Conditioning Regimens in Patients with β-Thalassemia Who Underwent Hematopoietic Stem Cell Transplantation: A Scoping Review
Abstract
1. Introduction
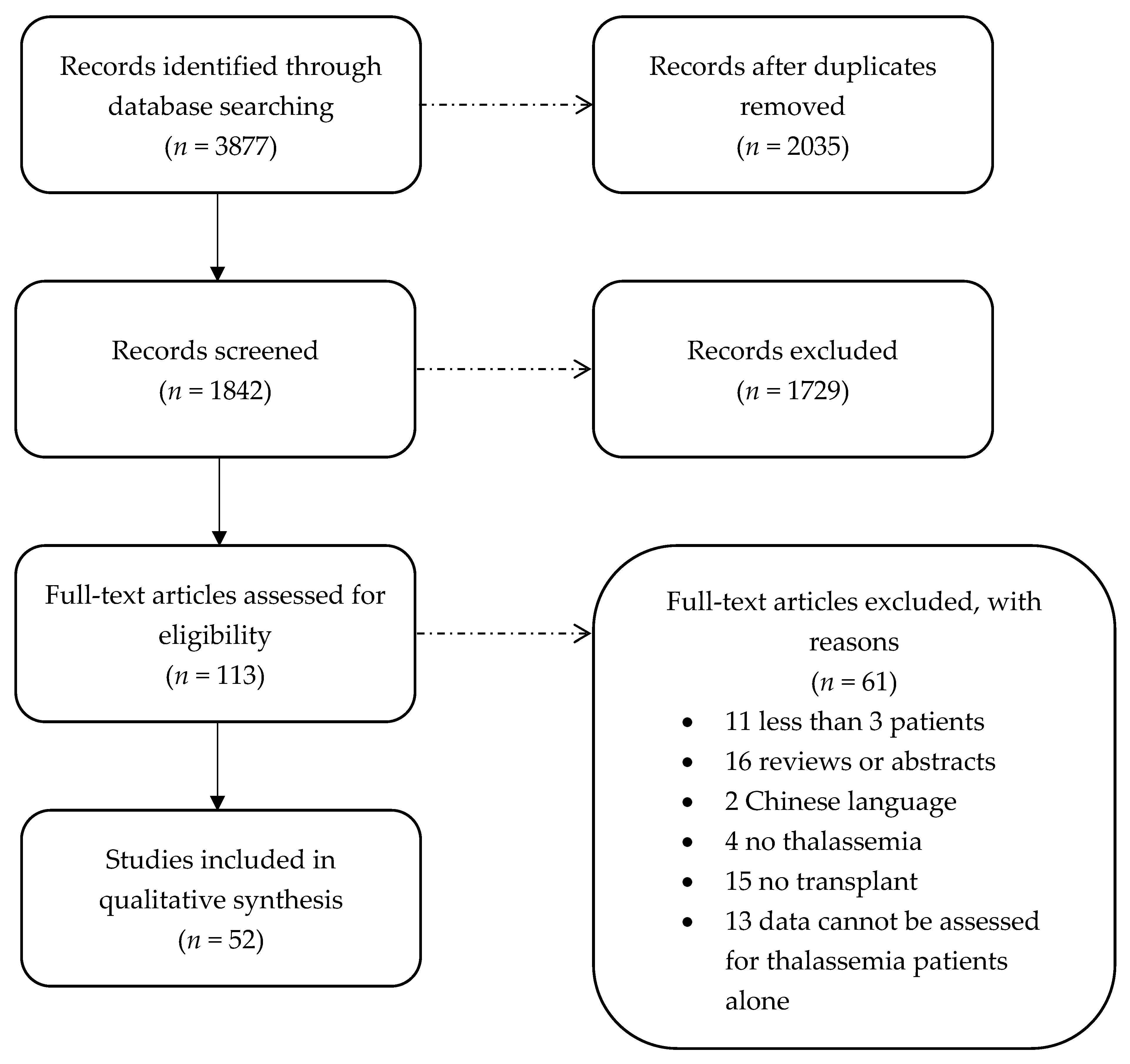
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Article Selection
2.4. Data Extraction
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. β-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef]
- Porter, J. Beyond transfusion therapy: New therapies in thalassemia including drugs, alternate donor transplant, and gene Therapy. Hematol. Am. Soc. Hematol. Educ. Program. 2018, 2018, 361–370. [Google Scholar] [CrossRef]
- Kattamis, A.; Forni, G.L.; Aydinok, Y.; Viprakasit, V. Changing patterns in the epidemiology of β-Thalassemia. Eur. J. Haematol. 2020, 105, 692–703. [Google Scholar] [CrossRef]
- Ali, S.; Mumtaz, S.; Shakir, H.A.; Khan, M.; Tahir, H.M.; Mumtaz, S.; Mughal, T.A.; Hassan, A.; Sadia, S.A.R.K.; Irfan, M.; et al. Current status of Beta-thalassemia and its treatment strategies. Mol. Genet. Genomic. Med. 2021, 9, e1788. [Google Scholar] [CrossRef]
- Kihm, A.J.; Kong, Y.; Hong, W.; Russell, J.E.; Rouda, S.; Adachi, K.; Simon, M.C.; Blobel, G.A.; Weiss, M.J. An abundant erythroid protein that stabilizes free α-haemoglobin. Nature 2002, 417, 758–763. [Google Scholar] [CrossRef]
- Hershko, C. Pathogenesis and management of iron toxicity in thalassemia. Ann. N. Y. Acad. Sci. 2010, 1202, 1–9. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Viprakasit, V.; Taher, A.T.; Georgiev, P.; Kuo, K.H.M.; Coates, T.; Voskaridou, E.; Liew, H.-K.; Pazgal-Kobrowski, I.; Forni, G.L.; et al. A phase 3 trial of luspatercept in patients with transfusion-dependent β-thalassemia. N. Engl. J. Med. 2020, 382, 1219–1231. [Google Scholar] [CrossRef]
- Thompson, A.A.; Walters, M.C.; Kwiatkowski, J.; Rasko, J.E.J.; Ribeil, J.-A.; Hongeng, S.; Magrin, E.; Schiller, G.J.; Payen, E.; Semeraro, M.; et al. Gene therapy in patients with transfusion-dependent β-thalassemia. N. Engl. J. Med. 2018, 378, 1479–1493. [Google Scholar] [CrossRef]
- Coquerelle, S.; Ghardallou, M.; Rais, S.; Taupin, P.; Touzot, F.; Boquet, L.; Blanche, S.; Benaouadi, S.; Brice, T.; Tuchmann-Durand, C.; et al. Innovative curative treatment of beta thalassemia: Cost-efficacy analysis of gene therapy versus allogenic hematopoietic stem-cell transplantation. Hum. Gene Ther. 2019, 30, 753–761. [Google Scholar] [CrossRef]
- Thomas, E.D.; Buckner, C.D.; Sanders, J.E.; Papayannopoulou, T.; Borgna-Pignatti, C.; De Stefano, P.; Sullivan, K.M.; Clift, R.A.; Storb, R. Marrow transplantation for thalassaemia. Lancet 1982, 2, 227–229. [Google Scholar] [CrossRef]
- Lucarelli, G.; Galimberti, M.; Polchi, P.; Angelucci, E.; Baronciani, D.; Giardini, C.; Politi, P.; Durazzi, S.M.; Muretto, P.; Albertini, F. Bone marrow transplantation in patients with thalassemia. N. Engl. J. Med. 1990, 322, 417–421. [Google Scholar] [CrossRef]
- Mathews, V.; George, B.; Deotare, U.; Lakshmi, K.M.; Viswabandya, A.; Daniel, D.; Chandy, M.; Srivastava, A. A new stratification strategy that identifies a subset of class III patients with an adverse prognosis among children with β thalassemia major undergoing a matched related allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2007, 13, 889–894. [Google Scholar] [CrossRef]
- Lucarelli, G.; Galimberti, M.; Giardini, C.; Polchi, P.; Angelucci, E.; Baronciani, D.; Erer, B.; Gaziev, D. Bone marrow transplantation in thalassemia: The experience of Pesaro. Ann. N. Y. Acad. Sci. 1998, 850, 270–275. [Google Scholar] [CrossRef]
- Hussein, A.A.; Al-Mousa, A.; Khattab, E.; Al-Zaben, A.; Frangoul, H. TLI-based reduced-intensity conditioning hematopoietic SCT for children and adolescents with high-risk nonmalignant disorders. Bone Marrow Transplant. 2015, 50, 452–454. [Google Scholar] [CrossRef][Green Version]
- Or, R.; Naparstek, E.; Cividalli, G.; Aker, M.; Engelhard, D.; Slavin, S.; Rachmilewitz, E.A. Bone marrow transplantation in β-thalassemia major. The Israeli experience. Hemoglobin 1988, 12, 609–614. [Google Scholar] [CrossRef]
- Lin, K.-H. Pediatric hematopoietic stem cell transplantation in Taiwan. Transplant. Proc. 1998, 30, 3477–3480. [Google Scholar] [CrossRef]
- Gaziev, D.; Galimberti, M.; Lucarelli, G.; Polchi, P.; Giardini, C.; Angelucci, E.; Baronciani, D.; Sodani, P.; Erer, B.; Biagi, M.D.; et al. Bone marrow transplantation from alternative donors for thalassemia: HLA-phenotypically identical relative and HLA-nonidentical sibling or parent transplants. Bone Marrow Transplant. 2000, 25, 815–821. [Google Scholar] [CrossRef]
- Hussein, A.A.; Al-Zaben, A.; Ghatasheh, L.; Natsheh, A.; Hammada, T.; Abdel-Rahman, F.; Abu-Jazar, H.; Sharma, S.; Najjar, R.; Frangoul, H. Risk adopted allogeneic hematopoietic stem cell transplantation using a reduced intensity regimen for children with thalassemia major. Pediatr. Blood Cancer 2013, 60, 1345–1349. [Google Scholar] [CrossRef]
- Suvatte, V.; Tanphaichitr, V.S.; Visuthisakchai, S.; Mahasandana, C.; Veerakul, G.; Chongkolwatana, V.; Chandanayingyong, D.; Issaragrisil, S. Bone marrow, peripheral blood and cord blood stem cell transplantation in children: Ten years’ experience at Siriraj Hospital. Int. J. Hematol. 1998, 68, 411–419. [Google Scholar] [CrossRef]
- Rosales, F.; Peylan-Ramu, N.; Cividalli, G.; Varadi, G.; Or, R.; Naparstek, E.; Slavin, S.; Nagler, A. The role of thiotepa in allogeneic bone marrow transplantation for genetic diseases. Bone Marrow Transplant. 1999, 23, 861–865. [Google Scholar] [CrossRef][Green Version]
- Khojasteh, H.N.; Zakerinia, M.; Ramzi, M.; Haghshenas, M. Bone marrow transplantation in thalassaemia patients in Shiraz, Islamic Republic of Iran. East. Mediterr. Health J. 2001, 7, 835–837. [Google Scholar] [CrossRef]
- La Nasa, G.; Giardini, C.; Argiolu, F.; Locatelli, F.; Arras, M.; De Stefano, P.; Ledda, A.; Pizzati, A.; Sanna, M.A.; Vacca, A.; et al. Unrelated donor bone marrow transplantation for thalassemia: The effect of extended haplotypes. Blood 2002, 99, 4350–4356. [Google Scholar] [CrossRef]
- Lawson, S.E.; Roberts, I.A.G.; Amrolia, P.; Dokal, I.; Szydlo, R.; Darbyshire, P.J. Bone marrow transplantation for β-thalassaemia major: The UK experience in two paediatric centres. Br. J. Haematol. 2003, 120, 289–295. [Google Scholar] [CrossRef]
- Sodani, P.; Gaziev, D.; Polchi, P.; Erer, B.; Giardini, C.; Angelucci, E.; Baronciani, D.; Andreani, M.; Manna, M.; Nesci, S.; et al. New approach for bone marrow transplantation in patients with class 3 thalassemia aged younger than 17 years. Blood 2004, 104, 1201–1203. [Google Scholar] [CrossRef]
- La Nasa, G.; Caocci, G.; Argiolu, F.; Giardini, C.; Locatelli, F.; Vacca, A.; Orofino, M.G.; Piras, E.; Addari, M.C.; Ledda, A.; et al. Unrelated donor stem cell transplantation in adult patients with thalassemia. Bone Marrow Transplant. 2005, 36, 971–975. [Google Scholar] [CrossRef]
- Chandy, M.; Balasubramanian, P.; Ramachandran, S.V.; Mathews, V.; George, B.; Dennison, D.; Krishnamoorthy, R.; Srivastava, A. Randomized trial of two different conditioning regimens for bone marrow transplantation in thalassemia—The role of busulfan pharmacokinetics in determining outcome. Bone Marrow Transplant. 2005, 36, 839–845. [Google Scholar] [CrossRef][Green Version]
- La Nasa, G.; Argiolu, F.; Giardini, C.; Pession, A.; Fagioli, F.; Caocci, G.; Vacca, A.; De Stefano, P.; Piras, E.; Ledda, A.; et al. Unrelated bone marrow transplantation for β-thalassemia patients: The experience of the Italian bone marrow transplant group. Ann. N. Y. Acad. Sci. 2005, 1054, 186–195. [Google Scholar] [CrossRef]
- Sauer, M.; Bettoni, C.; Lauten, M.; Ghosh, A.; Rehe, K.; Grigull, L.; Beilken, A.; Welte, K.; Sykora, K.W. Complete substitution of cyclophosphamide by fludarabine and ATG in a busulfan-based preparative regimen for children and adolescents with β-thalassemia. Bone Marrow Transplant. 2005, 36, 383–387. [Google Scholar] [CrossRef][Green Version]
- Elhasid, R.; Arush, M.B.; Zaidman, I.; Leiba, R.; Barak, A.B.; Postovsky, S.; Haddad, N.; Katz, T.; Pollack, S.; Sami, I.; et al. Safe and efficacious allogeneic bone marrow transplantation for nonmalignant disorders using partial T cell depletion and no posttransplantation graft-versus-host-disease prophylaxis. Biol. Blood Marrow Transplant. 2007, 13, 329–338. [Google Scholar] [CrossRef]
- Hongeng, S.; Pakakasama, S.; Chuansumrit, A.; Sirachainan, N.; Kitpoka, P.; Udomsubpayakul, U.; Ungkanont, A.; Jootar, S. Outcomes of transplantation with related- and unrelated-donor stem cells in children with severe thalassemia. Biol. Blood Marrow Transplant. 2006, 12, 683–687. [Google Scholar] [CrossRef]
- Smythe, J.; Armitage, S.; McDonald, D.; Pamphilon, D.; Guttridge, M.; Brown, J.; Green, A.; Brown, C.; Warwick, R.M.; Lankester, A.; et al. Directed sibling cord blood banking for transplantation: The 10-year experience in the national blood service in England. Stem Cells 2007, 25, 2087–2093. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmed, P.; Raza, S.; Satti, T.; Nisa, Q.; Mirza, S.; Akhtar, F.; Kamal, M.K.; Akhtar, F.M. Allogeneic stem cell transplantation in hematological disorders: Single center experience from Pakistan. Transplant. Proc. 2007, 39, 3347–3357. [Google Scholar] [CrossRef]
- La Nasa, G.; Littera, R.; Locatelli, F.; Giardini, C.; Ventrella, A.; Mulargia, M.; Vacca, A.; Orrù, N.; Orrù, S.; Piras, E.; et al. Status of donor-recipient HLA class I ligands and not the KIR genotype is predictive for the outcome of unrelated hematopoietic stem cell transplantation in beta-thalassemia patients. Biol. Blood Marrow Transplant. 2007, 13, 1358–1368. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Hatoum, H.A.; Mugharbel, A.; Otrock, Z.K.; Yassine, N.; Muwakkit, S.; Salem, Z.; Shebbo, W.; Jisr, T.; Abboud, M.; et al. Hematopoietic stem cell transplantation in Lebanon: First comprehensive report. Bone Marrow Transplant. 2008, 42, S96–S102. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Zecca, M.; Piras, E.; Vacca, A.; Giorgiani, G.; Cugno, C.; Caocci, G.; Comoli, P.; Mastronuzzi, A.; Merli, P.; et al. Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in patients with thalassaemia major. Br. J. Haematol. 2008, 143, 548–551. [Google Scholar] [CrossRef]
- Ghavamzadeh, A.; Iravani, M.; Ashouri, A.; Mousavi, S.A.; Mahdavi, N.; Shamshiri, A.; Hadjibabaie, M.; Namdar, R.; Nedaeifard, L.; Ghaffari, H.; et al. Peripheral blood versus bone marrow as a source of hematopoietic stem cells for allogeneic transplantation in children with class I and II beta thalassemia major. Biol. Blood Marrow Transplant. 2008, 14, 301–308. [Google Scholar] [CrossRef]
- Dennison, D.; Al Kindi, S.; Pathare, A.; Daar, S.; Nusrat, N.; Ur Rehman, J.; Zia, F.; Khan, H.; Khan, M.; Alghazaly, A.; et al. Hematopoietic stem cell transplantation in Oman. Bone Marrow Transplant. 2008, 42, S109–S113. [Google Scholar] [CrossRef]
- Di Bartolomeo, P.; Santarone, S.; Di Bartolomeo, E.; Olioso, P.; Bavaro, P.; Papalinetti, G.; Di Carlo, P.; Papola, F.; Nicolucci, A.; Di Nicola, M.; et al. Long-term results of survival in patients with thalassemia major treated with bone marrow transplantation. Am. J. Hematol. 2008, 83, 528–530. [Google Scholar] [CrossRef]
- Ghavamzadeh, A.; Alimoghaddam, K.; Naderi, A.; Hamidieh, A.A.; Bahar, B.; Mousavi, S.A.; Iravani, M. Outcome of related and unrelated cord-blood transplantation in children at hematopoietic stem cell transplantation research center of Shariati Hospital. Int. J. Hematol. Oncol. Stem Cell Res. 2009, 3, 17–20. [Google Scholar]
- Kumar, R.; Naithani, R.; Mishra, P.; Mahapatra, M.; Seth, T.; Dolai, T.K.; Bhargava, R.; Saxena, R. Allogeneic hematopoietic SCT performed in non-HEPA filter rooms: Initial experience from a single center in India. Bone Marrow Transplant. 2009, 43, 115–119. [Google Scholar] [CrossRef]
- Ramzi, M.; Nourani, H.; Zakerinia, M.; Dehghani, M.; Vojdani, R.; Haghshenas, M. Results of hematopoietic stem cell transplant in Shiraz: 15 years experience in Southern Iran. Off. J. Middle East Soc. Organ Transplant. 2010, 8, 61–65. [Google Scholar]
- Chiesa, R.; Cappelli, B.; Crocchiolo, R.; Frugnoli, I.; Biral, E.; Noè, A.; Evangelio, C.; Fossati, M.; Roccia, T.; Biffi, A.; et al. Unpredictability of intravenous busulfan pharmacokinetics in children undergoing hematopoietic stem cell transplantation for advanced beta thalassemia: Limited toxicity with a dose-adjustment policy. Biol. Blood Marrow Transplant. 2010, 16, 622–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caocci, G.; Efficace, F.; Ciotti, F.; Roncarolo, M.G.; Vacca, A.; Piras, E.; Littera, R.; Dawood Markous, R.S.; Collins, G.S.; Ciceri, F.; et al. Prospective assessment of health-related quality of life in pediatric patients with beta-thalassemia following hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2011, 17, 861–866. [Google Scholar] [CrossRef]
- Sodani, P.; Isgrò, A.; Gaziev, D.; Paciaroni, K.; Marziali, M.; Simone, M.D.; Roveda, A.; Alfieri, C.; De Angelis, G.; Gallucci, C.; et al. T Cell-depleted hla-haploidentical stem cell transplantation in thalassemia young patients. Pediatr. Rep. 2011, 3, 34–37. [Google Scholar] [CrossRef]
- Ruggeri, A.; Eapen, M.; Scaravadou, A.; Cairo, M.S.; Bhatia, M.; Kurtzberg, J.; Wingard, J.R.; Fasth, A.; Lo Nigro, L.; Ayas, M.; et al. Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol. Blood Marrow Transplant. 2011, 17, 1375–1382. [Google Scholar] [CrossRef]
- Jaing, T.-H.; Hung, I.-J.; Yang, C.-P.; Chen, S.-H.; Chung, H.-T.; Tsay, P.-K.; Wen, Y.-C. Unrelated cord blood transplantation for thalassaemia: A single-institution experience of 35 patients. Bone Marrow Transplant. 2012, 47, 33–39. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Feng, X.; He, Y.; Liu, H.; Pei, F.; Liao, J.; He, L.; Shi, L.; Li, N.; et al. A novel conditioning regimen improves outcomes in β-thalassemia major patients using unrelated donor peripheral blood stem cell transplantation. Blood 2012, 120, 3875–3881. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Piras, E.; Vacca, A.; Giorgiani, G.; Zecca, M.; Bertaina, A.; Pagliara, D.; Contoli, B.; Pinto, R.M.; Caocci, G.; et al. Allogeneic hematopoietic stem cell transplantation in thalassemia major: Results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood 2012, 120, 473–476. [Google Scholar] [CrossRef]
- Goussetis, E.; Peristeri, I.; Kitra, V.; Vessalas, G.; Paisiou, A.; Theodosaki, M.; Petrakou, E.; Dimopoulou, M.N.; Graphakos, S. HLA-matched sibling stem cell transplantation in children with β-thalassemia with anti-thymocyte globulin as part of the preparative regimen: The Greek experience. Bone Marrow Transplant. 2012, 47, 1061–1066. [Google Scholar] [CrossRef]
- Choudhary, D.; Sharma, S.K.; Gupta, N.; Kharya, G.; Pavecha, P.; Handoo, A.; Setia, R.; Katewa, S. Treosulfan-thiotepa-fludarabine-based conditioning regimen for allogeneic transplantation in patients with thalassemia major: A single-center experience from North India. Biol. Blood Marrow Transplant. 2013, 19, 492–495. [Google Scholar] [CrossRef]
- Mathews, V.; George, B.; Viswabandya, A.; Abraham, A.; Ahmed, R.; Ganapule, A.; Sindhuvi, E.; Lakshmi, K.M.; Srivastava, A. Improved clinical outcomes of high risk β thalassemia major patients undergoing a HLA matched related allogeneic stem cell transplant with a treosulfan based conditioning regimen and peripheral blood stem cell grafts. PLoS ONE 2013, 8, e61637. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, J.; Marziali, M.; Isgrò, A.; Sodani, P.; Paciaroni, K.; Gallucci, C.; Andreani, M.; Testi, M.; De Angelis, G.; Alfieri, C.; et al. Bone marrow transplantation for thalassemia from alternative related donors: Improved outcomes with a new approach. Blood 2013, 122, 2751–2756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parikh, S.H.; Mendizabal, A.; Benjamin, C.L.; Komanduri, K.V.; Antony, J.; Petrovic, A.; Hale, G.; Driscoll, T.A.; Martin, P.L.; Page, K.M.; et al. A novel reduced-intensity conditioning regimen for unrelated umbilical cord blood transplantation in children with nonmalignant diseases. Biol. Blood Marrow Transplant. 2014, 20, 326–336. [Google Scholar] [CrossRef]
- Anurathapan, U.; Pakakasama, S.; Mekjaruskul, P.; Sirachainan, N.; Songdej, D.; Chuansumrit, A.; Charoenkwan, P.; Jetsrisuparb, A.; Sanpakit, K.; Pongtanakul, B.; et al. Outcomes of thalassemia patients undergoing hematopoietic stem cell transplantation by using a standard myeloablative versus a novel reduced-toxicity conditioning regimen according to a new risk stratification. Biol. Blood Marrow Transplant. 2014, 20, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- King, A.A.; Kamani, N.; Bunin, N.; Sahdev, I.; Brochstein, J.; Hayashi, R.J.; Grimley, M.; Abraham, A.; Dioguardi, J.; Wah Chan, K.; et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am. J. Hematol. 2015, 90, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Shah, K.M.; Patel, K.A.; Anand, A.S.; Talati, S.S.; Panchal, H.P.; Patel, A.A.; Parikh, S.K.; Parekh, B.B.; Shukla, S.N.; et al. Unrelated umbilical cord blood transplant for children with β-thalassemia major. Indian J. Hematol. Blood Transfus. 2015, 31, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Anurathapan, U.; Hongeng, S.; Pakakasama, S.; Sirachainan, N.; Songdej, D.; Chuansumrit, A.; Charoenkwan, P.; Jetsrisuparb, A.; Sanpakit, K.; Rujkijyanont, P.; et al. Hematopoietic stem cell transplantation for homozygous β-thalassemia and β-thalassemia/hemoglobin e patients from haploidentical donors. Bone Marrow Transplant. 2016, 51, 813–818. [Google Scholar] [CrossRef]
- Zaidman, I.; Rowe, J.M.; Khalil, A.; Ben-Arush, M.; Elhasid, R. Allogeneic stem cell transplantation in congenital hemoglobinopathies using a tailored busulfan-based conditioning regimen: Single-center experience. Biol. Blood Marrow Transplant. 2016, 22, 1043–1048. [Google Scholar] [CrossRef]
- Gabr, H.; Youssry, I.; El-Ansary, Y.; Mosallam, G.; Riad, N.M.; Hanna, M.O.F. Chimerism in pediatric hematopoietic stem cell transplantation and its correlation with the clinical outcome. Transpl. Immunol. 2017, 45, 53–58. [Google Scholar] [CrossRef]
- Caocci, G.; Orofino, M.G.; Vacca, A.; Piroddi, A.; Piras, E.; Addari, M.C.; Caria, R.; Pilia, M.P.; Origa, R.; Moi, P.; et al. Long-term survival of beta thalassemia major patients treated with hematopoietic stem cell transplantation compared with survival with conventional treatment. Am. J. Hematol. 2017, 92, 1303–1310. [Google Scholar] [CrossRef]
- Park, B.-K.; Kim, H.-S.; Kim, S.; Lee, J.-W.; Park, Y.S.; Jang, P.-S.; Chung, N.-G.; Jeong, D.-C.; Cho, B. Allogeneic hematopoietic stem cell transplantation in congenital hemoglobinopathies with myeloablative conditioning and rabbit anti-thymocyte globulin. Blood Res. 2018, 53, 145–151. [Google Scholar] [CrossRef]
- Benakli, M.; Ahmed Nacer, R.; Mehdid, F.; Belhadj, R.; Talbi, A.; Rahmoune, N.; Niederwieser, C.; Baazizi, M.; Akhrouf, S.; Ait Ouali, D.; et al. Two decades of experience in a combined adult/pediatric allogeneic hematopoietic stem cell transplantation center in Algiers, Algeria. Ann. Hematol. 2020, 99, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kharya, G.; Bakane, A.N.; Rauthan, A.M. Pretransplant myeloid and immune suppression, reduced toxicity conditioning with posttransplant cyclophosphamide: Initial outcomes of novel approach for matched unrelated donor hematopoietic stem cell transplant for hemoglobinopathies. Pediatr. Blood Cancer 2021, 68, e28909. [Google Scholar] [CrossRef] [PubMed]
- La Nasa, G.; Littera, R.; Locatelli, F.; Lai, S.; Alba, F.; Caocci, G.; Lisini, D.; Nesci, S.; Vacca, A.; Piras, E.; et al. The human leucocyte antigen-g 14-basepair polymorphism correlates with graft-versus-host disease in unrelated bone marrow transplantation for thalassaemia. Br. J. Haematol. 2007, 139, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Anasetti, C.; Etzioni, R.; Petersdorf, E.W.; Martin, P.J.; Hansen, J.A. Marrow transplantation from unrelated volunteer donors. Annu. Rev. Med. 1995, 46, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Gaziev, J. Advances in the allogeneic transplantation for thalassemia. Blood Rev. 2008, 22, 53–63. [Google Scholar] [CrossRef]
- Baronciani, D.; Angelucci, E.; Potschger, U.; Gaziev, J.; Yesilipek, A.; Zecca, M.; Orofino, M.G.; Giardini, C.; Al-Ahmari, A.; Marktel, S.; et al. Hemopoietic stem cell transplantation in thalassemia: A report from the european society for blood and bone marrow transplantation hemoglobinopathy registry, 2000–2010. Bone Marrow Transplant. 2016, 51, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Zino, E.; Frumento, G.; Marktel, S.; Sormani, M.P.; Ficara, F.; Terlizzi, S.D.; Parodi, A.M.; Sergeant, R.; Martinetti, M.; Bontadini, A.; et al. A T-cell epitope encoded by a subset of hla-dpb1 alleles determines nonpermissive mismatches for hematologic stem cell transplantation. Blood 2004, 103, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Fleischhauer, K.; Locatelli, F.; Zecca, M.; Orofino, M.G.; Giardini, C.; De Stefano, P.; Pession, A.; Iannone, A.M.; Carcassi, C.; Zino, E.; et al. Graft rejection after unrelated donor hematopoietic stem cell transplantation for thalassemia is associated with nonpermissive HLA-DPB1 disparity in host-versus-graft direction. Blood 2006, 107, 2984–2992. [Google Scholar] [CrossRef]
- Littera, R.; Orrù, N.; Caocci, G.; Sanna, M.; Mulargia, M.; Piras, E.; Vacca, A.; Giardini, C.; Orofino, M.G.; Visani, G.; et al. Interactions between killer immunoglobulin-like receptors and their human leucocyte antigen class i ligands influence the outcome of unrelated haematopoietic stem cell transplantation for thalassaemia: A novel predictive algorithm. Br. J. Haematol. 2012, 156, 118–128. [Google Scholar] [CrossRef]
- Bertaina, A.; Merli, P.; Rutella, S.; Pagliara, D.; Bernardo, M.E.; Masetti, R.; Pende, D.; Falco, M.; Handgretinger, R.; Moretta, F.; et al. HLA-haploidentical stem cell transplantation after removal of αβ+ t and b cells in children with nonmalignant disorders. Blood 2014, 124, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulou, C.; Goussetis, E. HSCT remains the only cure for patients with transfusion-dependent thalassemia until gene therapy strategies are proven to be safe. Bone Marrow Transplant. 2021, 56, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
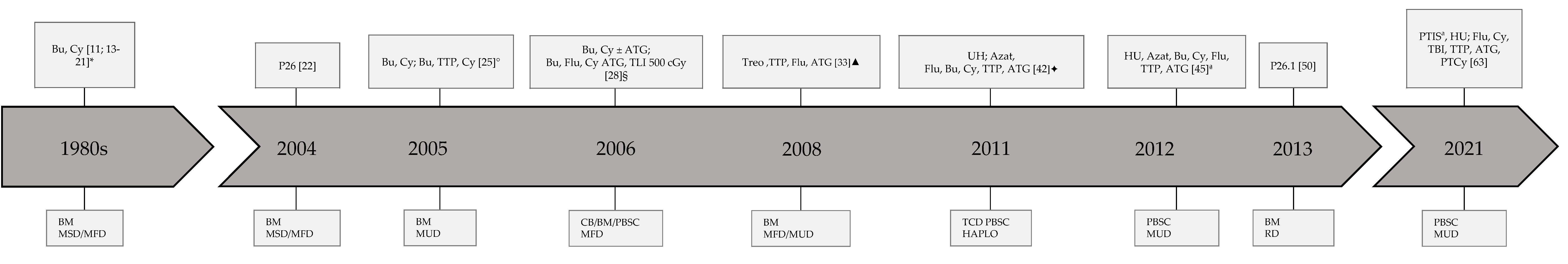
| Author | Year | Country | N | Median Age Years | Sex M:F | Risk Score | Conditioning Regimen | GVHD Prophylaxis | Graft Source | Donor Type | aGVHD I–II/III–IV % | cGVHD % | TRM % | GF % | Other Complications | OS % | EFS % | FU M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Or [15] | 1988 | Israel | 10 | 2 | 6:4 | NA | TLI 200 cGy/dx4, BU 4 mg/kg/dx4, Cy 50 mg/kg/dx4 | CSA | TCD BM | MSD, MFD | 0 | 0 | 30 | 10 | IH, IP | 70 | 90 | 30 |
| Lucarelli [11] | 1990 | Italy | 116 | 6.5/10.5/12 | NA | I-39/II-36/III-24 | Bu 3.5 mg/kg/dx4, Cy 50 mg/kg/dx4 | CSA, MTX, Cy | BM | MFD | 4.3 | 0.8 | 5.1/19.4/37.5 | 0/8.3/16 | CMV, ARDS | 94/80/61 | 94/77/53 | 12 |
| Lin [16] | 1998 | Taiwan | 38 | 7 | NA | NA | Bu 4 mg/kg/dx4, Cy 200 mg/kg ± TBI or TLI | CSA, MTX | NA | MFD, MUD | 3/3 | 3 | 25 | 5.2 | CMV, IP, IH, CHF | 79 | 45 | 82 |
| Suvatte [19] | 1998 | Thailand | 35 | NA | NA | NA | Bu 3.5 mg/kg/dx4, Cy 50 mg/kg/dx4; Bu 150 mg/m2/dx4, Cy 50 mg/kg/dx4 | NA | BM, CB | MFD, MSD | 0/3 | 0 | 3 | 14.3 | IH, S | 85.7 | 77.2 | 42 |
| Lucarelli [13] | 1998 | Italy | 393 | <17 | NA | I-121/II-272 | Bu 3.5 mg/kg/dx4, Cy 50 mg/kg/dx4 | CSA | BM | MFD | NA | NA | 5/15 | 5/4 | NA | 95/85 | 90/81 | NA |
| Lucarelli [13] | 1998 | Italy | 125 | <17 | NA | III | Bu 3.5 mg/kg/dx4, Cy 30–40 mg/kg/dx4 | CSA, MTX | BM | MFD | NA | NA | 19 | 33 | NA | 78 | 54 | NA |
| Rosales [20] | 1999 | Israel | 14 | NA | NA | NA | Bu 4 mg/kg/dx4, TTP 5 mg/kg/dx2, Cy 60 mg/kg/dx2 | ALE, CSA | NA | NA | 7/7 | 0 | 7.14 | 7.14 | NA | 92.8 | NA | 60 |
| Gaziev [17] | 2000 | Italy | 29 | 6 | 14:15 | I-6/II-III 23 | Bu 2–4 mg/kg/dx4, Cy 30–50 mg/dx4 ± TBI 300 400 cGy ± TLI ± ATG | CSA-MTX | NA | MFD, HAPLO | 47.3 | 37.5 | 34 | 55 | CMV | 65 | 21 | 90 |
| Khojasteh [21] | 2001 | Iran | 63 | 11 | 37:26 | II-8/III-55 | Bu 3.75 mg/kg/dx4, Cy 50 mg/kg/dx4, ATG 40 mg/kg | CSA, PND | BM | NA | 3/3 | 14 | 6.3 | 7.9 | S | 75 | 79.3 | 132 |
| La Nasa [22] | 2002 | Italy | 32 | 14 | 21:11 | I-4/II-11/III-17 | Bu 4 mg/kg/dx4, Cy 30–50 mg/kg/dx4; Bu 4 mg/kg/dx4, TTP 10 mg/kg, Cy 30–50 mg/kg/dx4 | CSA, MTX | BM | MUD | 41 | 25 | 19 | 12.5 | VOD, CMV, IH, HF | 79 | 66 | 30 |
| Lawson [23] | 2003 | UK | 55 | 6.4 | 26:29 | I-17/II-27/III-11 | Bu 3.5–4 mg/kg/dx4, Cy 30–50 mg/kg/dx4, ± FLU ± ATG | CSA, MTX | NA | MFD | 84/15 | 16 | 5.4 | 13.2 | VOD, CMV | 94.5 | 81.8 | 75 |
| Sodani [24] | 2004 | Italy | 33 | 16 | 16:17 | III | P26 | CSA, PDN, MTX, CY | BM | MSD | 9/0 | NA | 6 | 6 | CMV | 93 | 85 | 36 |
| La Nasa [25] | 2005 | Italy | 68 | 15 | 33:35 | I-14/II-16/III-38 | Bu 4 mg/kg/dx4, Cy 50 mg/kg/dx4; Bu 4 mg/kg/dx4, TTP 10 mg/kg, Cy 50 mg/kg/dx4; Bu 4 mg/kg/dx4, TTP 10 mg/kg, Flu 40 mg/m2/dx4 | CSA, MTX | BM | MUD | 40 | 18 | 20 | 13 | NA | 79.3 | 65.8 | 40 |
| Chandy [26] | 2005 | India | 47 | 6.74 | 31:16 | II-21/III-25 | Bu 150 mg/m2/dx4, Cy 50 mg/kg/dx4 | CSA, MTX | NA | NA | 30/19 | NA | 32 | 4 | VOD, HC | 68 | 68 | 63 |
| Chandy [26] | 2005 | India | 47 | 7.55 | 30:17 | II-22/III-25 | Bu 4 mg/kg/dx4, Cy 50 mg/kg/dx4, ATG 30 mg/kg/dx3 | CSA, MTX | NA | NA | 30/19 | NA | 28 | 9 | VOD, HC | 47 | 64 | 52 |
| La Nasa [27] | 2005 | Italy | 27 | 22 | 15:12 | NA | Bu 3.5 mg/kg/dx4,Cy30–40 mg/kg/dx4; Bu 3.5 mg/kg/dx4 -TT 10 mg/kg- Cy 30–40 mg/kg/dx4 | CSA-MTX ± ATG | BM | MUD | 37 | 26 | 30 | 3.7 | VOD CHF, CMV, IP, HF | 70 | 70 | 43 |
| Sauer [28] | 2005 | Germany | 5 | 11.5 | 3:2 | II-4/III-1 | Bu 3.5mg/kg/dx4, Flu 30 mg/m2/dx6 ± ATG | CSA, MTX | BM | MSD | 0 | 0 | 0 | 0 | VOD | 100 | 100 | 25 |
| Elhasid [29] | 2006 | Israel | 6 | 4.5 | NA | I | Bu 4 mg/kg/dx4, Cy 30 mg/kg/dx4, Flu 40mg/m2/dx5, ATG 5 mg/kg/dx5 | NONE | TCD PBSC | MSD | 0 | 17 | 0 | 0 | VOD, CLS, AHA | 100 | 100 | 39.8 |
| Hongeng [30] | 2006 | Thailand | 28 | 7.2 | 13:15 | I-15/II-III13 | Bu 4 mg/kg/dx4, Cy 50 mg/kg/dx4 ± ATG 40 mg/kg; Bu 8 mg/kg, Flu 175 mg/m2, Cy 50 mg/Kg/dx4, ATG 20 mg/kg, TLI 500 cGy | CSA, TCM, MTX, MTP | BM, PBSC, CB | MFD | 32/11 | 14 | 14.3 | 14 | HC, S, AHA, VOD | 92 | 82 | 51 |
| Hongeng [30] | 2006 | Thailand | 21 | 4 | 14:7 | I-13/II-III18 | Bu 4 mg/kg/dx4, Cy 50 mg/kg/dx4 ± ATG40 mg/kg | CSA, TCM MTX | BM | MUD | 43/14 | 14 | 7.1 | 14 | HC, S, VOD | 82 | 71 | 35 |
| Smythe [31] | 2007 | UK | 7 | 5,8 | NA | NA | Bu, Cy | NA | CB | MSD | 0 | 0 | 0 | 28,5 | NA | 100 | NA | 45 |
| Ullah [32] | 2007 | Pakistan | 40 | 4 | 28:12 | I-25/II-10/III-5 | Bu 3.5 mg/kg/dx4, Cy 50 mg/kg/dx4; P26 in III | CSA, PND | BM, PBSC | MSD | 40 | 13 | 20 | 12.5 | VOD, HC, S, TB, CMV | 80 | 72.5 | 48.9 |
| La Nasa [33] | 2007 | Italy | 45 | 33 | 25:20 | I-14/II-18/III-13 | Bu 3.5 mg/kg/dx4-TTP 10mg/kg-Cy 50 mg/kg dx4 or Cy 60 mg/kg/dx2; Bu 3.5 mg/kg/dx4, TT10 mg/kg Flu 40 mg/m2/dx4 | CSA-MTX | BM | MUD | 44 | NA | 13.3 | 15.6 | NA | 86.7 | 71.4 | 55 |
| La Nasa [33] | 2007 | Italy | 53 | 12 | NA | I-18/II-21/III-14 | Bu 3.5mg/kg/dx4, Cy 30–50mg/kg/dx4; Bu 3.5 mg/kg/dx4, TTP10 mg/kg, Flu 40 mg/m2/dx4 | CSA-MTX | BM | MUD | 30 | NA | 11.3 | 15.1 | CMV, IP | 88.7 | 73.6 | NA |
| Bazarbachi [34] | 2008 | Lebanon | 10 | 5 | 4:6 | III | Bu/Cy/ ± ATG | NA | NA | NA | 10/10 | NA | 10 | 10 | VOD, CMV, S | 89 | 67 | 65 |
| Bernardo [35] | 2008 | Italy | 20 | 13 | 14:6 | I-7/II-4/III-9 | Treo 14 g/m2/dx3, TTP 8 mg/kg, Flu 40 mg/m2/dx4, ATG 10 mg/kg/dx3 | CSA, MTX | BM | MFD, MUD | 10/5 | 5 | 5 | 10 | NA | 95 | 85 | 20 |
| Ghavamzadeh [36] | 2008 | Iran | 96 | 6 | 49:47 | I-40/II-56 | Bu 4 mg/kg/dx4, Cy 50 mg/dx4 | CSA, MTX | BM | MFD | 35 | 18 | 11 | 3.1 | NA | 89 | 76 | 29 |
| Ghavamzadeh [36] | 2008 | Iran | 87 | 5 | 57:30 | I-48/II-39 | Bu 4 mg/kg/dx4, Cy 50 mg/dx4 | CSA, MTX | PBSC | MFD | 72 | 48 | 17 | 0 | NA | 83 | 76 | 60 |
| Dennison [37] | 2008 | Oman | 41 | 9 | NA | II-23 | Bu–Cy 50 mg/kg/dx4 ± ATG; Bu 3.5mg/kg/dx4, Flu 30 mg/m2/dx6 ± ATG | NA | BM CB-BM | NA | 19 | 5 | 10 | 7 | NA | 88 | 88 | 72 |
| Di Bartolomeo [38] | 2008 | Italy | 115 | 9 | 57:58 | NA | Bu 3.5–4mg/kg/dx4, Cy 50mg/kg/dx4 | CSA, MTX | BM | MFD | 43 | 20 | 8.7 | 6.7 | E, CHF | 89.2 | 85.7 | 180 |
| Ghavamzadeh [39] | 2009 | Iran | 9 | 6.7 | NA | I-4/II-5 | Bu 3.5–4 mg/kg/dx4, Cy 50 mg/dx4 | CSA, MTX | CB | MSD | 0 | 0 | 11.1 | 55.5 | NONE | 88.9 | 33.3 | NA |
| Kumar [40] | 2009 | India | 4 | 6 | 4:0 | II-1/III-3 | Bu 4 mg/kg/dx4, Cy 50 mg/kg/dx4, ATG 30 mg/kg | CY, MTX | BM, PBSC | NA | NA | NA | 0 | NA | NA | 100 | NA | 18 |
| Ramzi [41] | 2009 | Iran | 155 | 9.5 | NA | I-43/II-53/III-59 | Bu 4 mg/kg/dx4, Cy 30–50 mg/kg/dx4, ATG 40 mg/kg | CSA, MTX | BM, PBSC | NA | NA | NA | 14.8 | 12.9 | NA | 85.1 | 74.1 | 97.2 |
| Chiesa [42] | 2010 | Italy | 53 | 8 | 29:24 | I-2/II-26/III-25 | Bu, Cy 50 mg/kg/dx4 ± TTP 10 mg/kg; P26, ¥Bu, and Cy 4 mg/kg/dx4 | CSA | BM | MSD MFD | 7 | 4 | 4 | 21 | VOD AKI | 96 | 79 | 18.5 |
| Caocci [43] | 2011 | Italy | 28 | 10 | 17:11 | II-4/III-24 | Treo, TTP, Flu; Bu Cy Bu, TTP, Cy | CSA -MTX | NA | MFD, HAPLO, MUD | 21/14 | 18 | 10.9 | 14.3 | NA | 89.3 | 78.6 | 24 |
| Sodani [44] | 2011 | Italy | 31 | NA | NA | NA | HU 60 mg/kg/dx50; Azat 3 mg/kg/dx48, Flu 30 mg/m2/dx5, Bu 3 mg/kg/dx4, Cy 50 mg/kg/dx4, TTP 10 mg/kg, ATG 12.5 mg/kg/dx4 | CSA | TCD PBSC | HAPLO | 0 | 0 | 6.5 | 22.5 | CMV, EBV | 93.5 | 71 | NA |
| Ruggeri [45] | 2011 | France | 35 | NA | 20:15 | NA | Bu 8 mg/kg or Bu 6.4 mg/kg, Cy, ATG; other regimens Bu, Cy ± Flu ± TTP ± ATG ± TLI ± TBI | CSA, MFM, MTX, ALE | CB | MUD | NA | NA | 14.2 | 57.1 | VOD, MOF, ARDS | 62 | 21 | 21 |
| Jaing [46] | 2012 | Taiwan | 35 | 5.5 | 16:19 | NA | Bu 3.5 mg/kg/dx4, Cy 50 mg/kg/dx4, ATG 30 mg/Kg/dx4 or thymoglobulin 3 mg/Kg/dx4 | CSA, MPN | CB | MUD | 51/46 | 40 | 11.4 | 17.1 | IH, S, IP | 88.3 | 73.9 | 36 |
| Li [47] | 2012 | China | 30 | 6 | 20:10 | II | HU 30 mg/kg/d, Azat 3 mg/kg/d, Bu 2.8–4.4 mg/kg/dx 4, Cy 60 mg/kg/dx2, Flu 40 mg/m2/dx4, TTP 10 mg/kg, ATG 15–30 mg/Kg/dx4 or TG 2.5 mg/Kg/dx4 | CSA, MFM, MTX | BM, CB-BM | MFD | 0/3.6 | 0 | 10 | 6.9 | CMV, VOD | 90 | 83.3 | 24 |
| Li [47] | 2012 | China | 52 | 6 | 36:16 | II | HU 30 mg/kg/d, Azat 3 mg/kg/d, BU 2.8–4.4 mg/kg/dx 4, Cy 60 mg/kg/dx2, Flu 40 mg/m2/dx4, TTP 10 mg/kg, ATG 15–30 mg/Kg/dx4 or TG 2.5 mg/Kg/dx4 | CSA, MFM, MTX | PBSC | MUD | 0/9.6 | 0 | 7.7 | 1.9 | CMV, VOD | 92.3 | 90.4 | 24 |
| Bernardo [48] | 2012 | Italy | 60 | 7 | 32:28 | I-27/II-17/III-4 | Treo 14 g/m2/dx3, TTP 8 mg/kg, Flu 40mg/m2/dx4, ATG 10 mg/kg/dx3 | CSA, MTX | BM, PBSC, CB | MFD, MUD | 7/7 | 2 | 6.6 | 9 | IP | 93 | 84 | 36 |
| Goussetis [49] | 2012 | Greece | 75 | 7 | 37:38 | I-16/II-38/III-17 | Bu 3,5–4 mg/kg/dx4, Cy 37.5–50 mg/kg/dx4, ± Flu 25 mg/m2/dx4, ATG | CSA, MTX | BM, BM + CB, PBSC | MFD | 9/4 | 13 | 4 | 4 | NA | 96 | 92 | 108 |
| Choudhary [50] | 2013 | India | 28 | 9,6 | 15:13 | II-7/III-21 | Treo 14 g/m2/dx3, TTP 8 mg/kg, Flu 40mg/m2/dx4 | CSA, MTX | NA | MFD | 4 | 2 | 21.4 | 7.14 | VOD | 78.5 | 71.4 | 13 |
| Mathews [51] | 2013 | India | 193 | >11 | 118:75 | III-139/IIIc-54 | Bu 4 mg/kg/dx4, Cy 50 mg/kg/dx4, ATG 30 mg/kg/dx3; Bu 150 mg/m2/dx4, Cy 50 mg/kg/dx4 | CSA, MTX | BM, PBSC | MFD, MUD | 44 | 18 | 28 | 12 | IH, VOD, HC, | 63.6/39.4 | 57.3/32.4 | 42 |
| Mathews [51] | 2013 | India | 74 | >11 | 46:28 | III-50/IIIc-24 | TTP 8 mg/kg, Treo 14 g/m2/dx3,Flu 30 mg/m2/dx4 | CSA, MTX | BM, PBSC | MFD, MUD | 35 | 11 | 12 | 8 | VOD | 87.4/86.6 | 78.8/77.8 | 42 |
| Gaziev [52] | 2013 | Italy | 16 | 9.6 | 10:6 | I-5/II-5/III-10 | P26.1 | CSA, MTX, PND, CY | BM | RD | 19/13 | 13 | 6 | 0 | HC | 94 | 94 | 72 |
| Gaziev [52] | 2013 | Italy | 66 | 10 | 37:29 | I-0/II-31/III-35 | Bu 3.5 mg/kg/dx4, Cy 50 mg/dx4 ± TTP; Bu 3.5 mg/kg/dx4, Cy 50 mg/dx4; HU30 mg/kg/d, Azat3 mg/kg/day, Flu20 mg/m2, Bu 3.5 mg/kg/dx4, Cy 22.5–40 mg/dx4 ± TTP | CSA, MTX, PND, CY | BM | MSD | 36/7 | 11 | 8 | 12 | VOD, HC | 92 | 82 | 80 |
| Hussein [18] | 2013 | Jordan | 44 | 8 | 20:24 | I-7/II-24/III-13 | Bu 5–4 mg/kg/dx4, Cy 50 mg/kg/dx4; Bu 2 mg/kg/dx5, Flu 35 mg/m2/dx5, TLI 500 cGy | CSA, MTX, MFM | BM, PBSC | MFD | 32 | 16 | 2.2 | 11,3 | NA | 97.8 | 86.4 | 64 |
| Parikh [53] | 2014 | US | 4 | 3.3 | 3:1 | NA | HU 30 mg/kg/dx12, Flu 30 mg/m2/dx5, TTP 200 mg/m2 × 1 | TCM, MFM, ALE | CB | MUD | 50/50 | 25 | 0 | 0 | AHA, EBV | 100 | NA | 19.7 |
| Anurathapan [54] | 2014 | Thailand/US | 76 | 8 | 44:32 | III | Bu 1 mg/kg/dx4 or Bu 0.95–1.2 mg/kg/dx 4, Cy 50 mg/kg/dx4; Bu 1 mg/kg/dx4 or Bu 0.95–1.2 mg/kg/dx 4, Cy 50 mg/kg/dx4, Flu 30 mg/m2/dx6, ATG 10 mg/Kg/dx4 | CSA, TCM, MTX | BM, PBSC | MFD, MUD | 17/7 | 11 | 7 | 8 | HC, S, AHA, VOD, CMV | 95 | 88 | 114 |
| Anurathapan [54] | 2014 | Thailand/US | 22 | 17 | 9:13 | IIIc | PTISbx2, Flu 35 mg/m2/dx6, Bu 130 mg/m2/dx4, ATG 1.5 mg/kg/dx3 | CSA, TCM, MFM | BM, PBSC | MFD, MUD | 9/14 | 18 | 9 | 0 | HC, VOD, CMV | 90 | 93 | 36 |
| King [55] | 2015 | US/Canada | 9 | NA | NA | NA | Flu 35–37.5 mg/m2/dx4, MEL 150 mg/m2 | CSA, TCM, MTX, PND, MFM, ALE | BM, CB | MSD | NA | NA | 0 | NA | NA | 100 | 100 | 41 |
| Hussein [14] | 2015 | Jordan/US | 29 | 13.9 | 14:15 | III | Bu 4 mg/kg/dx2, Flu 35 mg/m2/dx5, ATG 30 mg/kg/dx5 in 7; ATG 2.5 mg/kg/dx3 in 22; TLI 500 cGy single dose | CSA, MFM | PBSC | MSD, MFD | 11 | 8 | 0 | 20.6 | VOD | 100 | NA | 45.3 |
| Shah [56] | 2015 | India | 9 | 3.8 | 8:1 | NA | Bu 4 mg/kg/dx 4, Cy 50 mg/kg/dx2, Flu 90 mg/kg/dx2, ATG 7.5 mg/Kg/dx3 | CSA, MPN | CB | MUD | 33/0 | 0 | 0 | 44 | CMV | 100 | 56 | 22.6 |
| Anurathapan [57] | 2016 | Thailand | 31 | 10.1 | 17:14 | I-7/II-9/IIIc-15 | PTISbx2, Flu 35 mg/m2/dx6, Bu 130 mg/m2/dx4, ATG 1.5 mg/kg/dx3 | CY, TCM or SIL, MFM | TCD-PBSC | HAPLO | 29/3.2 | 16.1 | 3.2 | 3.2 | VOD, CMV | 95 | 94 | 12 |
| Zaidman [58] | 2016 | Israel | 34 | 8 | NA | I-7/II-6/III-21 | P26; P26.1; BU 3.5 mg/kg/dx4, Cy 50 mg/kg/dx4;Bu 4 mg/kg/dx4, Cy 30 mg/kg/dx4, Flu 40 mg/m2/dx5, ATG 5 mg/kg/dx5 | CSA, MTX, TTP, ATG | BM, PBSC, CB ± TCD | MFD, MUD | NA | NA | 14.7 | 8.8 | VOD, CLS | 90.5 | 81.7 | 129 |
| Gabr [59] | 2017 | Egypt | 6 | 5.5 | 3:3 | II | Bu 4 mg/kg/dx4, Cy 30 mg/kg/dx4, ATG11 mg/kg/dx4 | DEX, MTP, CSA | PBSC, CB | MSD | 17/0 | NA | 16.7 | 33.3 | NA | 83.3 | 50 | 24 |
| Caocci [60] | 2017 | Italy | 258 | 12 | 140:118 | I-57/II-83/III-21 | Bu Cy 30–50 mg/kg/dx4; Bu –Cy 30–50 mg/kg/dx4,TTP; Treo-TT-Flu, ATG | CSA, TCM, MPD, MTX | NA | MFD, MUD | 23.6 | 12.9 | 13.8 | 6.9 | VOD, IH | 82.6 | 77.8 | 132 |
| Park [61] | 2018 | Korea | 15 | 6.2 | 6:9 | NA | Bu 130 mg/m2/dx 4, Cy 60 mg/kg/dx2, ATG2.5mg/kg/dx3 | CSA, MTX | BM, PBSC | NA | 0/7 | 0 | 0 | 0 | VOD | 100 | NA | 27 |
| Benakly [62] | 2020 | Algeria | 47 | 7.6 | NA | NA | Bu 3 mg/kg/dx4, Cy 30–50 mg/kg/dx4, ATG10mg/kg | CSA, MTX | BM, PBSC, CB | MUD | 28 | 9 | NA | 14.8 | NA | 75.7 | 66.8 | 180 |
| Kharya [63] | 2021 | India | 4 | 6 | 1:2 | III | PTISa x2 cycles, HU 20 mg/kg/dx50; F30 mg/m2/dx5, CY 14.5 mg/kg/dx2, TBI 2 Gyx1, TTP 10 mg/kg/dx1, ATG 1.5 mg/kg/dx3, PTCY dx2 | PTCY, SIL, MFM | PBSC | MUD | 25/0 | 0 | 0 | 0 | NONE | 100 | 100 | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulas, O.; Mola, B.; Caocci, G.; La Nasa, G. Conditioning Regimens in Patients with β-Thalassemia Who Underwent Hematopoietic Stem Cell Transplantation: A Scoping Review. J. Clin. Med. 2022, 11, 907. https://doi.org/10.3390/jcm11040907
Mulas O, Mola B, Caocci G, La Nasa G. Conditioning Regimens in Patients with β-Thalassemia Who Underwent Hematopoietic Stem Cell Transplantation: A Scoping Review. Journal of Clinical Medicine. 2022; 11(4):907. https://doi.org/10.3390/jcm11040907
Chicago/Turabian StyleMulas, Olga, Brunella Mola, Giovanni Caocci, and Giorgio La Nasa. 2022. "Conditioning Regimens in Patients with β-Thalassemia Who Underwent Hematopoietic Stem Cell Transplantation: A Scoping Review" Journal of Clinical Medicine 11, no. 4: 907. https://doi.org/10.3390/jcm11040907
APA StyleMulas, O., Mola, B., Caocci, G., & La Nasa, G. (2022). Conditioning Regimens in Patients with β-Thalassemia Who Underwent Hematopoietic Stem Cell Transplantation: A Scoping Review. Journal of Clinical Medicine, 11(4), 907. https://doi.org/10.3390/jcm11040907






