [CrossRef]
- Koyama, N.; Hata, J.; Sato, T.; Tomiyama, Y.; Hino, K. Assessment of hepatic fibrosis with superb microvascular imaging in hepatitis C virus-associated chronic liver diseases. Hepatol. Res. 2017, 47, 593–597. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Ishige, N. Signal Intensity of Superb Microvascular Imaging Correlates with the Severity of Acute Cholecystitis. Case Rep. Gastroenterol. 2016, 10, 452–458. [Google Scholar] [CrossRef]
- Zhan, J.; Diao, X.H.; Jin, J.M.; Chen, L.; Chen, Y. Superb Microvascular Imaging-A new vascular detecting ultrasonographic technique for avascular breast masses: A preliminary study. Eur. J. Radiol. 2016, 85, 915–921. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, M.J.; Han, S.W.; Lee, H.S.; Im, Y.J.; Shin, H.J.; Lee, M.J. Superb microvascular imaging for the detection of parenchymal perfusion in normal and undescended testes in young children. Eur. J. Radiol. 2016, 85, 649–656. [Google Scholar] [CrossRef]
- Karaca, L.; Oral, A.; Kantarci, M.; Sade, R.; Ogul, H.; Bayraktutan, U.; Okur, A.; Yuce, I. Comparison of the superb microvascular imaging technique and the color Doppler techniques for evaluating children’s testicular blood flow. Eur. Rev. Med. Pharm. 2016, 20, 1947–1953. [Google Scholar]
- Kuroda, H.; Abe, T.; Kakisaka, K.; Fujiwara, Y.; Yoshida, Y.; Miyasaka, A.; Ishida, K.; Ishida, H.; Sugai, T.; Takikawa, Y. Visualizing the hepatic vascular architecture using superb microvascular imaging in patients with hepatitis C virus: A novel technique. World J. Gastroenterol. 2016, 22, 6057–6064. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, I.; Suh, S.; You, S.H.; Seol, H.Y. Usefulness of Microvascular Ultrasonography in Differentiating Metastatic Lymphadenopathy from Tuberculous Lymphadenitis. Ultrasound Med. Biol. 2016, 42, 2189–2195. [Google Scholar] [CrossRef]
- Available online: https://webcache.googleusercontent.com/search?q=cache:tsGbq2rzUyAJ:https://us.medical.canon/download/aplio-500-wp-smi-seeing-the-unseen+&cd=1&hl=en&ct=clnk&gl=il (accessed on 7 September 2021).
- Toshiba. Available online: http://www.toshibamedicalsystems.com/products/us/aplio_smi.html. (accessed on 7 September 2021).
- Gao, J.; Thai, A.; Erpelding, T. Comparison of superb microvascular imaging to conventional color Doppler ultrasonography in depicting renal cortical microvasculature. Clin. Imaging 2019, 58, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R. Renal vascular structure and rarefaction. Compr. Physiol. 2013, 3, 817–831. [Google Scholar]
- Sun, D.; Eirin, A.; Ebrahimi, B.; Textor, S.C.; Lerman, A.; Lerman, L.O. Early atherosclerosis aggravates renal microvascular loss and fibrosis in swine renal artery stenosis. J. Am. Soc. Hypertens. 2016, 10, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Shebel, H.M.; Akl, A.; Dawood, A.; El-Diasty, T.A.; Shokeir, A.A.; Ghoneim, M.A. Power doppler sonography in early renal transplantation: Does it differentiate acute graft rejection from acute tubular necrosis? Saudi J. Kidney Dis. Transplant. 2014, 25, 733–740. [Google Scholar]
- Wang, H.K.; Chou, Y.H.; Yang, A.H.; Chiou, S.Y.; Chiou, H.J.; Wu, T.H.; Loong, C.C.; Chang, C.Y. Evaluation of cortical perfusion in renal transplants: Application of quantified power Doppler ultrasonography. Transplant. Proc. 2008, 40, 2330–2332. [Google Scholar] [CrossRef]
- Lane, P.H.; Steffes, M.W.; Mauer, S.M. Glomerular structure in IDDM women with low glomerular filtration rate and normal urinary albumin excretion. Diabetes 1992, 41, 581–586. [Google Scholar] [CrossRef]
- Tsalamandris, C.; Allen, T.J.; Gilbert, R.E.; Sinha, A.; Panagiotopoulos, S.; Cooper, M.E.; Jerums, G. Progressive Decline in Renal-Function in Diabetic-Patients with and without Albuminuria. Diabetes 1994, 43, 649–655. [Google Scholar] [CrossRef]
| n | % | ||
|---|---|---|---|
| Group | C | 17 | 24.6% |
| Pts | 52 | 75.4% | |
| Gender | F | 33 | 47.8% |
| M | 36 | 52.2% | |
| Stage | 1 | 11 | 21.6% |
| 2 | 13 | 25.5% | |
| 3a | 11 | 21.6% | |
| 3b | 12 | 23.5% | |
| 4 | 3 | 5.9% | |
| 5 | 1 | 2.0% | |
| Impression | G | 44 | 63.8% |
| INT | 16 | 23.2% | |
| Po | 9 | 13.0% | |
| SHAPE | Nor | 44 | 64.7% |
| SD | 17 | 25.0% | |
| VD | 7 | 10.3% | |
| Mean | Standard Deviation | Percentile 25 | Median | Percentile 75 | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|
| Age | 52.21 | 18.37 | 34.00 | 56.00 | 67.00 | 20.00 | 85.00 |
| Hb (gr/dL) | 13.21 | 1.70 | 11.90 | 13.10 | 14.50 | 9.30 | 17.00 |
| Sr. Creatinine | 1.22 | 0.57 | 0.80 | 1.13 | 1.49 | 0.49 | 3.50 |
| eGFR-MDRD | 73.75 | 34.49 | 43.93 | 64.12 | 105.90 | 14.07 | 158.51 |
| eGFR-EPI | 73.29 | 34.43 | 43.30 | 65.50 | 107.00 | 13.30 | 133.60 |
| CCT | 70.26 | 45.22 | 45.00 | 61.00 | 99.00 | 6.00 | 177.00 |
| Proteinuria | 1.74 | 1.55 | 0.44 | 1.54 | 2.30 | 0.09 | 6.60 |
| Kidney size | 11.19 | 1.52 | 10.30 | 11.20 | 12.00 | 8.30 | 17.20 |
| Kidney Index | 55.39 | 18.50 | 46.50 | 55.00 | 70.00 | 20.00 | 90.00 |
| Group | Mann Whitney U (Z, p.Value) | ||||
|---|---|---|---|---|---|
| C (n = 17) | P (n = 52) | ||||
| Mean ± SD | Median ± IQR | Mean ± SD | Median ± IQR | ||
| Age | 36.13 ± 12.32 | 33.00 + [26.00,49.00] | 56.85 ± 17.24 | 59.00 + [45.00,72.50] | Z = −3.90, p < 0.01 |
| Hb (gr/dL) | 14.29 ± 1.15 | 14.45 + [13.50,15.30] | 12.88 ± 1.72 | 12.80 + [11.60,13.90] | Z = −2.87, p < 0.01 |
| Sr. Creatinine (mg%) | 0.78 ± 0.11 | 0.80 + [0.74,0.84] | 1.33 ± 0.59 | 1.27 + [0.88,1.62] | Z = −3.64, p < 0.01 |
| eGFR-MDRD (mL/min) | 113.44 ± 16.59 | 110.40 + [105.90,117.61] | 63.06 ± 29.95 | 55.04 + [41.01,85.79] | Z = −4.58, p < 0.01 |
| eGFR-EPI (mL/min) | 108.32 ± 21.66 | 115.00 + [99.50,119.10] | 62.79 ± 30.43 | 53.60 + [40.50,93.30] | Z = −4.24, p < 0.01 |
| Creatinine Clearance (CCT) (mL/min) | 70.26 ± 45.22 | 61.00 + [45.00,99.00] | |||
| Proteinuria (gr/d) | 1.74 ± 1.55 | 1.54 + [0.44,2.30] | |||
| Kidney Size (cm) | 11.19 ± 0.90 | 11.20 + [11.00,11.80] | 11.19 ± 1.69 | 11.00 + [10.05,12.00] | Z = −0.47, p = 0.64 |
| SMI Index (%) | 72.16 ± 12.92 | 74.00 + [60.00,82.00] | 49.91 ± 16.72 | 50.00 + [33.75,61.90] | Z = −4.41, p < 0.01 |
| Sr Creatinine | eGFR-MDRD | eGFR-EPI | CCT | Proteinuria | Kidney Size | Impression | Shape | Index | |
|---|---|---|---|---|---|---|---|---|---|
| Sr Creatinine | −0.939 | −0.904 | −0.658 | 0.693 | −0.123 | 0.645 | 0.646 | −0.601 | |
| eGFR-MDRD | −0.939 | 0.966 | 0.705 | −0.571 | 0.143 | −0.678 | −0.671 | 0.631 | |
| eGFR-EPI | −0.904 | 0.966 | 0.692 | −0.558 | 0.199 | −0.656 | −0.644 | 0.593 | |
| Creatinine Clearance (CCT) | −0.658 | 0.705 | 0.692 | −0.411 | −0.017 | −0.553 | −0.519 | 0.434 | |
| Proteinuria | 0.693 | −0.571 | −0.558 | −0.411 | −0.381 | 0.309 | 0.455 | −0.251 | |
| Kidney Size | −0.123 | 0.143 | 0.199 | −0.017 | −0.381 | −0.180 | −0.208 | 0.148 | |
| Impression | 0.645 | −0.678 | −0.656 | −0.553 | 0.309 | −0.180 | 0.996 | −0.827 | |
| Shape | 0.646 | −0.671 | −0.644 | −0.519 | 0.455 | −0.208 | 0.996 | −0.815 | |
| Kidney Index | −0.601 | 0.631 | 0.593 | 0.434 | −0.251 | 0.148 | −0.827 | −0.815 |
| Sr Creatinine | eGFR-MDRD | eGFR-EPI | CCT | Proteinuria | Kidney Size | Impression | Shape | Index | |
|---|---|---|---|---|---|---|---|---|---|
| Serum Creatinine | −0.953 | −0.939 | −0.658 | 0.693 | −0.203 | 0.574 | 0.582 | −0.540 | |
| eGFR-MDRD | −0.953 | 0.980 | 0.705 | −0.571 | 0.212 | −0.621 | −0.613 | 0.560 | |
| eGFR-EPI | −0.939 | 0.980 | 0.692 | −0.558 | 0.280 | −0.642 | −0.627 | 0.564 | |
| CCT | −0.658 | 0.705 | 0.692 | −0.411 | −0.017 | −0.553 | −0.519 | 0.434 | |
| Proteinuria | 0.693 | −0.571 | −0.558 | −0.411 | −0.381 | 0.309 | 0.455 | −0.251 | |
| Kidney Size | −0.203 | 0.212 | 0.280 | −0.017 | −0.381 | −0.184 | −0.228 | 0.144 | |
| Impression | 0.574 | −0.621 | −0.642 | −0.553 | 0.309 | −0.184 | 0.992 | −0.870 | |
| Shape | 0.582 | −0.613 | −0.627 | −0.519 | 0.455 | −0.228 | 0.992 | −0.853 | |
| Index | −0.540 | 0.560 | 0.564 | 0.434 | −0.251 | 0.144 | −0.870 | −0.853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armaly, Z.; Abu-Rahme, M.; Kinaneh, S.; Hijazi, B.; Habbasshi, N.; Artul, S. An Innovative Ultrasound Technique for Early Detection of Kidney Dysfunction: Superb Microvascular Imaging as a Reference Standard. J. Clin. Med. 2022, 11, 925. https://doi.org/10.3390/jcm11040925
Armaly Z, Abu-Rahme M, Kinaneh S, Hijazi B, Habbasshi N, Artul S. An Innovative Ultrasound Technique for Early Detection of Kidney Dysfunction: Superb Microvascular Imaging as a Reference Standard. Journal of Clinical Medicine. 2022; 11(4):925. https://doi.org/10.3390/jcm11040925
Chicago/Turabian StyleArmaly, Zaher, Munai Abu-Rahme, Safa Kinaneh, Basem Hijazi, Nayef Habbasshi, and Suheil Artul. 2022. "An Innovative Ultrasound Technique for Early Detection of Kidney Dysfunction: Superb Microvascular Imaging as a Reference Standard" Journal of Clinical Medicine 11, no. 4: 925. https://doi.org/10.3390/jcm11040925
APA StyleArmaly, Z., Abu-Rahme, M., Kinaneh, S., Hijazi, B., Habbasshi, N., & Artul, S. (2022). An Innovative Ultrasound Technique for Early Detection of Kidney Dysfunction: Superb Microvascular Imaging as a Reference Standard. Journal of Clinical Medicine, 11(4), 925. https://doi.org/10.3390/jcm11040925






