Risk Factors for Acute Kidney Injury Following Cardiac Surgery and Performance of Leicester Score in a Spanish Cohort
Abstract
:1. Introduction
2. Material and Methods
2.1. Data Colection and Definitions
2.2. Definitions
2.3. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Surgical Characteristics and Post-Intervention Lengh of Stay
3.3. AKI Characteristics
3.4. Preoperative and Intraoperative Risk Factors for CSA-AKI
3.5. Leicester Score Performance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neal, J.B.; Shaw, A.D.; Billings, F.T. Acute kidney injury following cardiac surgery: Current understanding and future directions. Crit. Care 2016, 20, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Liu, B.; Liang, Z.; Yang, Z.; Ma, F.; Yang, Y.; Hu, W. Acute Kidney Injury following Cardiopulmonary Bypass: A Challenging Picture. Oxidative Med. Cell. Longev. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Crosina, J.; Lerner, J.; Ho, J.; Tangri, N.; Komenda, P.; Hiebert, B.; Choi, N.; Arora, R.C.; Rigatto, C. Improving the Prediction of Cardiac Surgery–Associated Acute Kidney Injury. Kidney Int. Rep. 2016, 2, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef] [Green Version]
- Birnie, K.; Verheyden, V.; Pagano, D.; Bhabra, M.; Tilling, K.; Sterne, J.A.; Murphy, G.J.; UK AKI in Cardiac Surgery Collabortors. Predictive models for kidney disease: Improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit. Care 2014, 18, 606. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ma, J. Mild AKI is associated with mortality of patients who underwent cardiopulmonary bypass surgery. Exp. Ther. Med. 2020, 20, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, P.; Möller, S.; Arneth, B.; Roth, P.; Niemann, B.; Renz, H.; Böning, A. Predicting Cardiac Surgery-Associated Acute Kidney Injury Using a Combination of Clinical Risk Scores and Urinary Biomarkers. Thorac. Cardiovasc. Surg. 2019, 68, 389–400. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [Green Version]
- Yi, Q.; Li, K.; Jian, Z.; Xiao, Y.-B.; Chen, L.; Zhang, Y.; Ma, R.-Y. Risk Factors for Acute Kidney Injury after Cardiovascular Surgery: Evidence from 2157 Cases and 49,777 Controls—A Meta-Analysis. Cardiorenal Med. 2016, 6, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.-Y.; Chou, N.-K.; Chen, Y.-S.; Yu, H.-Y. Risk factor for acute kidney injury in patients with chronic kidney disease receiving valve surgery with cardiopulmonary bypass. Asian J. Surg. 2020, 44, 229–234. [Google Scholar] [CrossRef]
- Lombardi, R.; Ferreiro, A. Risk factors profile for acute kidney injury after cardiac surgery is different according to the level of baseline renal function. Ren. Fail. 2008, 30, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-J.; Gardiner, B.S.; Smith, D.W. A cardiovascular model for renal perfusion during cardiopulmonary bypass surgery. Comput. Biol. Med. 2020, 119, 103676. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Spina, S.; Lei, C.; Pinciroli, R.; Berra, L. Hemolysis and Kidney Injury in Cardiac Surgery: The Protective Role of Nitric Oxide Therapy. Semin. Nephrol. 2019, 39, 484–495. [Google Scholar] [CrossRef]
- Issitt, R.; James, T.; Walsh, B.; Voegeli, D. Do lipid microemboli induce acute kidney injury during cardiopulmonary bypass? Perfusion 2017, 32, 466–473. [Google Scholar] [CrossRef]
- Kumar, A.B.; Suneja, M.; Bayman, E.O.; Weide, G.D.; Tarasi, M. Association between Postoperative Acute Kidney Injury and Duration of Cardiopulmonary Bypass: A Meta-Analysis. J. Cardiothorac. Vasc. Anesthesia 2012, 26, 64–69. [Google Scholar] [CrossRef]
- Ranucci, M.; Aloisio, T.; Carboni, G.; Ballotta, A.; Pistuddi, V.; Menicanti, L.; Frigiola, A. Acute Kidney Injury and Hemodilution during Cardiopulmonary Bypass: A Changing Scenario. Ann. Thorac. Surg. 2015, 100, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Mazzone, A.L.; Baker, R.A.; Gleadle, J.M. Mending a broken heart but breaking the kidney. Nephrology 2016, 21, 812–820. [Google Scholar] [CrossRef] [Green Version]
- Kanji, H.D.; Schulze, C.J.; Hervas-Malo, M.; Wang, P.; Ross, D.B.; Zibdawi, M.; Bagshaw, S.M. Difference between pre-operative and cardiopulmonary bypass mean arterial pressure is independently associated with early cardiac surgery-associated acute kidney injury. J. Cardiothorac. Surg. 2010, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Rihal, C.S.; Sutton-Tyrrell, K.; Guo, P.; Keller, N.M.; Jandova, R.; Sellers, M.A.; Schaff, H.V.; Holmes, D.R., Jr. Increased incidence of periprocedural complications among patients with peripheral vascular disease undergoing myocardial revascularization in the bypass angioplasty revascularization investigation. Circulation 1999, 100, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-asscited AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Euroscore II | Cleveland Clinic Score | Leicester Score |
|---|---|---|
| Age | --- | Age |
| Gender | ||
| Preoperative renal function (Cockroft–Gault formula, ml/min): >85, 50–85, <50, dialysis treatment | Preoperative renal function (creatinine, mg/dL): <1.2 mg/dL, 1.2–2.1, ≥2.1 | Renal function (Cockroft–Gault formula, ml/min): >90–60–89, 30–59, <30 |
| Poor mobility | --- | --- |
| Chronic lung disease | COPD requiring treatment | --- |
| Previous cardiac surgery | Previous cardiac surgery | --- |
| Active endocarditis | --- | --- |
| Critical preoperative state | Preoperative use of IABP | --- |
| Diabetes mellitus on insulin therapy | Diabetes mellitus on insulin therapy | Diabetes mellitus |
| NYHA class (I–IV) | Heart failure | NYHA class (I–IV) |
| Class IV angina a | --- | --- |
| Left ventricular function (%): >50, 31–50, 21–30, <21 | Left ventricular function <35% | Left ventricular function (%): ≥50, 40–49, <40 |
| Recent miocardial infarction (90 days) | --- | --- |
| Pulmonary hypertension: ystolic arterial pressure 31–55 mmHg, >55 | --- | --- |
| Urgency; (elective, urgent, emergency, salvage) | Emergency surgery | Urgency (elective, urgent, emergency) |
| Type of surgery: isolated CABG, non-CABG, 2 procedures, 3 procedures | Type of surgery: CABG, valve, CABG + valve, other | Type of surgery: CABG, single valve, CABG + valve, other/multiple |
| Surgery on thoracic aorta | --- | --- |
| --- | --- | Body mass index (kg/m2): <20, 20–24, 25–29, 30–34, >34 |
| --- | --- | Smoking habit: never, ex-smoker, current |
| --- | --- | Hypertension |
| --- | --- | Peripheral vascular disease |
| --- | --- | Preoperative hemoglobin (g/dL) (<10, 10–11.9, ≥12) |
| --- | --- | Triple vessel disease |
| --- | --- | Time from catheterism to surgery |
| n = 444 | n (%)/Median (IQR)/Mean+/−SD |
|---|---|
| Sex (%man) | 285 (64.2) |
| Age (years) ≥75 years | 69 (61–76) 137 (30.9) |
| History of smoking habit | 213 (49) |
| Diabetes Diabetes with insulin therapy | 157 (35.36) 42 (9.45) |
| Hypertension | 338 (76.1) |
| BMI (kg/m2) BMI ≥ 30 | 28.33+/−4.47 136 (34.1) |
| Anemia Hemoglobin (g/L) Hematocrit (%) | 87 (19.6) 134 (123–143) 39 (36–42) |
| Peripheral vascular disease | 42 (9.5) |
| Low ejection fraction (<40%) | 45 (10.13) |
| Creatinine (mg/dL) EGFR(ml/min) EGFR<60 mL/min CKD EIII CKD EIV | 0.9 (0.73–1.06) 83.3 (65–91) 86 (19,37) 75 (87.2) 11 (12.8) |
| Previous cardiac surgery | 47 (10.6) |
| Procedure | Valve surgery: 199 (44.8), CABG: 171 (38.5) Valve + CABG: 74 (16.7) |
| Charlson index | 4 (3–5) |
| Euroscore II | 1.77 (1.08–3.02) |
| Cleveland Clinic Score | 0.4 (0.4–1.8) |
| Leicester Score | 18.45 (11.12–30.94) |
| AKI Stages | Days between Surgery and AKI Start |
| AKI stage 1: 105 (61,4%)→49 met only the “>0.3 mg/dL in 48h” criteria (46.2%) AKI stage 2: 40 (23.4%) AKI stage 3: 26 (15,2%)→15 with dialysis requirement (57.7%) | Median time from surgery to AKI (1 (1–2) First 24 h: 114 (66.7%) 48 h: 36 (22.8%) 72 h: 10 (5.85%) >72 h: 11(6.4%) |
| AKI duration | Dialysis technique |
| Median duration time (days): 3 (1–6) 24 h: 50 patients (29.24%) 48 h: 26 patients (15.2%) 72 h: 23patients (13.45%) >72 h: 72 patients(42.1%) | Intermittent hemodialysis: 6 patients CRRT: 5 patients Both: 4 patients * Median intermittent hemodialysis sessions: 2 (IQR 1–4) Median CRRT treatment (days): 3 (1–4) |
| Total (n = 444) | No AKI (n = 273, 61.5%) | AKI (n = 171, 38.5%) | OR (IQR) | p Value | |
|---|---|---|---|---|---|
| PREOPERATIVE | |||||
| Age ≥ 75 (years) | 137 (30.9) | 70 (25.6) | 67 (39.2) | 1.868 (1.24–2.815) | 0.003 |
| Sex (%Male) | 285 (64.2) | 168 (61.5) | 117 (68.4) | 1.354 (0.904–2.029) | 0.142 |
| BMI > 30 | 136 (34.1) | 76 (31.3) | 60 (35.1) | 1.373 (0.901–2.093) | 0.140 |
| Ever smoked | 213 (49) | 130 (48.7) | 83 (48.5) | 1.029 (0.699–1.514) | 0.884 |
| Diabetes | 157 (35.36) | 89 (32.6) | 67 (39.4) | 1.365 (0.917–2.031) | 0.125 |
| Hypertension | 338 (76.1) | 194 (71.1) | 144 (84.2) | 2.172 (1.334–3.535) | 0.002 |
| Peripheral vascular disease | 42 (9.5) | 13 (4.8) | 29 (16.9) | 4.085 (2.058–8.106) | <0.001 |
| EF < 40% | 45 (10.13) | 22 (8.1) | 23 (13.4) | 1.773 (0.9455–3.292) | 0.070 |
| Anemia | 87 (19.6) | 41 (15) | 46 (26.9) | 2.099 (1.307–3.372) | 0.002 |
| Creatinine (mg/dL) | 0.9 (0.73–1.06) | 0.86 (0.7–1) | 0.99 (0.79–1.25) | 6.778 (3.405–13.49) | <0.001 |
| eGFR (ml/min) | 83.3 (65–91) | 85 (71–91) | 72 (53–86) | 0.964 (0.955–0.975) | <0.001 |
| EGFR < 60 mL/min | 86 (19.37) | 32 (11.7) | 54 (31.6) | 3.571 (2.190–5.822) | <0.001 |
| Only CABG | 171 (38.5) | 116 (42.5) | 55 (32.2) | 0.642 (0.43–0.958) | 0.03 |
| Leicester score | 18.45 (11.12–30.94) | 15.17 (9.2–22.45) | 26.81 (16.4–41.42) | 1.058 (1.042–1.073) | <0.001 |
| Euroscore II | 1.77 (1.08–3.02) | 1.42 (0.95–2.61) | 2.34 (1.34–3.89) | 1.203 (1.103–1.306) | <0.001 |
| Cleveland Clinic Score | 0.4 (0.4–1.8) | 0.4 (0.4–1.8) | 1.8 (0,4–1.8) | 1.188 (1.081–1.306) | <0.001 |
| Charlson Index | 4 (3–5) | 3 (2–5) | 4 (3–6) | 1.373 (1.226–1.537) | <0.001 |
| INTRAOPERATIVE | |||||
| Blood transfusion | 115 (26) | 61 (22.4) | 54 (31.6) | 1.610 (1.047–2.477) | 0.030 |
| Vasodilator agents | 153 (34.5) | 107 (39.2) | 46 (26.9) | 0.576 (0.379–0.873) | 0.009 |
| Dobutamine | 198 (45.2) | 118 (43.2) | 78 (45.6) | 1.114 (0.758–1.637) | 0.584 |
| Furosemide use | 114 (25.7) | 62 (22.7) | 52 (30.4) | 1.5 (0.974–2.310) | 0.066 |
| Vasoconstrictor agents | 298 (67.12) | 150 (54.9) | 148 (86.5) | 1.543 (1.039–2.292) | 0.032 |
| CPB time (min) | 91.5 (72–117) | 88 (71–110) | 100 (74–127) | 1.007 (1.002–1.012) | 0.005 |
| CPB time > 90 min | 226 (51.4) | 124 (45.8) | 102 (59.6) | 1.805 (1.222–2.666) | 0.003 |
| Ischemia time | 65 (50–80.25) | 60 (48–80) | 75 (54–92) | 1.012 (1.005–1.018) | <0.001 |
| Ischemia time > 70 min | 179 (41.2) | 91 (33.8) | 88 (51.5) | 2.235 (1.504–3.324) | <0.001 |
| Variable | OR (CI) | p-Value |
|---|---|---|
| Age ≥ 75 years | 1.483 (0.928–2.371) | 0.099 |
| Hypertension | 1.883 (1.086–3.265) | 0.024 |
| EGFR < 60mL/min | 2.365 (1.375–4.070) | 0.002 |
| Anemia | 1.642 (0.918–2.939) | 0.095 |
| Bypass | 0.838 (0,503–1.397) | 0.499 |
| Peripheral vascular disease | 4.66 (2.134–10.177) | <0.001 |
| Blood transfusion | 0.87 (0.509–1.487) | 0.608 |
| Vasopressors agents | 1.261 (0.784–2.027) | 0.34 |
| Vasodilators agents | 0.694 (0.412–1.168) | 0.169 |
| CPB time >90 min | 1.019 (0.553–1.879) | 0.951 |
| Isquemia time >70 min | 1.844 (0.979–3.473) | 0.058 |
| Discrimination | Calibration | ||
|---|---|---|---|
| AUC (95% CI) | p Value | Chi Square p Value a | |
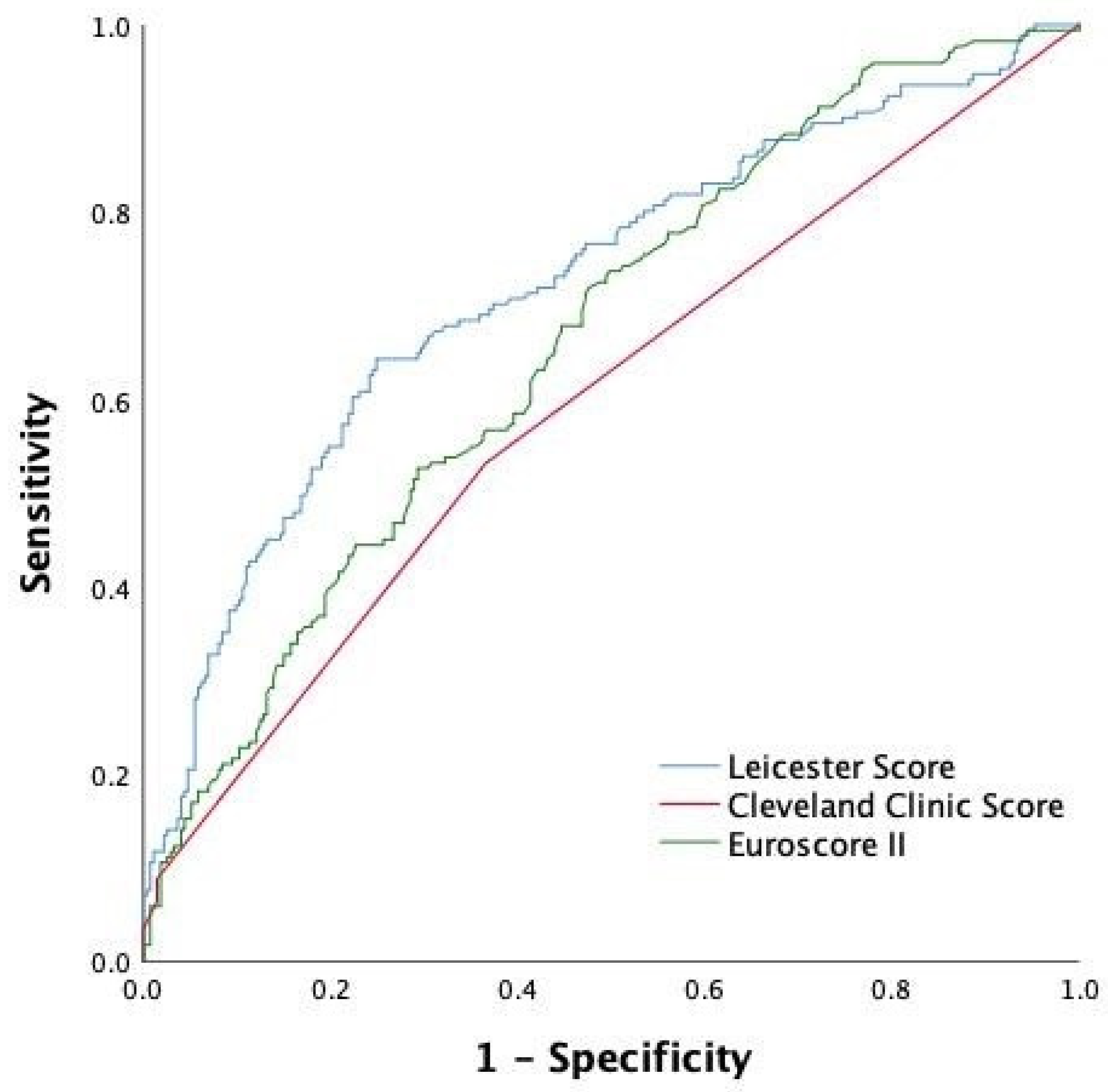
| Leicester score | 0.721 (0.671–0.771) | <0.001 | 10.1 0.225 |
| Cleveland Clinic Score | 0.595 (0.54–0.65) | 0.001 | 2.631 0.105 |
| Euroscore | 0.662 (0.611–0.713) | <0.001 | 11.48 0.176 |
| CCS-Euroscore II | CCS-LS | Euroscore II-LS | |
|---|---|---|---|
| Difference between areas | 0.067 | 0.126 | 0.059 |
| Stadard Error (CI) | 0.025 (0.017–0.112) | 0.030 (0.067–0.185) | 0.027 (0.006–0.112) |
| Z statistic | 2.633 | 4.203 | 2.199 |
| p-Value | 0.009 | <0.001 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina Andújar, A.; Lucas, A.; Escudero, V.J.; Rovira, I.; Matute, P.; Ibañez, C.; Blasco, M.; Sandoval, E.; Ruiz, J.; Chorda Sánchez, M.; et al. Risk Factors for Acute Kidney Injury Following Cardiac Surgery and Performance of Leicester Score in a Spanish Cohort. J. Clin. Med. 2022, 11, 904. https://doi.org/10.3390/jcm11040904
Molina Andújar A, Lucas A, Escudero VJ, Rovira I, Matute P, Ibañez C, Blasco M, Sandoval E, Ruiz J, Chorda Sánchez M, et al. Risk Factors for Acute Kidney Injury Following Cardiac Surgery and Performance of Leicester Score in a Spanish Cohort. Journal of Clinical Medicine. 2022; 11(4):904. https://doi.org/10.3390/jcm11040904
Chicago/Turabian StyleMolina Andújar, Alícia, Alvaro Lucas, Victor Joaquin Escudero, Irene Rovira, Purificación Matute, Cristina Ibañez, Miquel Blasco, Elena Sandoval, Jesús Ruiz, Marina Chorda Sánchez, and et al. 2022. "Risk Factors for Acute Kidney Injury Following Cardiac Surgery and Performance of Leicester Score in a Spanish Cohort" Journal of Clinical Medicine 11, no. 4: 904. https://doi.org/10.3390/jcm11040904
APA StyleMolina Andújar, A., Lucas, A., Escudero, V. J., Rovira, I., Matute, P., Ibañez, C., Blasco, M., Sandoval, E., Ruiz, J., Chorda Sánchez, M., Piñeiro, G. J., Quintana, E., & Poch, E. (2022). Risk Factors for Acute Kidney Injury Following Cardiac Surgery and Performance of Leicester Score in a Spanish Cohort. Journal of Clinical Medicine, 11(4), 904. https://doi.org/10.3390/jcm11040904







